

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50190686 ((R)-3'-(2,3-dimethylbenzo[b]thiophen-5-yl)spiro[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Displacement of [125I]alpha-BTX from alpha-7 nAChR in rat hippocampus membrane | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50190677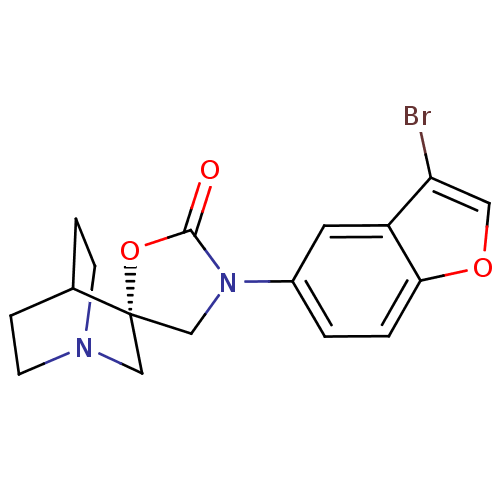 ((R)-3'-(3-bromobenzo[b]thiophen-5-yl)spiro[1-azabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Displacement of [125I]alpha-BTX from alpha-7 nAChR in rat hippocampus membrane | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164606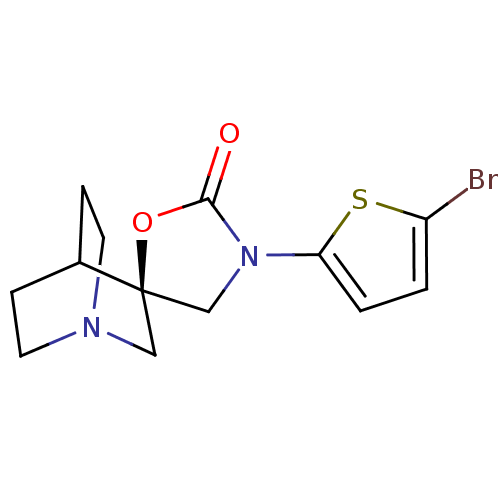 ((2R)-3'-(5-bromothien-2-yl)-2'H-spiro[4-azabicyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50190696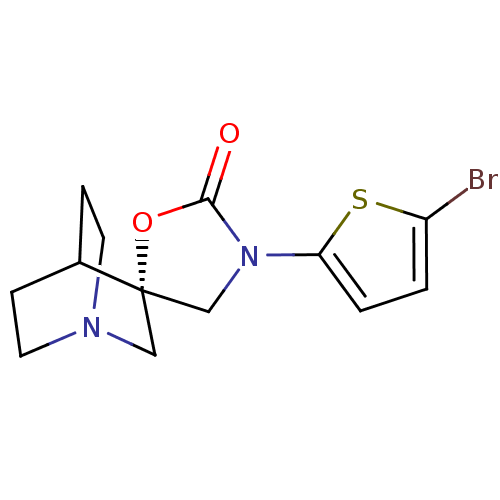 ((2S)-3'-(5-bromothien-2-yl)-2'H-spiro[4-azabicyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Displacement of [125I]alpha-BTX from alpha-7 nAChR in rat hippocampus membrane | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164618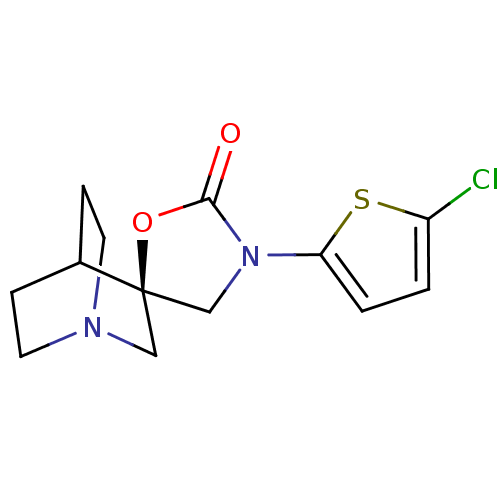 ((2R)-3'-(5-chlorothien-2-yl)-2'H-spiro[4-azabicycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164607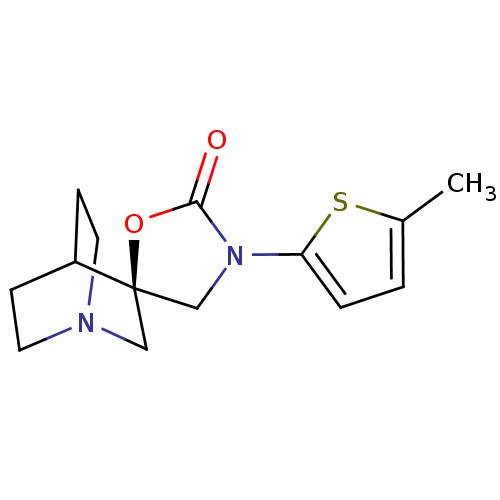 ((2R)-3'-(5-methylthien-2-yl)-2'H-spiro[4-azabicycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50149146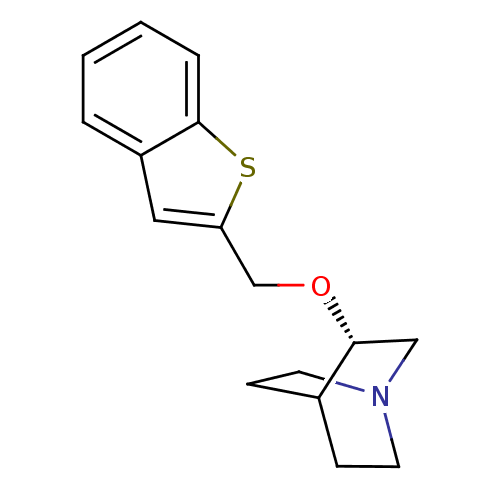 ((S)-3-(Benzo[b]thiophen-2-ylmethoxy)-1-aza-bicyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164611 ((2R)-3'-(5-ethylthien-2-yl)-2'H-spiro[4-azabicyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164621 ((2R)-3'-(2-naphthyl)-2'H-spiro[4-azabicyclo[2.2.2]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50149147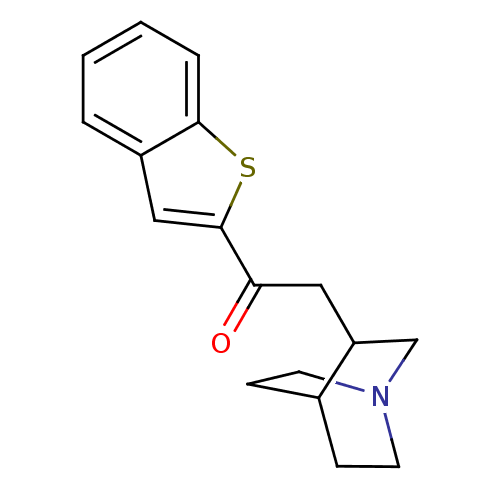 (2-(1-Aza-bicyclo[2.2.2]oct-3-yl)-1-benzo[b]thiophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164627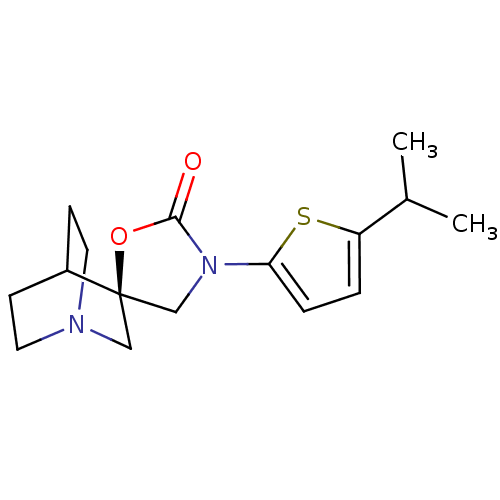 ((2R)-3'-(5-isopropylthien-2-yl)-2'H-spiro[4-azabic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164622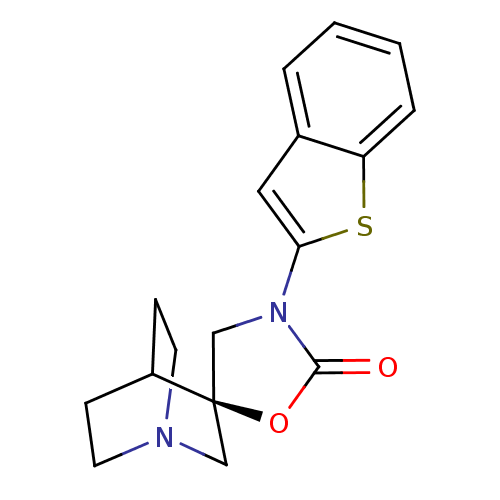 ((2R)-3'-(1-benzothien-2-yl)-2'H-spiro[4-azabicyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164612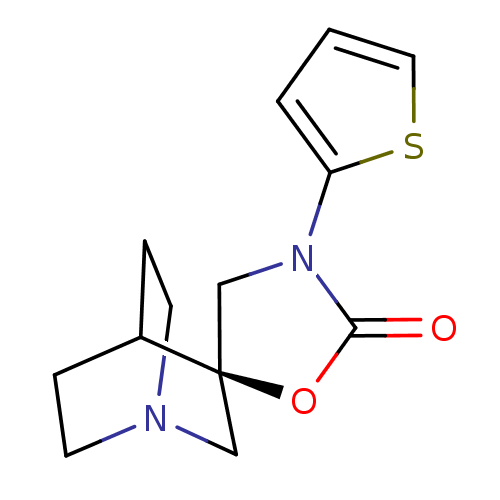 ((2R)-3'-thien-2-yl-2'H-spiro[4-azabicyclo[2.2.2]oc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164619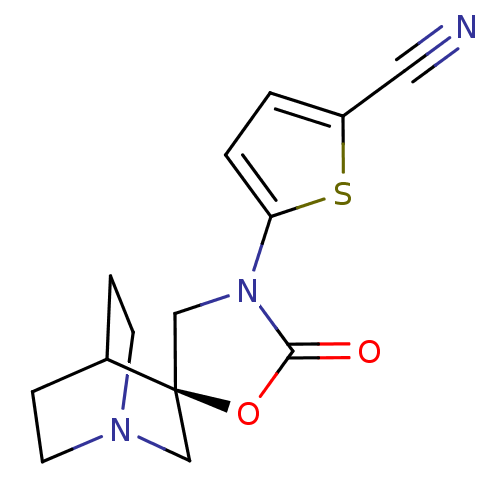 (5-[(2R)-2'-oxo-3'H-spiro[4-azabicyclo[2.2.2]octane...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164628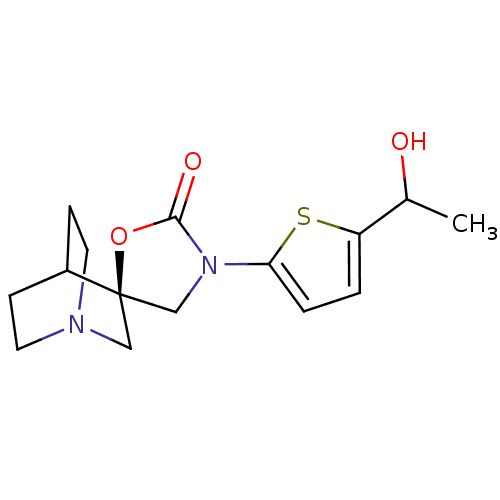 ((2R)-3'-[5-(1-hydroxyethyl)thien-2-yl]-2'H-spiro[4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164614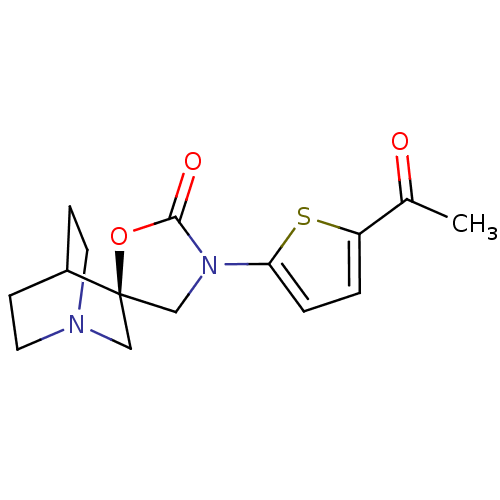 ((2R)-3'-(5-acetylthien-2-yl)-2'H-spiro[4-azabicycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50190687 ((R)-3'-(indan-5-yl)spiro[-1-azabicyclo[2.2.2]octan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Displacement of [125I]alpha-BTX from alpha-7 nAChR in rat hippocampus membrane | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164608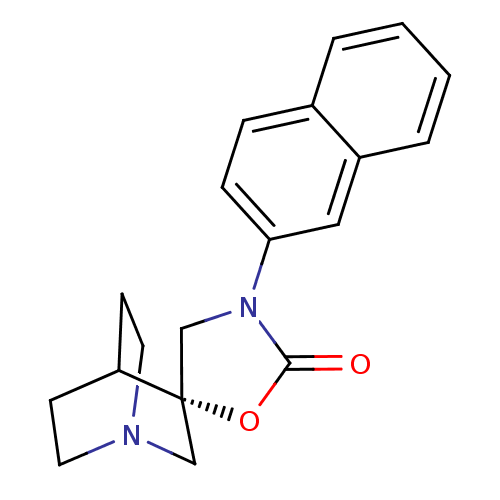 ((2S)-3'-(2-naphthyl)-2'H-spiro[4-azabicyclo[2.2.2]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50061564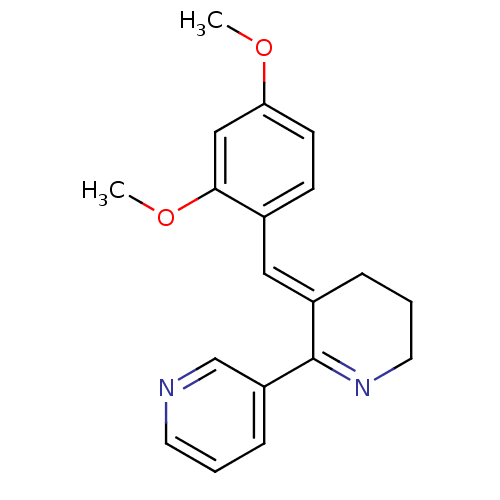 ((3E)-3-[(2,4-DIMETHOXYPHENYL)METHYLIDENE]-3,4,5,6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50093255 ((+)-Spiro[1-azabicyclo[2.2.2]octane-3,5'-oxazolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164629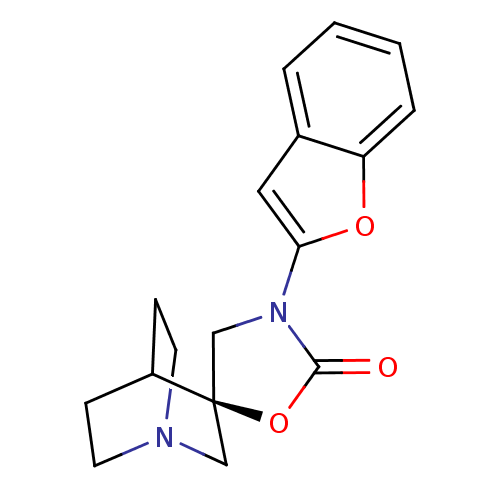 ((2R)-3'-(1-benzofuran-2-yl)-2'H-spiro[4-azabicyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164615 ((2R)-3'-(1,3-benzothiazol-2-yl)-2'H-spiro[4-azabic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164624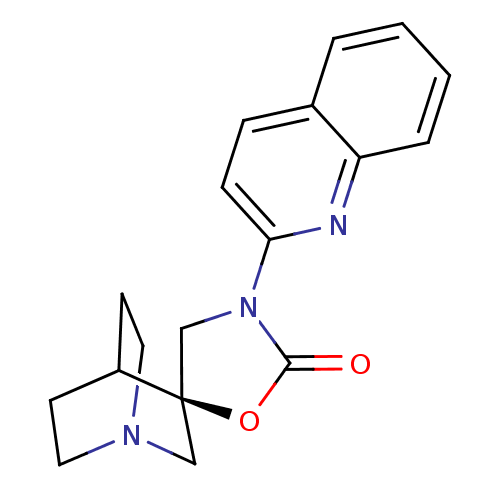 ((2R)-3'-quinolin-2-yl-2'H-spiro[4-azabicyclo[2.2.2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity mitogen-activated protein kinase kinase 1 (Mus musculus) | BDBM50170831 (CHEMBL188343 | N*6*-Cyclohexyl-N*2*-(4-morpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of MEK1 in mouse C2C12 by ERK2 phosphorylation assay | Proc Natl Acad Sci U S A 104: 10482-7 (2007) Article DOI: 10.1073/pnas.0704360104 BindingDB Entry DOI: 10.7270/Q2QR4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50190684 ((R)-3'-(3-methyl-2-oxo-2,3-dihydro-1,3-benzothiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Displacement of [125I]alpha-BTX from alpha-7 nAChR in rat hippocampus membrane | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164625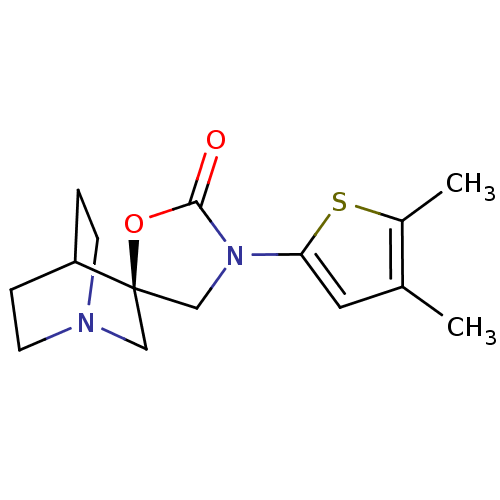 ((2R)-3'-(4,5-dimethylthien-2-yl)-2'H-spiro[4-azabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164623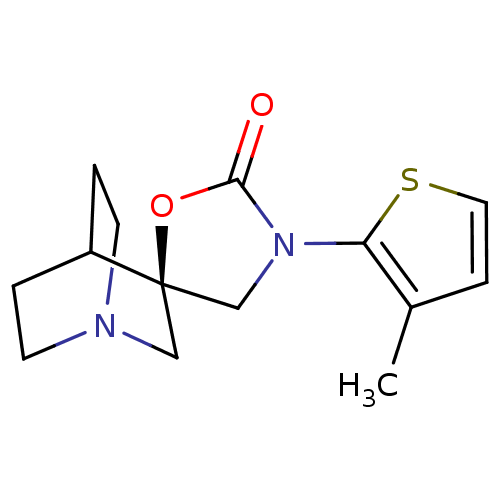 ((2R)-3'-(3-methylthien-2-yl)-2'H-spiro[4-azabicycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164620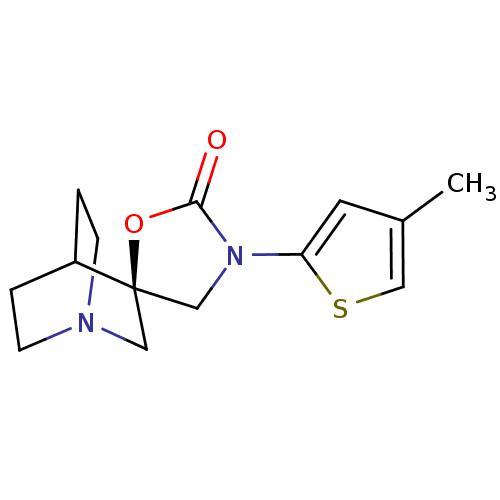 ((2R)-3'-(4-methylthien-2-yl)-2'H-spiro[4-azabicycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164617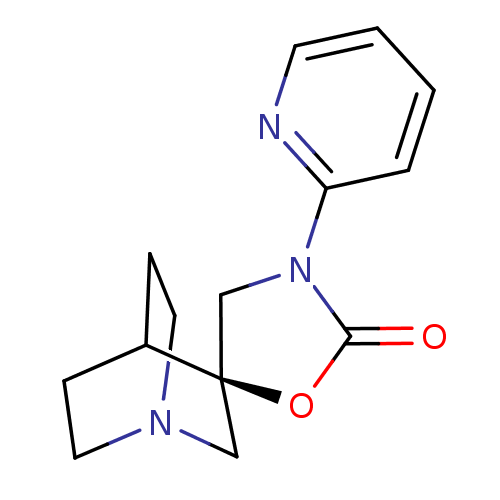 ((2R)-3'-pyridin-2-yl-2'H-spiro[4-azabicyclo[2.2.2]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164616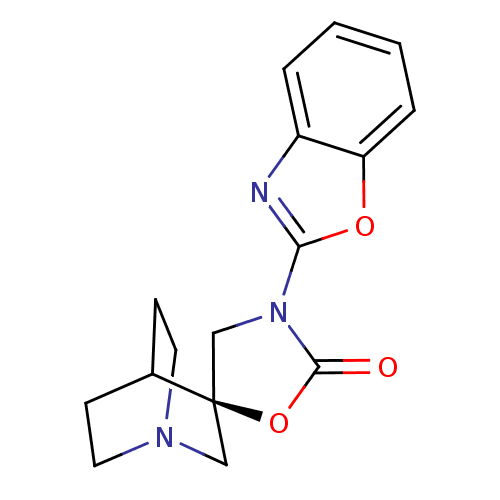 ((2R)-3'-(1,3-benzoxazol-2-yl)-2'H-spiro[4-azabicyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164610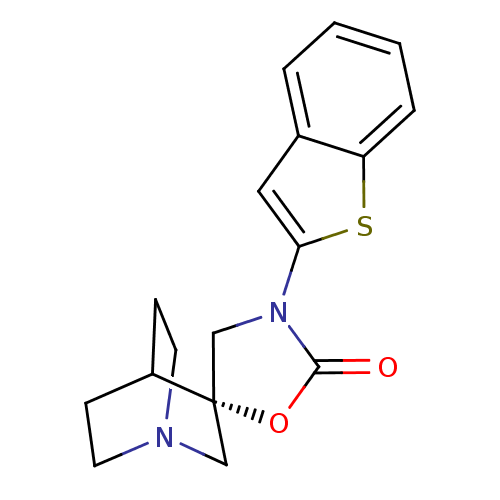 ((2S)-3'-(1-benzothien-2-yl)-2'H-spiro[4-azabicyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164609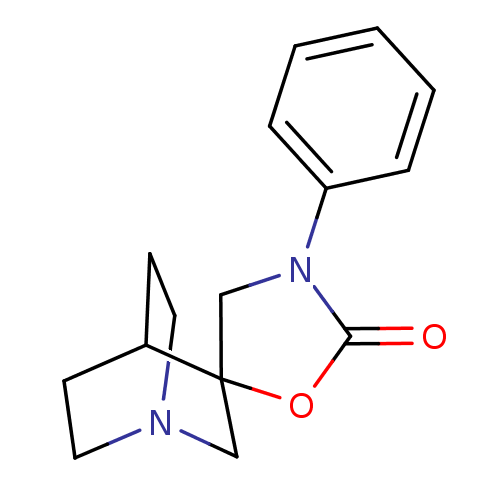 (3'-phenyl-2'H-spiro[4-azabicyclo[2.2.2]octane-2,5'...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164626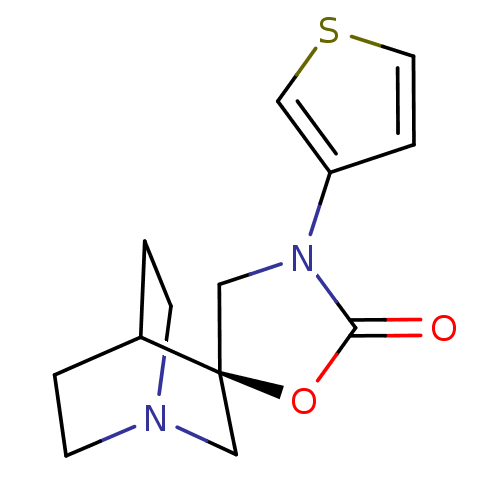 ((2R)-3'-thien-3-yl-2'H-spiro[4-azabicyclo[2.2.2]oc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50190698 ((R)-3'-(3-methyl-2-oxo-2,3-dihydro-1,3-benzoxazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Displacement of [125I]alpha-BTX from alpha-7 nAChR in rat hippocampus membrane | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50190695 ((R)-3'-(quinolin-6-yl)spiro[1-azabicyclo[2.2.2]oct...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Displacement of [125I]alpha-BTX from alpha-7 nAChR in rat hippocampus membrane | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50164618 ((2R)-3'-(5-chlorothien-2-yl)-2'H-spiro[4-azabicycl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50190696 ((2S)-3'-(5-bromothien-2-yl)-2'H-spiro[4-azabicyclo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50410988 (CHEMBL2113241) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Binding affinity to histamine H1 receptor | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50410986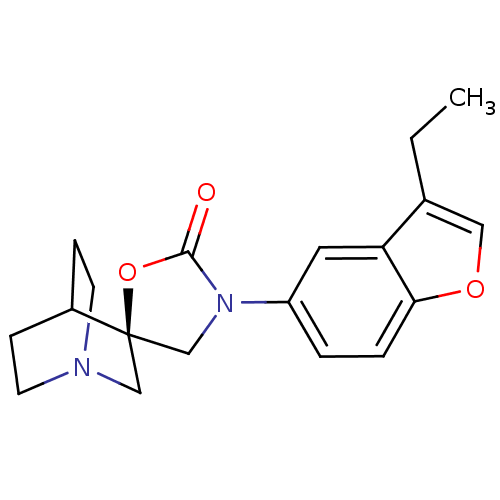 (CHEMBL2113242) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50410987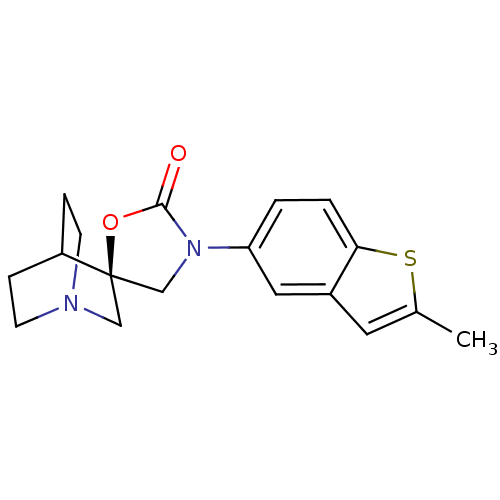 (CHEMBL2113238) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50190677 ((R)-3'-(3-bromobenzo[b]thiophen-5-yl)spiro[1-azabi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50190686 ((R)-3'-(2,3-dimethylbenzo[b]thiophen-5-yl)spiro[1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50410992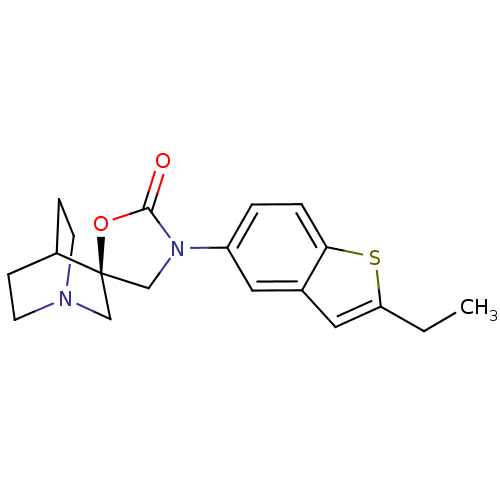 (CHEMBL2113239) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50410989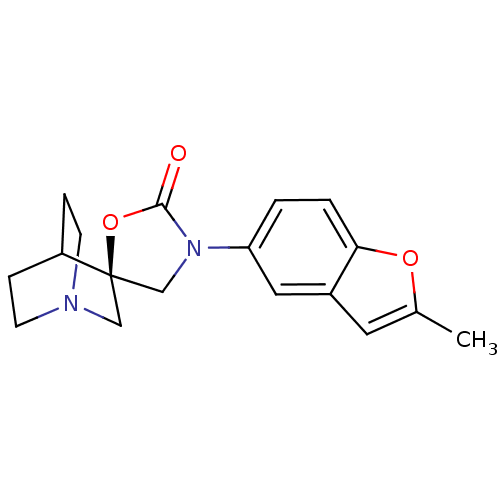 (CHEMBL2113233) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50410990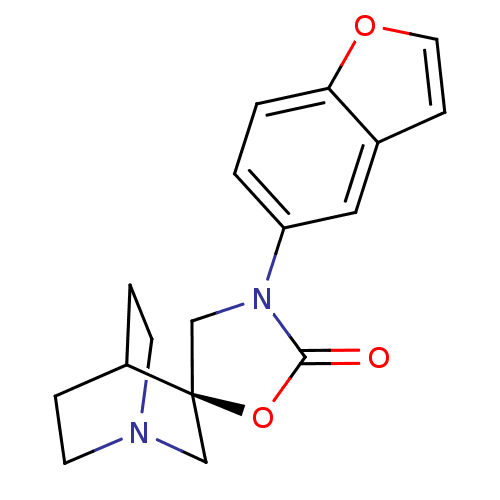 (CHEMBL2113228) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50410991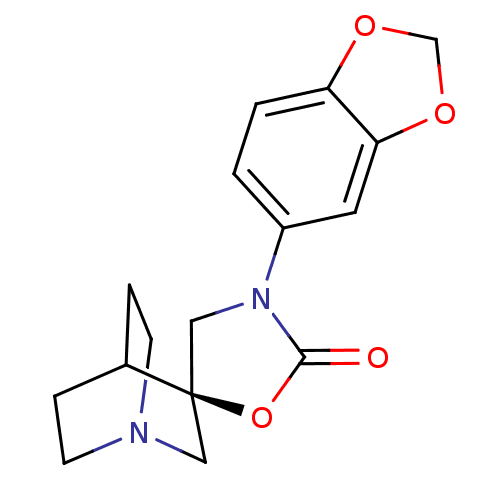 (CHEMBL2113236) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50393256 (CHEMBL2113232) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50410988 (CHEMBL2113241) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50410993 (CHEMBL2113240) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosylation factor 1 (Homo sapiens (Human)) | BDBM50373058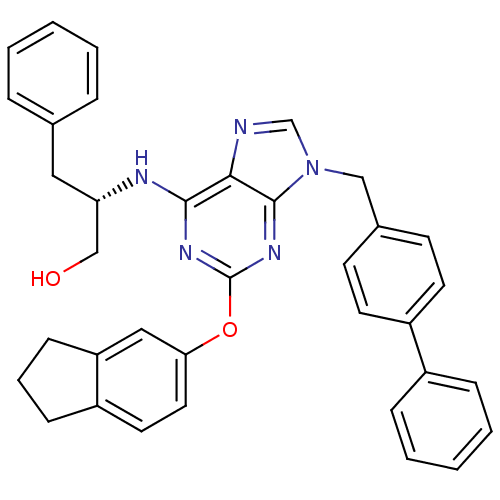 (CHEMBL259181) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Binding affinity to ADP-ribosylation factor 1 expressed HEK293 cells by surface plasmon resonance analysis | Proc Natl Acad Sci USA 104: 7444-8 (2007) Article DOI: 10.1073/pnas.0702136104 BindingDB Entry DOI: 10.7270/Q2SJ1MGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||