

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249758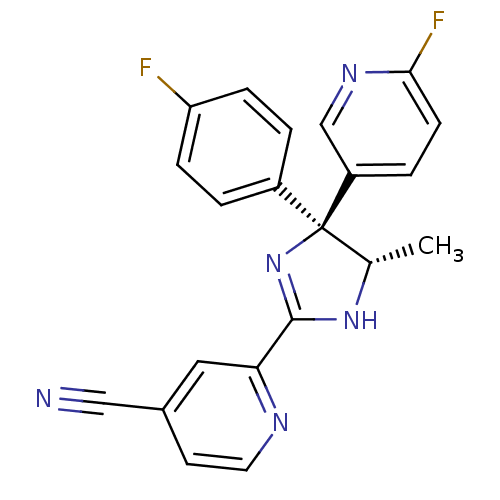 (2-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity to human NPYY5 receptor | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249833 (6-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346211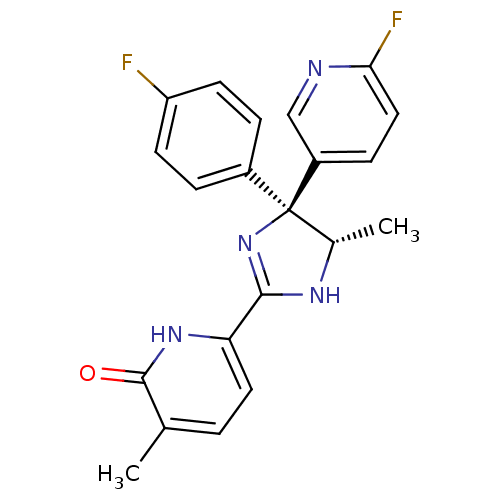 (6-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoropyridin-3...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346228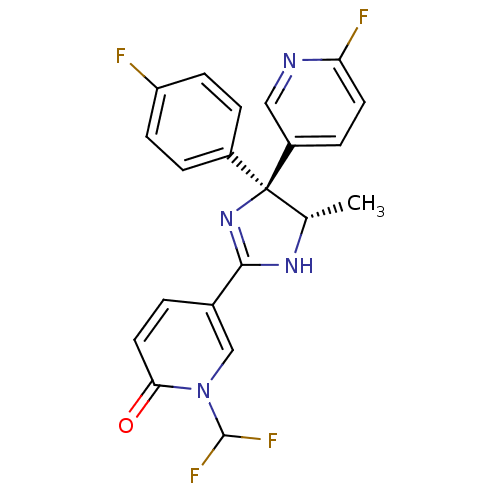 (1-(Difluoromethyl)-5-[(4S,5S)-4-(4-fluorophenyl)-4...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346229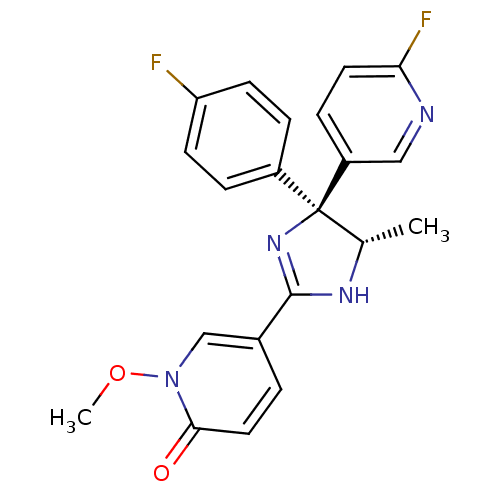 (5-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoropyridin-3...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346225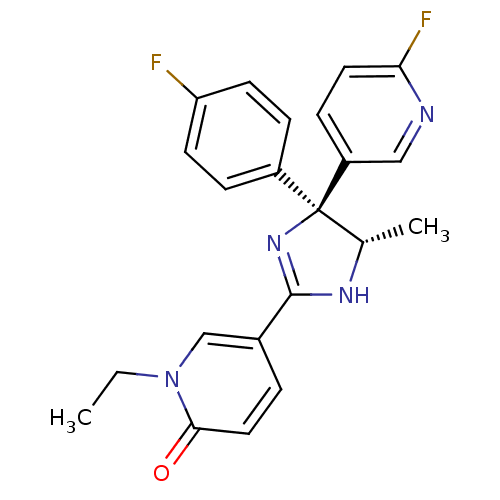 (1-Ethyl-5-[(4S,5S)-4-(4-fluorophenyl)-4-(6-fluorop...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346221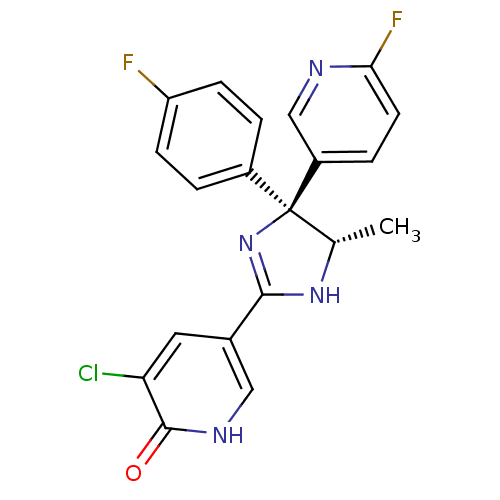 (3-Chloro-5-[(4S,5S)-4-(4-fluorophenyl)-4-(6-fluoro...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346227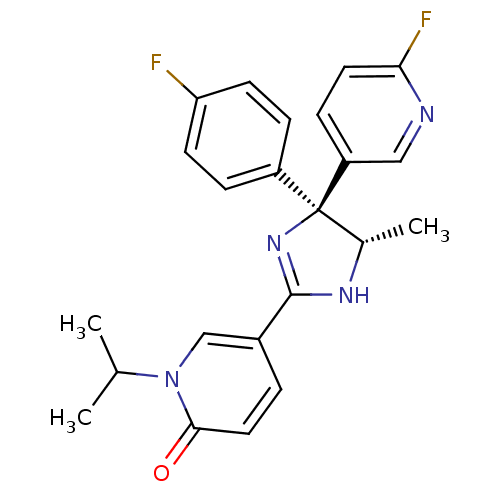 (5-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoropyridin-3...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346222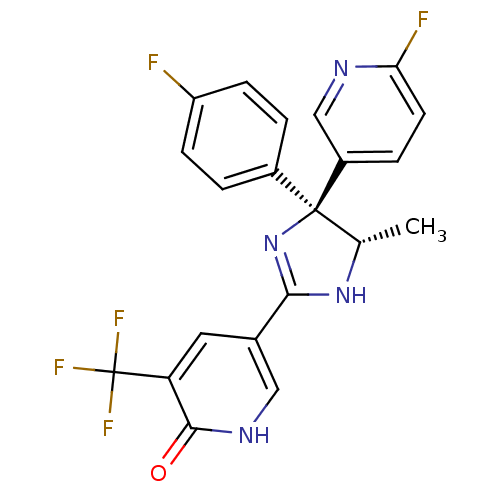 (5-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoropyridin-3...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346217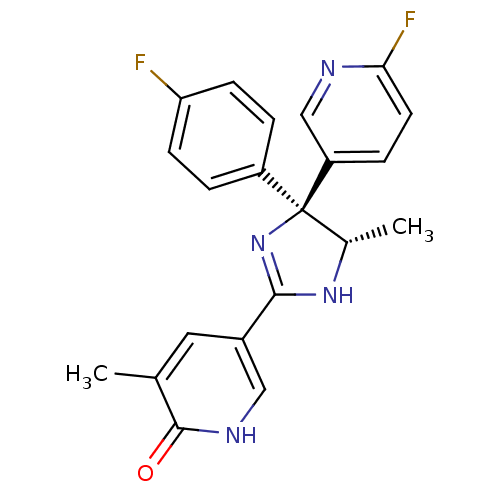 (5-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoropyridin-3...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346226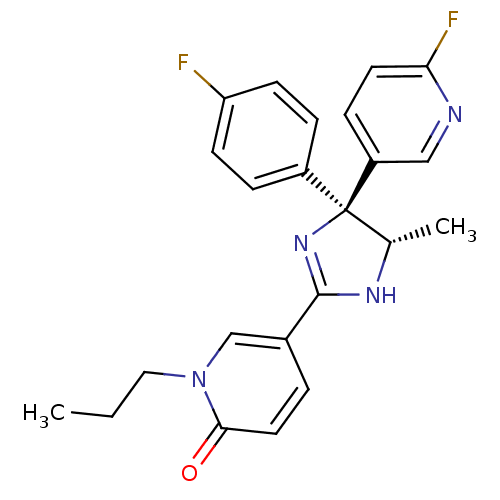 (5-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoropyridin-3...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346223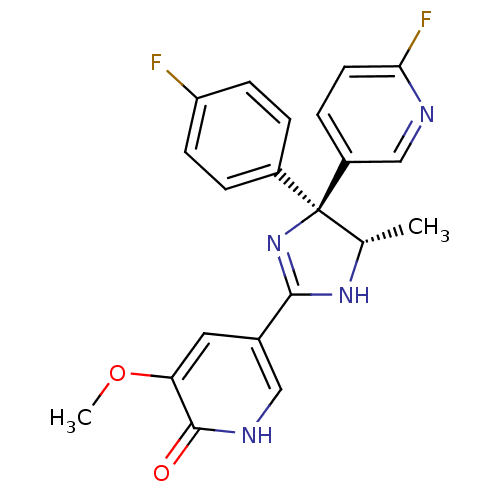 (5-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoropyridin-3...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346231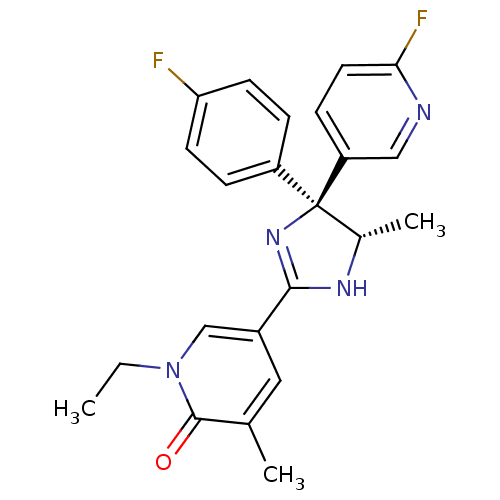 (1-Ethyl-5-[(4S,5S)-4-(4-fluorophenyl)-4-(6-fluorop...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346214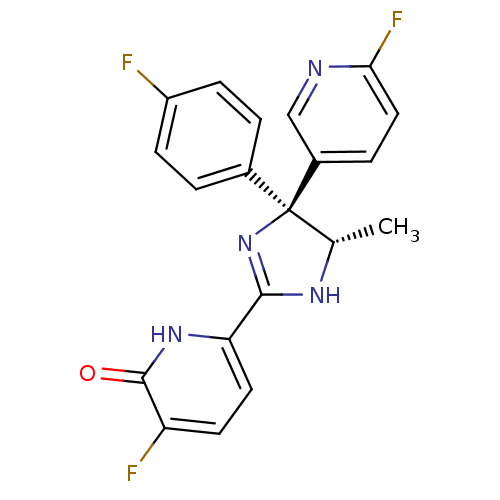 (3-Fluoro-6-[(4S,5S)-4-(4-fluorophenyl)-4-(6-fluoro...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346230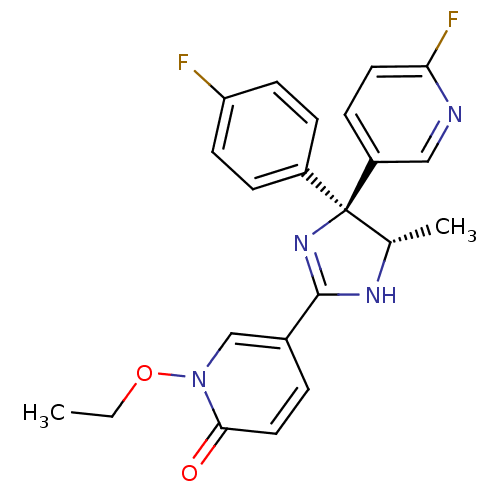 (1-Ethoxy-5-[(4S,5S)-4-(4-fluorophenyl)-4-(6-fluoro...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346233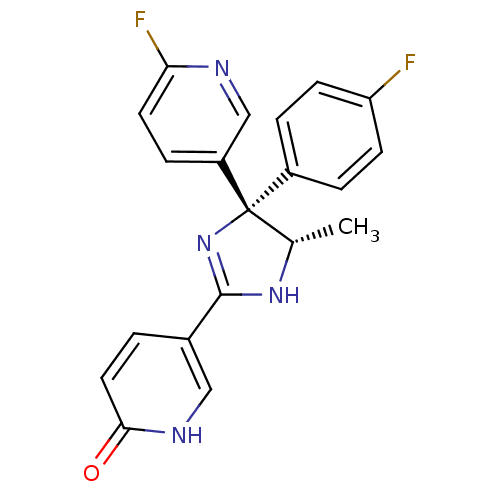 (5-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoropyridin-3...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346232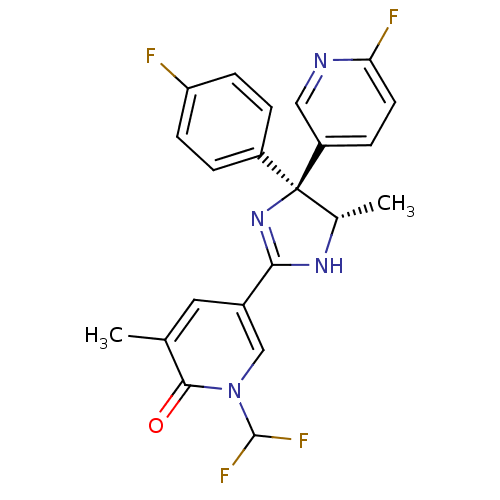 (1-(Difluoromethyl)-5-[(4S,5S)-4-(4-fluorophenyl)-4...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346212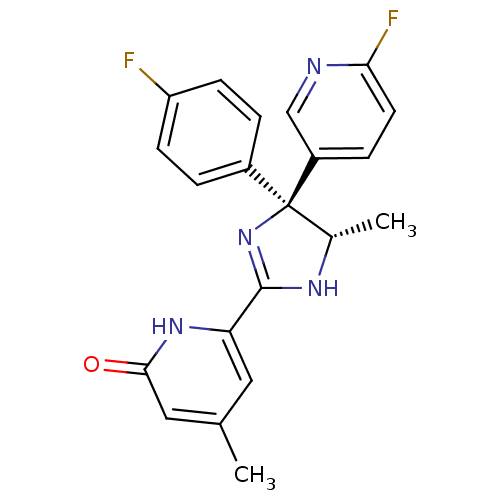 (6-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoropyridin-3...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346215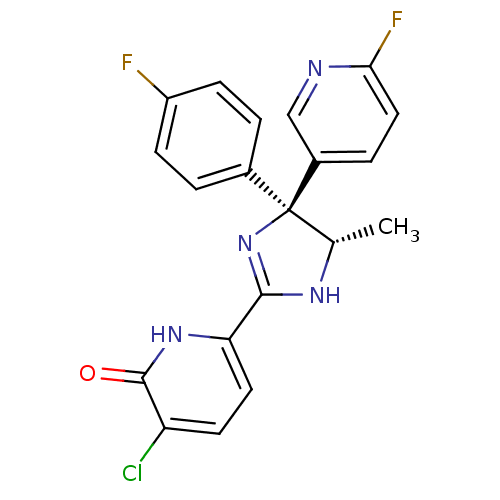 (3-Chloro-6-[(4S,5S)-4-(4-fluorophenyl)-4-(6-fluoro...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346220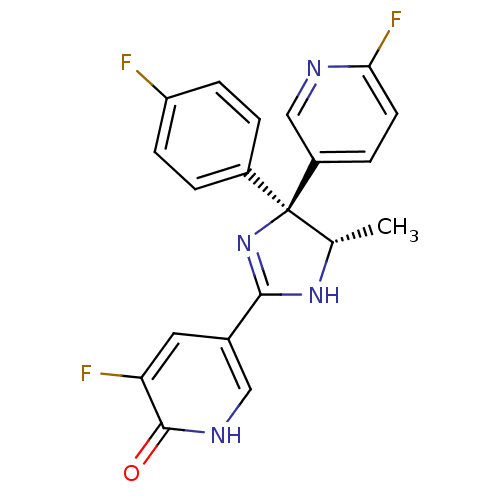 (3-Fluoro-5-[(4S,5S)-4-(4-fluorophenyl)-4-(6-fluoro...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346224 (5-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoropyridin-3...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346213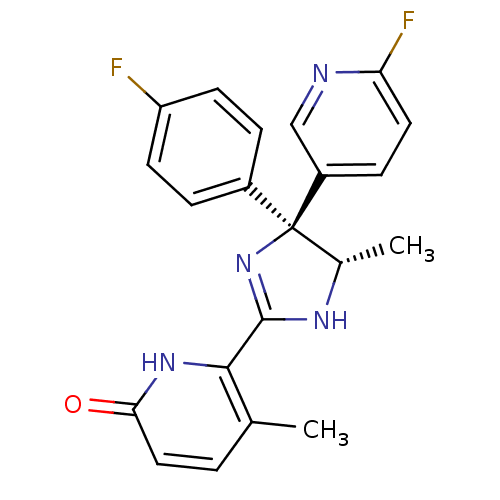 (6-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoropyridin-3...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346210 (4-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoropyridin-3...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50344644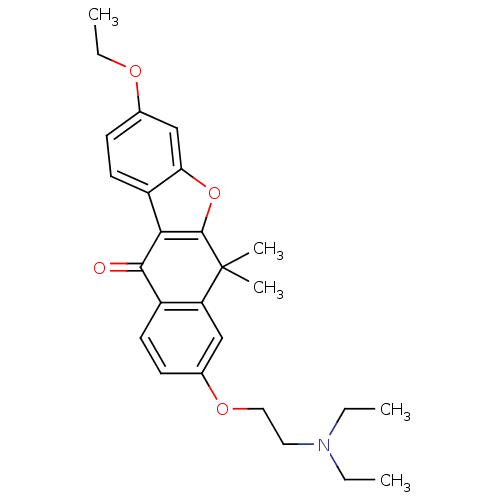 (8-(2-(diethylamino)ethoxy)-3-ethoxy-6,6-dimethylbe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of ALK phosphorylation by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 3788-93 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.04.020 BindingDB Entry DOI: 10.7270/Q2Q81DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346216 (6-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoropyridin-3...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346234 (3-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoropyridin-3...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346218 (5-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoropyridin-3...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346219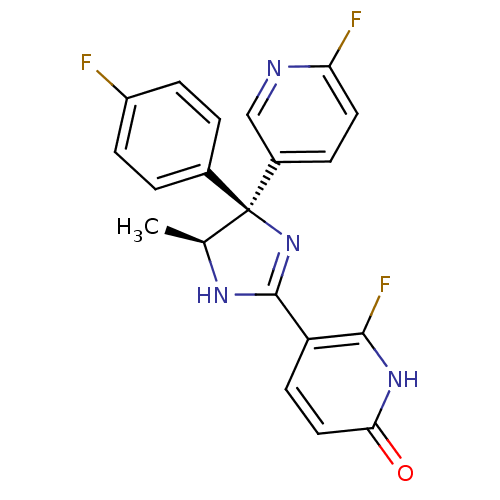 (6-Fluoro-5-[(4S,5S)-4-(4-fluorophenyl)-4-(6-fluoro...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269429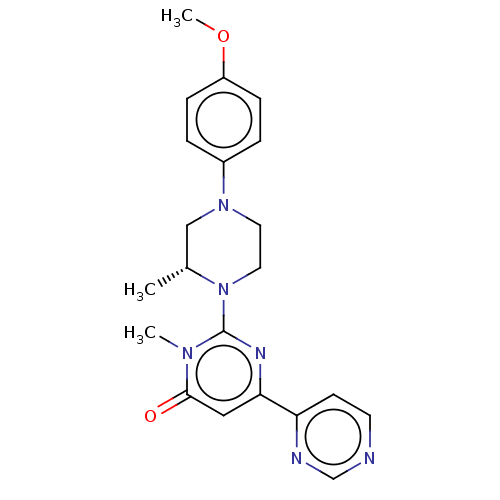 (CHEMBL4084855) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269437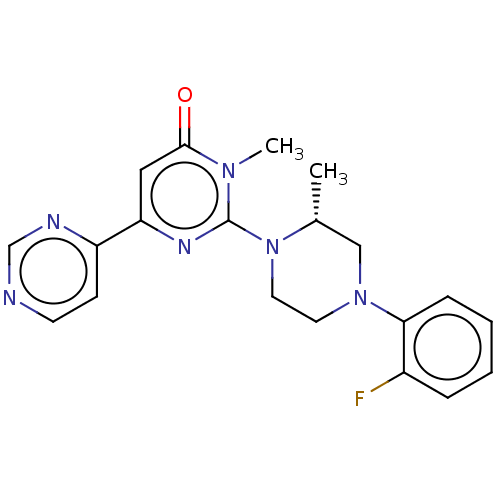 (CHEMBL4077376) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269428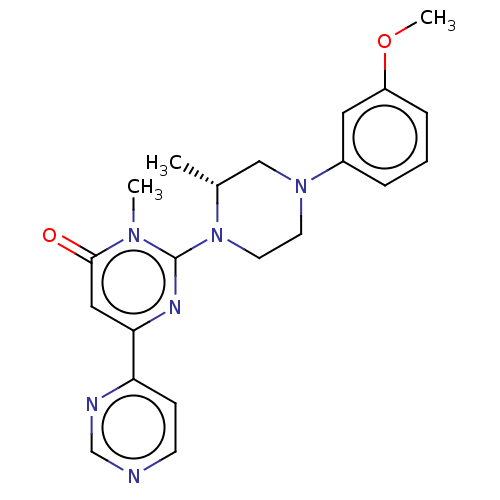 (CHEMBL4063206) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269447 (CHEMBL4076186) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000 Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GSK-3beta using prephosphorylated GS-1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scinti... | Bioorg Med Chem Lett 27: 3726-3732 (2017) Article DOI: 10.1016/j.bmcl.2017.06.078 BindingDB Entry DOI: 10.7270/Q2T43WKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269447 (CHEMBL4076186) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000 Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GSK-3beta using prephosphorylated GS-1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scinti... | Bioorg Med Chem Lett 27: 3726-3732 (2017) Article DOI: 10.1016/j.bmcl.2017.06.078 BindingDB Entry DOI: 10.7270/Q2T43WKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269455 (CHEMBL4087402) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000 Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GSK-3beta using prephosphorylated GS-1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scinti... | Bioorg Med Chem Lett 27: 3726-3732 (2017) Article DOI: 10.1016/j.bmcl.2017.06.078 BindingDB Entry DOI: 10.7270/Q2T43WKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269438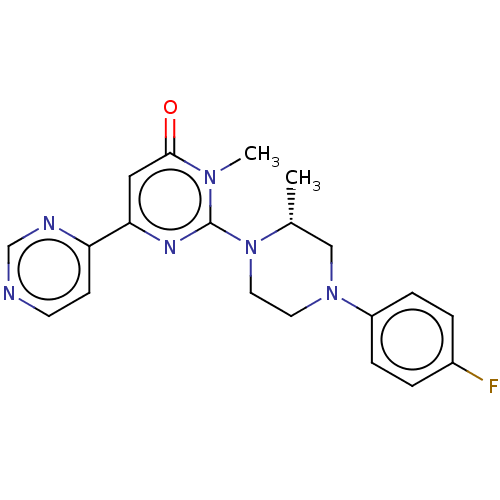 (CHEMBL4076060) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269455 (CHEMBL4087402) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000 Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GSK-3beta using prephosphorylated GS-1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scinti... | Bioorg Med Chem Lett 27: 3726-3732 (2017) Article DOI: 10.1016/j.bmcl.2017.06.078 BindingDB Entry DOI: 10.7270/Q2T43WKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269443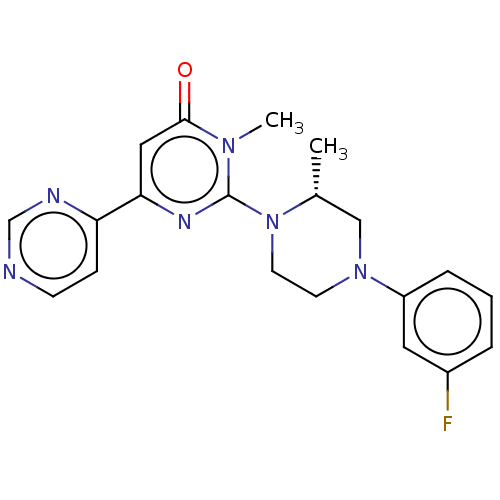 (CHEMBL4095848) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269440 (CHEMBL4065818) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM244381 (US9550763, Compound I-133) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Stably expressing cell line (C6BU-1 cell transfected with human P2X3 receptor gene (GenBank accession number Y07683) was used. The cells were seeded ... | US Patent US9550763 (2017) BindingDB Entry DOI: 10.7270/Q2V126TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM244285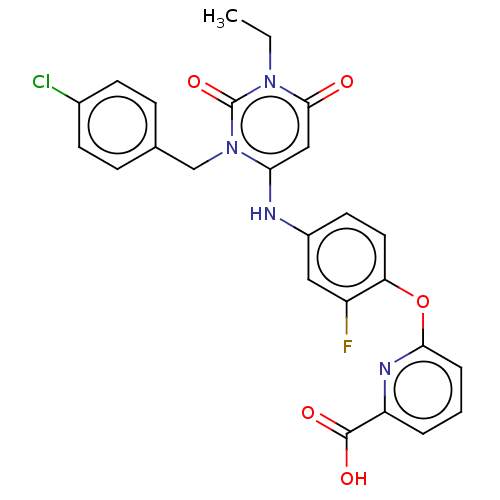 (US9550763, Compound I-033) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Stably expressing cell line (C6BU-1 cell transfected with human P2X3 receptor gene (GenBank accession number Y07683) was used. The cells were seeded ... | US Patent US9550763 (2017) BindingDB Entry DOI: 10.7270/Q2V126TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM244385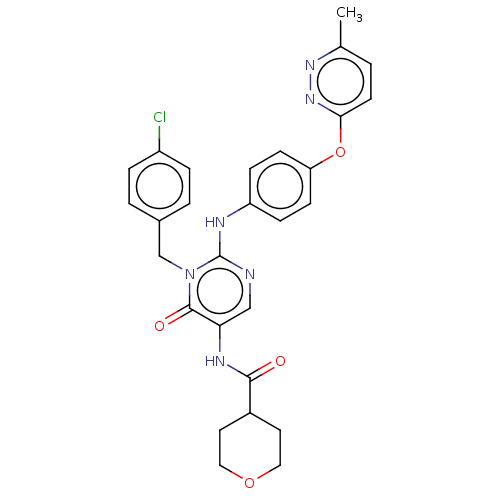 (US9550763, Compound I-137) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Stably expressing cell line (C6BU-1 cell transfected with human P2X3 receptor gene (GenBank accession number Y07683) was used. The cells were seeded ... | US Patent US9550763 (2017) BindingDB Entry DOI: 10.7270/Q2V126TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM244280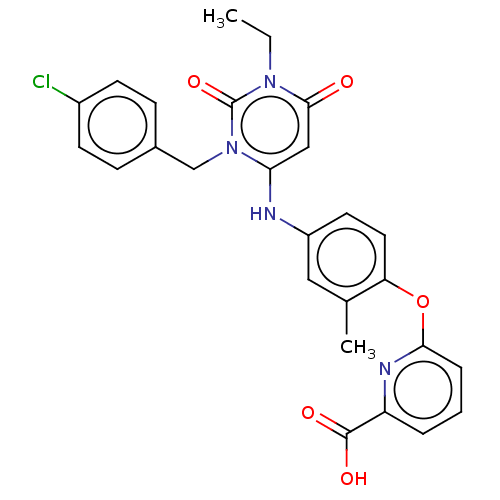 (US9550763, Compound I-028) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Stably expressing cell line (C6BU-1 cell transfected with human P2X3 receptor gene (GenBank accession number Y07683) was used. The cells were seeded ... | US Patent US9550763 (2017) BindingDB Entry DOI: 10.7270/Q2V126TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269492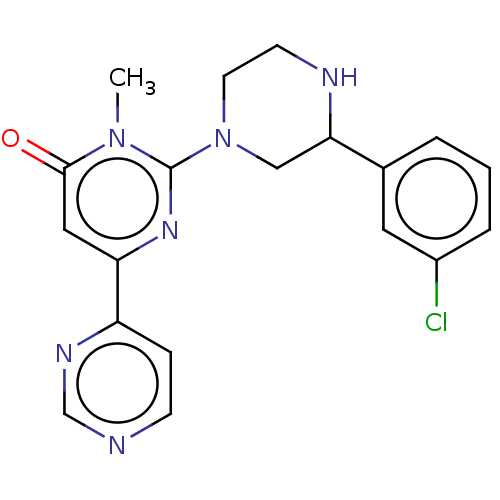 (CHEMBL4067532) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000 Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GSK-3beta using prephosphorylated GS-1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scinti... | Bioorg Med Chem Lett 27: 3726-3732 (2017) Article DOI: 10.1016/j.bmcl.2017.06.078 BindingDB Entry DOI: 10.7270/Q2T43WKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269452 (CHEMBL4105346) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000 Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GSK-3beta using prephosphorylated GS-1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scinti... | Bioorg Med Chem Lett 27: 3726-3732 (2017) Article DOI: 10.1016/j.bmcl.2017.06.078 BindingDB Entry DOI: 10.7270/Q2T43WKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269439 (CHEMBL4077048) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296002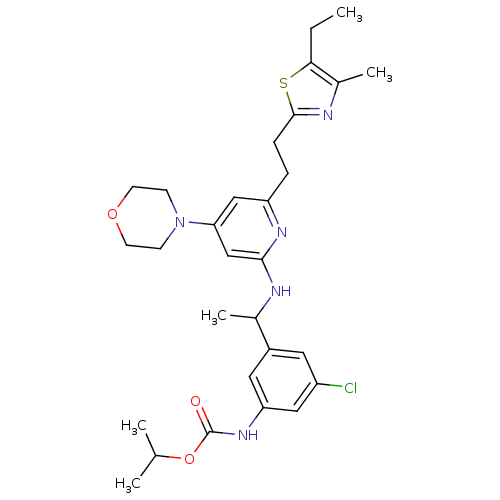 (CHEMBL564536 | isopropyl 3-chloro-5-(1-(6-(2-(5-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant NPY Y1 receptor expressed in CHO cells assessed as inhibition of [35S]GTPgammaS binding by scinitill... | Bioorg Med Chem Lett 19: 4325-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.069 BindingDB Entry DOI: 10.7270/Q21G0M97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269426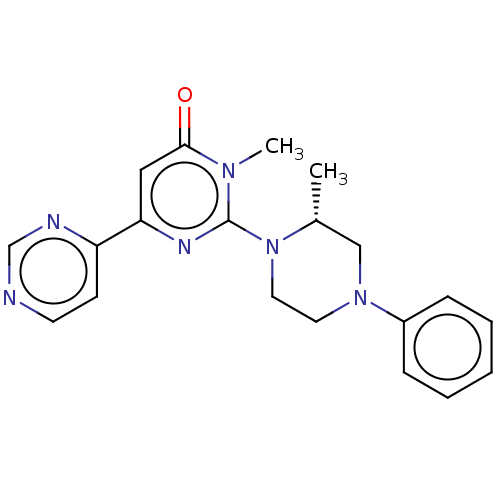 (CHEMBL3719193) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50344664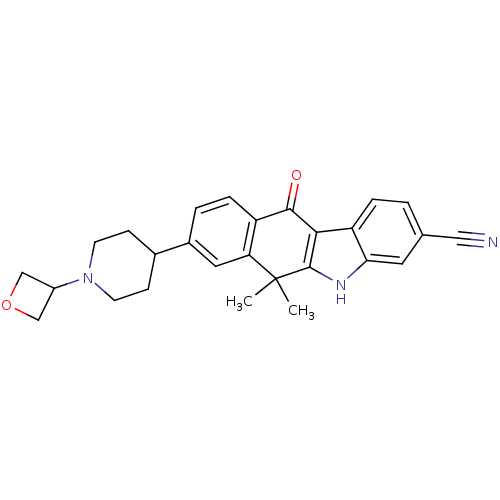 (6,6-dimethyl-8-(1-(oxetan-3-yl)piperidin-4-yl)-11-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of ALK assessed as biotin-EGPWLEEEEEAYGWMDF peptide phosphorylation by TR-FRET assay | Bioorg Med Chem Lett 21: 3788-93 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.04.020 BindingDB Entry DOI: 10.7270/Q2Q81DF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269494 (CHEMBL4104714) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000 Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GSK-3beta using prephosphorylated GS-1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scinti... | Bioorg Med Chem Lett 27: 3726-3732 (2017) Article DOI: 10.1016/j.bmcl.2017.06.078 BindingDB Entry DOI: 10.7270/Q2T43WKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269485 (CHEMBL4071429) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000 Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GSK-3beta using prephosphorylated GS-1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scinti... | Bioorg Med Chem Lett 27: 3726-3732 (2017) Article DOI: 10.1016/j.bmcl.2017.06.078 BindingDB Entry DOI: 10.7270/Q2T43WKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1148 total ) | Next | Last >> |