 Found 2758 hits with Last Name = 'tei' and Initial = 'l'
Found 2758 hits with Last Name = 'tei' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075807
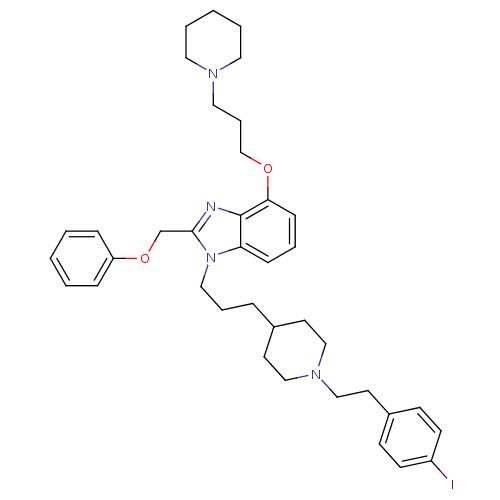
(1-(3-{1-[2-(4-Iodo-phenyl)-ethyl]-piperidin-4-yl}-...)Show SMILES Ic1ccc(CCN2CCC(CCCn3c(COc4ccccc4)nc4c(OCCCN5CCCCC5)cccc34)CC2)cc1 Show InChI InChI=1S/C38H49IN4O2/c39-33-17-15-32(16-18-33)21-28-42-26-19-31(20-27-42)10-8-25-43-35-13-7-14-36(44-29-9-24-41-22-5-2-6-23-41)38(35)40-37(43)30-45-34-11-3-1-4-12-34/h1,3-4,7,11-18,31H,2,5-6,8-10,19-30H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075796
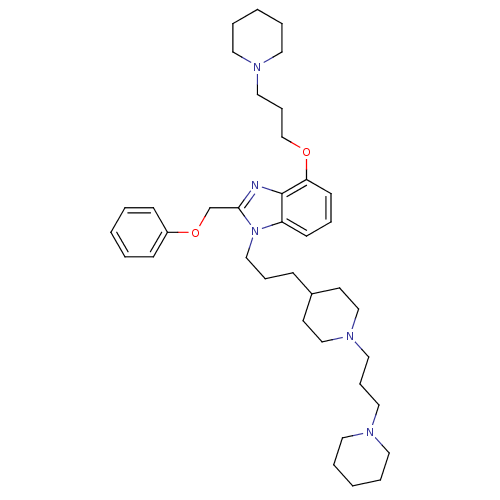
(2-Phenoxymethyl-4-(3-piperidin-1-yl-propoxy)-1-{3-...)Show SMILES C(COc1cccc2n(CCCC3CCN(CCCN4CCCCC4)CC3)c(COc3ccccc3)nc12)CN1CCCCC1 Show InChI InChI=1S/C38H57N5O2/c1-4-15-34(16-5-1)45-32-37-39-38-35(17-10-18-36(38)44-31-13-27-41-23-8-3-9-24-41)43(37)28-11-14-33-19-29-42(30-20-33)26-12-25-40-21-6-2-7-22-40/h1,4-5,10,15-18,33H,2-3,6-9,11-14,19-32H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075811

(3-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-propo...)Show SMILES O=C(CCN1CCC(CCCn2c(COc3ccccc3)nc3c(OCCCN4CCCCC4)cccc23)CC1)c1ccccc1 Show InChI InChI=1S/C39H50N4O3/c44-36(33-14-4-1-5-15-33)22-29-42-27-20-32(21-28-42)13-11-26-43-35-18-10-19-37(45-30-12-25-41-23-8-3-9-24-41)39(35)40-38(43)31-46-34-16-6-2-7-17-34/h1-2,4-7,10,14-19,32H,3,8-9,11-13,20-31H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075803
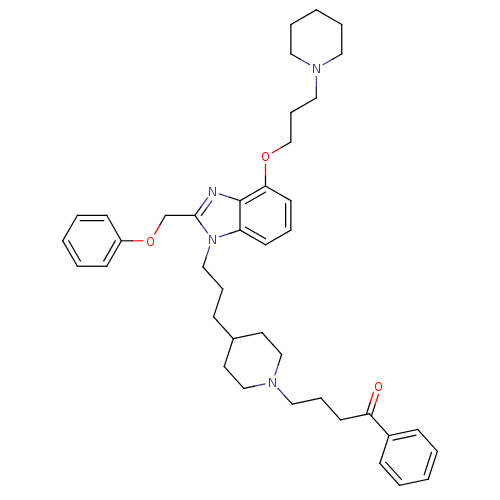
(4-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-propo...)Show SMILES O=C(CCCN1CCC(CCCn2c(COc3ccccc3)nc3c(OCCCN4CCCCC4)cccc23)CC1)c1ccccc1 Show InChI InChI=1S/C40H52N4O3/c45-37(34-15-4-1-5-16-34)20-12-26-43-29-22-33(23-30-43)14-11-28-44-36-19-10-21-38(46-31-13-27-42-24-8-3-9-25-42)40(36)41-39(44)32-47-35-17-6-2-7-18-35/h1-2,4-7,10,15-19,21,33H,3,8-9,11-14,20,22-32H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075812
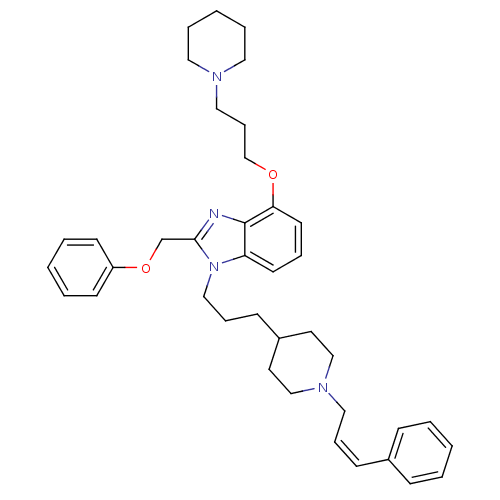
(2-Phenoxymethyl-1-{3-[1-((Z)-3-phenyl-allyl)-piper...)Show SMILES C(COc1cccc2n(CCCC3CCN(C\C=C/c4ccccc4)CC3)c(COc3ccccc3)nc12)CN1CCCCC1 Show InChI InChI=1S/C39H50N4O2/c1-4-14-33(15-5-1)16-11-26-42-29-22-34(23-30-42)17-12-28-43-36-20-10-21-37(44-31-13-27-41-24-8-3-9-25-41)39(36)40-38(43)32-45-35-18-6-2-7-19-35/h1-2,4-7,10-11,14-16,18-21,34H,3,8-9,12-13,17,22-32H2/b16-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075809

(1-[3-(1-Phenethyl-piperidin-4-yl)-propyl]-2-phenox...)Show SMILES C(COc1cccc2n(CCCC3CCN(CCc4ccccc4)CC3)c(COc3ccccc3)nc12)CN1CCCCC1 Show InChI InChI=1S/C38H50N4O2/c1-4-13-32(14-5-1)20-27-41-28-21-33(22-29-41)15-11-26-42-35-18-10-19-36(43-30-12-25-40-23-8-3-9-24-40)38(35)39-37(42)31-44-34-16-6-2-7-17-34/h1-2,4-7,10,13-14,16-19,33H,3,8-9,11-12,15,20-31H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394157
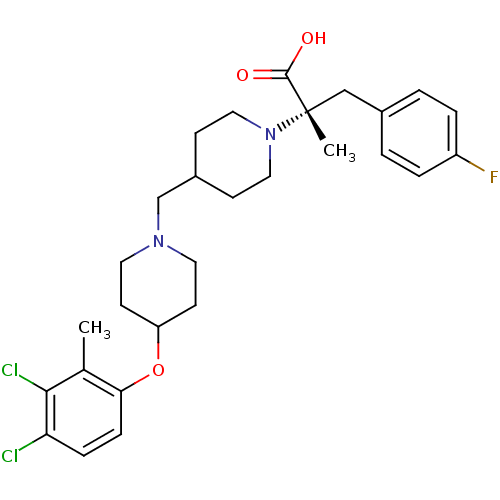
(CHEMBL2158790)Show SMILES Cc1c(Cl)c(Cl)ccc1OC1CCN(CC2CCN(CC2)[C@@](C)(Cc2ccc(F)cc2)C(O)=O)CC1 |r| Show InChI InChI=1S/C28H35Cl2FN2O3/c1-19-25(8-7-24(29)26(19)30)36-23-11-13-32(14-12-23)18-21-9-15-33(16-10-21)28(2,27(34)35)17-20-3-5-22(31)6-4-20/h3-8,21,23H,9-18H2,1-2H3,(H,34,35)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR3 expressed in CHOK1 cells by radioligand displacement assay |
Bioorg Med Chem Lett 22: 6694-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.103
BindingDB Entry DOI: 10.7270/Q2Z320SD |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075797

(1-{3-[1-(2-Cyclohexyl-ethyl)-piperidin-4-yl]-propy...)Show SMILES C(COc1cccc2n(CCCC3CCN(CCC4CCCCC4)CC3)c(COc3ccccc3)nc12)CN1CCCCC1 Show InChI InChI=1S/C38H56N4O2/c1-4-13-32(14-5-1)20-27-41-28-21-33(22-29-41)15-11-26-42-35-18-10-19-36(43-30-12-25-40-23-8-3-9-24-40)38(35)39-37(42)31-44-34-16-6-2-7-17-34/h2,6-7,10,16-19,32-33H,1,3-5,8-9,11-15,20-31H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.285 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075806

(1-{3-[1-(3-Methyl-butyl)-piperidin-4-yl]-propyl}-2...)Show SMILES CC(C)CCN1CCC(CCCn2c(COc3ccccc3)nc3c(OCCCN4CCCCC4)cccc23)CC1 Show InChI InChI=1S/C35H52N4O2/c1-29(2)17-24-38-25-18-30(19-26-38)12-10-23-39-32-15-9-16-33(40-27-11-22-37-20-7-4-8-21-37)35(32)36-34(39)28-41-31-13-5-3-6-14-31/h3,5-6,9,13-16,29-30H,4,7-8,10-12,17-28H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075802
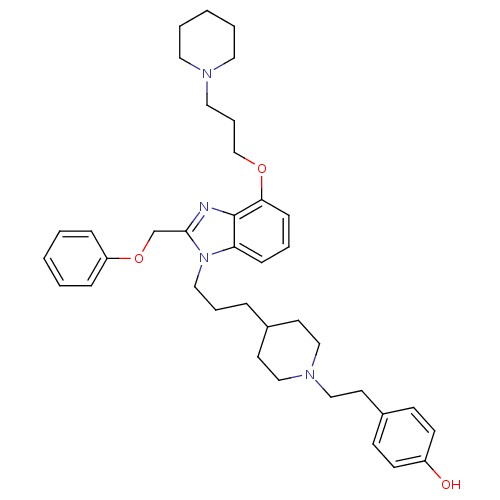
(4-[2-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-pr...)Show SMILES Oc1ccc(CCN2CCC(CCCn3c(COc4ccccc4)nc4c(OCCCN5CCCCC5)cccc34)CC2)cc1 Show InChI InChI=1S/C38H50N4O3/c43-33-17-15-32(16-18-33)21-28-41-26-19-31(20-27-41)10-8-25-42-35-13-7-14-36(44-29-9-24-40-22-5-2-6-23-40)38(35)39-37(42)30-45-34-11-3-1-4-12-34/h1,3-4,7,11-18,31,43H,2,5-6,8-10,19-30H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075799
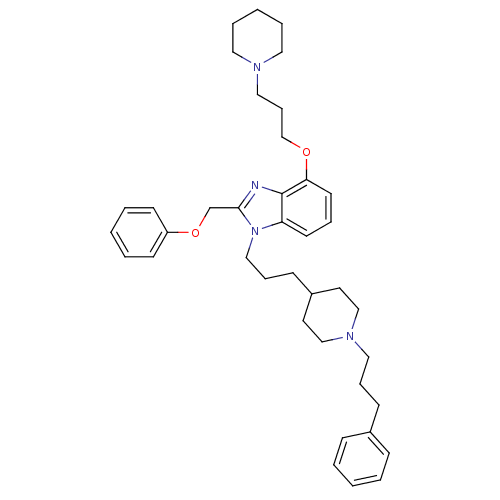
(2-Phenoxymethyl-1-{3-[1-(3-phenyl-propyl)-piperidi...)Show SMILES C(COc1cccc2n(CCCC3CCN(CCCc4ccccc4)CC3)c(COc3ccccc3)nc12)CN1CCCCC1 Show InChI InChI=1S/C39H52N4O2/c1-4-14-33(15-5-1)16-11-26-42-29-22-34(23-30-42)17-12-28-43-36-20-10-21-37(44-31-13-27-41-24-8-3-9-25-41)39(36)40-38(43)32-45-35-18-6-2-7-19-35/h1-2,4-7,10,14-15,18-21,34H,3,8-9,11-13,16-17,22-32H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.361 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075798
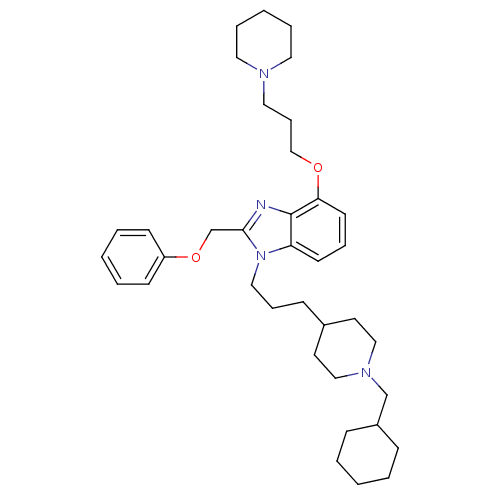
(1-[3-(1-Cyclohexylmethyl-piperidin-4-yl)-propyl]-2...)Show SMILES C(COc1cccc2n(CCCC3CCN(CC4CCCCC4)CC3)c(COc3ccccc3)nc12)CN1CCCCC1 Show InChI InChI=1S/C37H54N4O2/c1-4-13-32(14-5-1)29-40-26-20-31(21-27-40)15-11-25-41-34-18-10-19-35(42-28-12-24-39-22-8-3-9-23-39)37(34)38-36(41)30-43-33-16-6-2-7-17-33/h2,6-7,10,16-19,31-32H,1,3-5,8-9,11-15,20-30H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.393 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394117
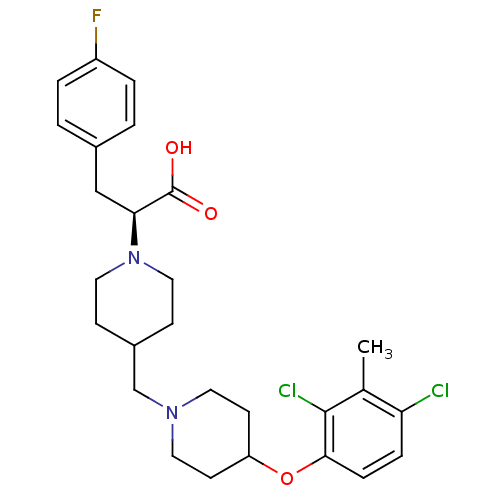
(CHEMBL2158783)Show SMILES Cc1c(Cl)ccc(OC2CCN(CC3CCN(CC3)[C@@H](Cc3ccc(F)cc3)C(O)=O)CC2)c1Cl |r| Show InChI InChI=1S/C27H33Cl2FN2O3/c1-18-23(28)6-7-25(26(18)29)35-22-10-12-31(13-11-22)17-20-8-14-32(15-9-20)24(27(33)34)16-19-2-4-21(30)5-3-19/h2-7,20,22,24H,8-17H2,1H3,(H,33,34)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR3 expressed in CHOK1 cells by radioligand displacement assay |
Bioorg Med Chem Lett 22: 6694-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.103
BindingDB Entry DOI: 10.7270/Q2Z320SD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394116
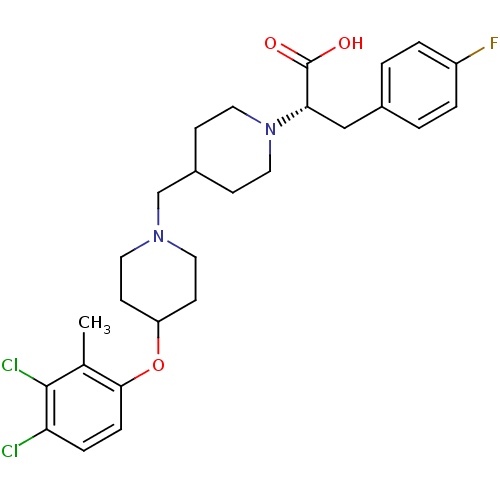
(CHEMBL2158784)Show SMILES Cc1c(Cl)c(Cl)ccc1OC1CCN(CC2CCN(CC2)[C@@H](Cc2ccc(F)cc2)C(O)=O)CC1 |r| Show InChI InChI=1S/C27H33Cl2FN2O3/c1-18-25(7-6-23(28)26(18)29)35-22-10-12-31(13-11-22)17-20-8-14-32(15-9-20)24(27(33)34)16-19-2-4-21(30)5-3-19/h2-7,20,22,24H,8-17H2,1H3,(H,33,34)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR3 expressed in CHOK1 cells by radioligand displacement assay |
Bioorg Med Chem Lett 22: 6694-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.103
BindingDB Entry DOI: 10.7270/Q2Z320SD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394114
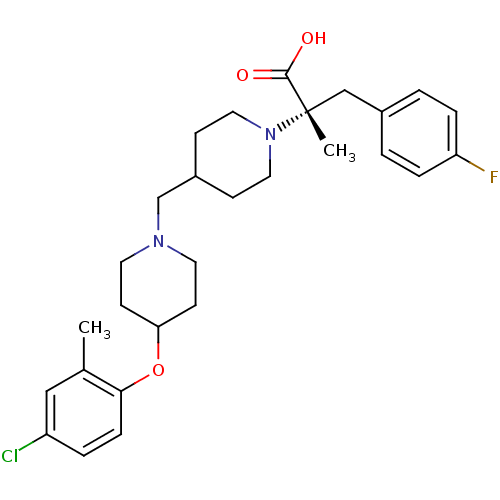
(CHEMBL2158791)Show SMILES Cc1cc(Cl)ccc1OC1CCN(CC2CCN(CC2)[C@@](C)(Cc2ccc(F)cc2)C(O)=O)CC1 |r| Show InChI InChI=1S/C28H36ClFN2O3/c1-20-17-23(29)5-8-26(20)35-25-11-13-31(14-12-25)19-22-9-15-32(16-10-22)28(2,27(33)34)18-21-3-6-24(30)7-4-21/h3-8,17,22,25H,9-16,18-19H2,1-2H3,(H,33,34)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR3 expressed in CHOK1 cells by radioligand displacement assay |
Bioorg Med Chem Lett 22: 6694-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.103
BindingDB Entry DOI: 10.7270/Q2Z320SD |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075795

(1-[3-(1-But-3-enyl-piperidin-4-yl)-propyl]-2-pheno...)Show SMILES C=CCCN1CCC(CCCn2c(COc3ccccc3)nc3c(OCCCN4CCCCC4)cccc23)CC1 Show InChI InChI=1S/C34H48N4O2/c1-2-3-20-37-25-18-29(19-26-37)13-11-24-38-31-16-10-17-32(39-27-12-23-36-21-8-5-9-22-36)34(31)35-33(38)28-40-30-14-6-4-7-15-30/h2,4,6-7,10,14-17,29H,1,3,5,8-9,11-13,18-28H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.707 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075808
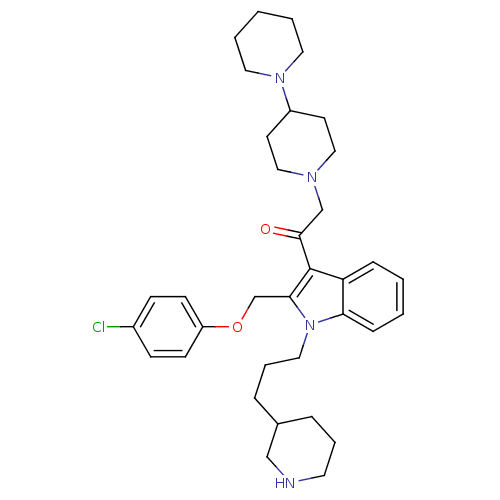
(2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...)Show SMILES Clc1ccc(OCc2c(C(=O)CN3CCC(CC3)N3CCCCC3)c3ccccc3n2CCCC2CCCNC2)cc1 Show InChI InChI=1S/C35H47ClN4O2/c36-28-12-14-30(15-13-28)42-26-33-35(34(41)25-38-22-16-29(17-23-38)39-19-4-1-5-20-39)31-10-2-3-11-32(31)40(33)21-7-9-27-8-6-18-37-24-27/h2-3,10-15,27,29,37H,1,4-9,16-26H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50060725

(2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...)Show SMILES Clc1ccc(OCc2c(C(=O)CN3CCC(CC3)N3CCCCC3)c3ccccc3n2CCC[C@@H]2CCCNC2)cc1 Show InChI InChI=1S/C35H47ClN4O2/c36-28-12-14-30(15-13-28)42-26-33-35(34(41)25-38-22-16-29(17-23-38)39-19-4-1-5-20-39)31-10-2-3-11-32(31)40(33)21-7-9-27-8-6-18-37-24-27/h2-3,10-15,27,29,37H,1,4-9,16-26H2/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY |
J Med Chem 41: 2709-19 (1998)
Article DOI: 10.1021/jm9706630
BindingDB Entry DOI: 10.7270/Q2TD9WGN |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394160
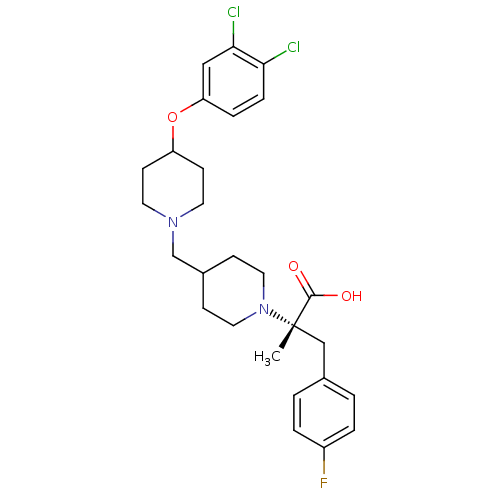
(CHEMBL2158787)Show SMILES C[C@@](Cc1ccc(F)cc1)(N1CCC(CN2CCC(CC2)Oc2ccc(Cl)c(Cl)c2)CC1)C(O)=O |r| Show InChI InChI=1S/C27H33Cl2FN2O3/c1-27(26(33)34,17-19-2-4-21(30)5-3-19)32-14-8-20(9-15-32)18-31-12-10-22(11-13-31)35-23-6-7-24(28)25(29)16-23/h2-7,16,20,22H,8-15,17-18H2,1H3,(H,33,34)/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR3 expressed in CHOK1 cells by radioligand displacement assay |
Bioorg Med Chem Lett 22: 6694-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.103
BindingDB Entry DOI: 10.7270/Q2Z320SD |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM85212
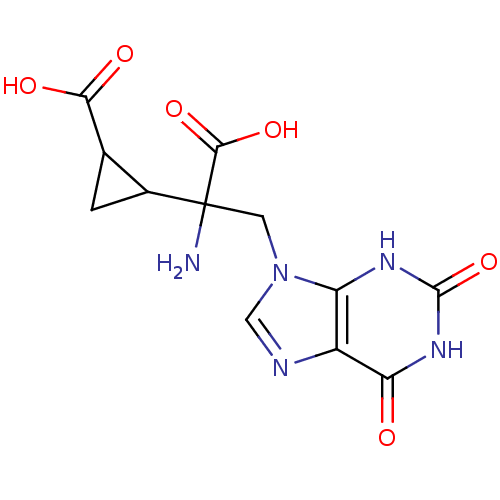
(CAS_5311260 | LY341495 | NSC_5311260)Show SMILES NC(Cn1cnc2c1[nH]c(=O)[nH]c2=O)(C1CC1C(O)=O)C(O)=O Show InChI InChI=1S/C12H13N5O6/c13-12(10(21)22,5-1-4(5)9(19)20)2-17-3-14-6-7(17)15-11(23)16-8(6)18/h3-5H,1-2,13H2,(H,19,20)(H,21,22)(H2,15,16,18,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 453-60 (2001)
BindingDB Entry DOI: 10.7270/Q2V986M2 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075804

(1-[3-(1-Isobutyl-piperidin-4-yl)-propyl]-2-phenoxy...)Show SMILES CC(C)CN1CCC(CCCn2c(COc3ccccc3)nc3c(OCCCN4CCCCC4)cccc23)CC1 Show InChI InChI=1S/C34H50N4O2/c1-28(2)26-37-23-17-29(18-24-37)12-10-22-38-31-15-9-16-32(39-25-11-21-36-19-7-4-8-20-36)34(31)35-33(38)27-40-30-13-5-3-6-14-30/h3,5-6,9,13-16,28-29H,4,7-8,10-12,17-27H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.829 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394115
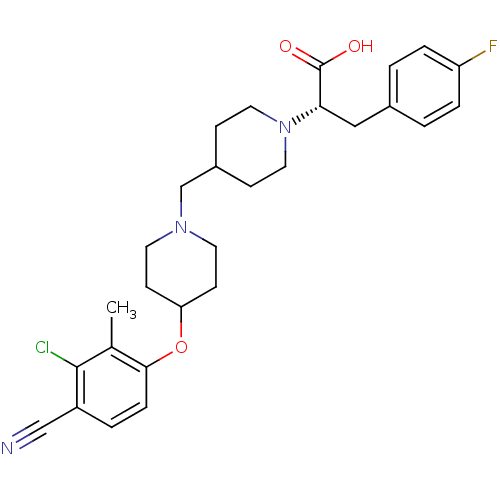
(CHEMBL2158785)Show SMILES Cc1c(Cl)c(ccc1OC1CCN(CC2CCN(CC2)[C@@H](Cc2ccc(F)cc2)C(O)=O)CC1)C#N |r| Show InChI InChI=1S/C28H33ClFN3O3/c1-19-26(7-4-22(17-31)27(19)29)36-24-10-12-32(13-11-24)18-21-8-14-33(15-9-21)25(28(34)35)16-20-2-5-23(30)6-3-20/h2-7,21,24-25H,8-16,18H2,1H3,(H,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR3 expressed in CHOK1 cells by radioligand displacement assay |
Bioorg Med Chem Lett 22: 6694-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.103
BindingDB Entry DOI: 10.7270/Q2Z320SD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394113
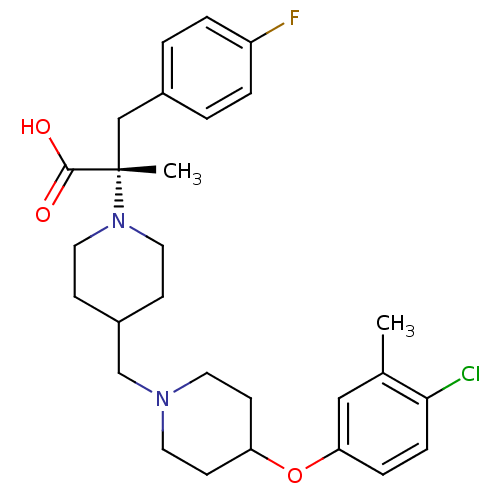
(CHEMBL2158792)Show SMILES Cc1cc(OC2CCN(CC3CCN(CC3)[C@@](C)(Cc3ccc(F)cc3)C(O)=O)CC2)ccc1Cl |r| Show InChI InChI=1S/C28H36ClFN2O3/c1-20-17-25(7-8-26(20)29)35-24-11-13-31(14-12-24)19-22-9-15-32(16-10-22)28(2,27(33)34)18-21-3-5-23(30)6-4-21/h3-8,17,22,24H,9-16,18-19H2,1-2H3,(H,33,34)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR3 expressed in CHOK1 cells by radioligand displacement assay |
Bioorg Med Chem Lett 22: 6694-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.103
BindingDB Entry DOI: 10.7270/Q2Z320SD |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50062522
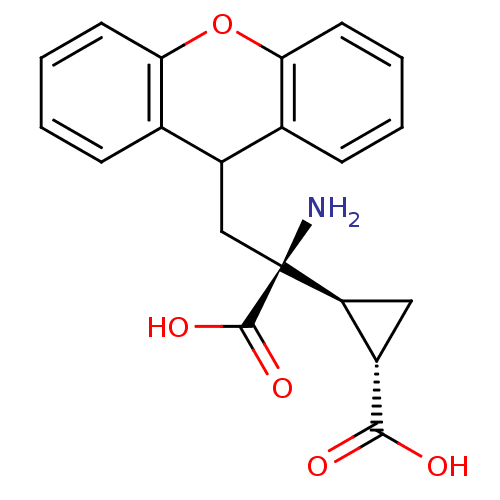
((1S,2S)-2-((S)-1-amino-1-carboxy-2-(9H-xanthen-9-y...)Show SMILES N[C@@](CC1c2ccccc2Oc2ccccc12)([C@H]1C[C@@H]1C(O)=O)C(O)=O Show InChI InChI=1S/C20H19NO5/c21-20(19(24)25,15-9-13(15)18(22)23)10-14-11-5-1-3-7-16(11)26-17-8-4-2-6-12(14)17/h1-8,13-15H,9-10,21H2,(H,22,23)(H,24,25)/t13-,15-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LY459477 from recombinant human mGlu3 receptor expressed in hamster AV12 cell membranes co-expressing rat EAAT1 incubated for 90... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2611419 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288674
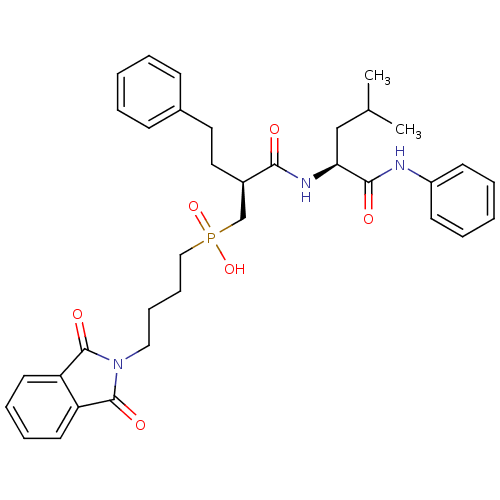
(CHEMBL420674 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H42N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,25,27,31H,11-12,19-24H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t27-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394158
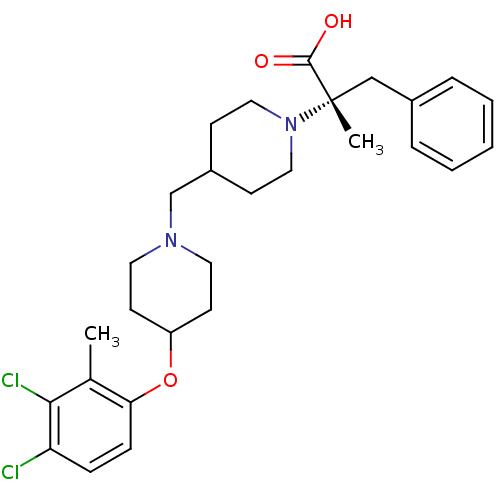
(CHEMBL2158789)Show SMILES Cc1c(Cl)c(Cl)ccc1OC1CCN(CC2CCN(CC2)[C@@](C)(Cc2ccccc2)C(O)=O)CC1 |r| Show InChI InChI=1S/C28H36Cl2N2O3/c1-20-25(9-8-24(29)26(20)30)35-23-12-14-31(15-13-23)19-22-10-16-32(17-11-22)28(2,27(33)34)18-21-6-4-3-5-7-21/h3-9,22-23H,10-19H2,1-2H3,(H,33,34)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR3 expressed in CHOK1 cells by radioligand displacement assay |
Bioorg Med Chem Lett 22: 6694-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.103
BindingDB Entry DOI: 10.7270/Q2Z320SD |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075800

(1-[3-(1-Allyl-piperidin-4-yl)-propyl]-2-phenoxymet...)Show SMILES C=CCN1CCC(CCCn2c(COc3ccccc3)nc3c(OCCCN4CCCCC4)cccc23)CC1 Show InChI InChI=1S/C33H46N4O2/c1-2-19-35-24-17-28(18-25-35)12-10-23-37-30-15-9-16-31(38-26-11-22-36-20-7-4-8-21-36)33(30)34-32(37)27-39-29-13-5-3-6-14-29/h2-3,5-6,9,13-16,28H,1,4,7-8,10-12,17-27H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075801
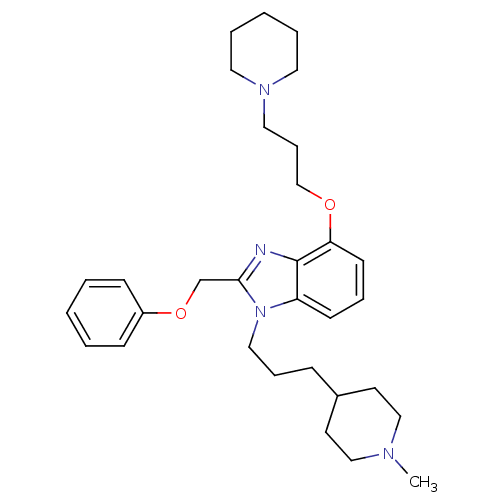
(1-[3-(1-Methyl-piperidin-4-yl)-propyl]-2-phenoxyme...)Show SMILES CN1CCC(CCCn2c(COc3ccccc3)nc3c(OCCCN4CCCCC4)cccc23)CC1 Show InChI InChI=1S/C31H44N4O2/c1-33-22-16-26(17-23-33)11-9-21-35-28-14-8-15-29(36-24-10-20-34-18-6-3-7-19-34)31(28)32-30(35)25-37-27-12-4-2-5-13-27/h2,4-5,8,12-15,26H,3,6-7,9-11,16-25H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075810
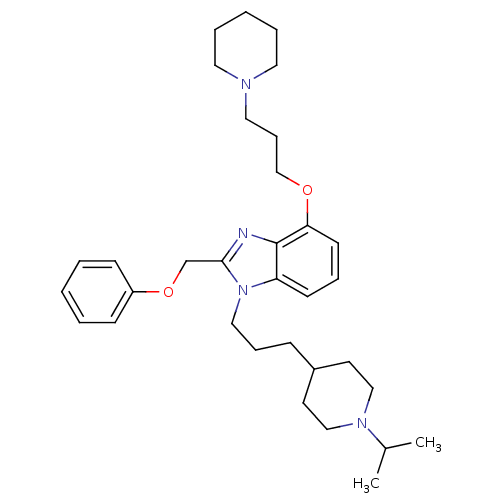
(1-[3-(1-Isopropyl-piperidin-4-yl)-propyl]-2-phenox...)Show SMILES CC(C)N1CCC(CCCn2c(COc3ccccc3)nc3c(OCCCN4CCCCC4)cccc23)CC1 Show InChI InChI=1S/C33H48N4O2/c1-27(2)36-23-17-28(18-24-36)12-10-22-37-30-15-9-16-31(38-25-11-21-35-19-7-4-8-20-35)33(30)34-32(37)26-39-29-13-5-3-6-14-29/h3,5-6,9,13-16,27-28H,4,7-8,10-12,17-26H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50065468
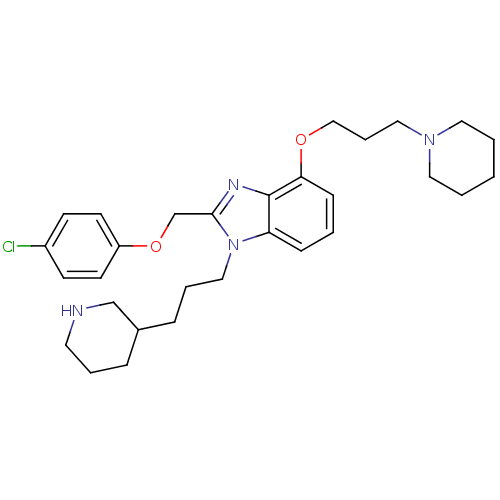
(2-(4-Chloro-phenoxymethyl)-4-(3-piperidin-1-yl-pro...)Show SMILES Clc1ccc(OCc2nc3c(OCCCN4CCCCC4)cccc3n2CCCC2CCCNC2)cc1 Show InChI InChI=1S/C30H41ClN4O2/c31-25-12-14-26(15-13-25)37-23-29-33-30-27(35(29)20-6-9-24-8-5-16-32-22-24)10-4-11-28(30)36-21-7-19-34-17-2-1-3-18-34/h4,10-15,24,32H,1-3,5-9,16-23H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50065468

(2-(4-Chloro-phenoxymethyl)-4-(3-piperidin-1-yl-pro...)Show SMILES Clc1ccc(OCc2nc3c(OCCCN4CCCCC4)cccc3n2CCCC2CCCNC2)cc1 Show InChI InChI=1S/C30H41ClN4O2/c31-25-12-14-26(15-13-25)37-23-29-33-30-27(35(29)20-6-9-24-8-5-16-32-22-24)10-4-11-28(30)36-21-7-19-34-17-2-1-3-18-34/h4,10-15,24,32H,1-3,5-9,16-23H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY |
J Med Chem 41: 2709-19 (1998)
Article DOI: 10.1021/jm9706630
BindingDB Entry DOI: 10.7270/Q2TD9WGN |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50065468

(2-(4-Chloro-phenoxymethyl)-4-(3-piperidin-1-yl-pro...)Show SMILES Clc1ccc(OCc2nc3c(OCCCN4CCCCC4)cccc3n2CCCC2CCCNC2)cc1 Show InChI InChI=1S/C30H41ClN4O2/c31-25-12-14-26(15-13-25)37-23-29-33-30-27(35(29)20-6-9-24-8-5-16-32-22-24)10-4-11-28(30)36-21-7-19-34-17-2-1-3-18-34/h4,10-15,24,32H,1-3,5-9,16-23H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Binding affinity of the compound at cloned Neuropeptide Y receptor type 1 expressed in AV-12 cells is evaluated. |
Bioorg Med Chem Lett 8: 473-6 (1999)
BindingDB Entry DOI: 10.7270/Q2GH9H33 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394159
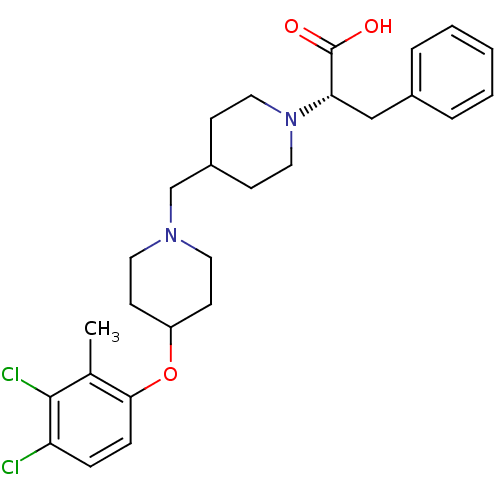
(CHEMBL2158788)Show SMILES Cc1c(Cl)c(Cl)ccc1OC1CCN(CC2CCN(CC2)[C@@H](Cc2ccccc2)C(O)=O)CC1 |r| Show InChI InChI=1S/C27H34Cl2N2O3/c1-19-25(8-7-23(28)26(19)29)34-22-11-13-30(14-12-22)18-21-9-15-31(16-10-21)24(27(32)33)17-20-5-3-2-4-6-20/h2-8,21-22,24H,9-18H2,1H3,(H,32,33)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR3 expressed in CHOK1 cells by radioligand displacement assay |
Bioorg Med Chem Lett 22: 6694-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.103
BindingDB Entry DOI: 10.7270/Q2Z320SD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394150
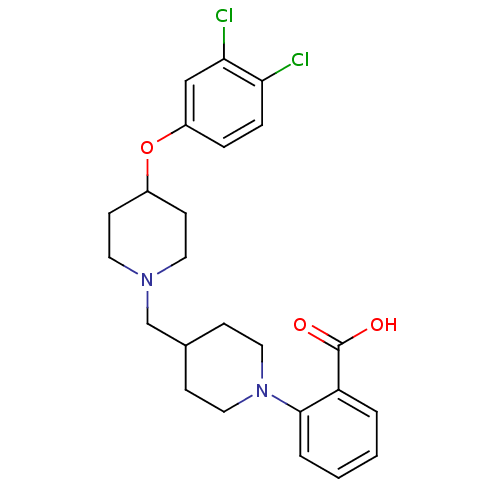
(CHEMBL2158822)Show SMILES OC(=O)c1ccccc1N1CCC(CN2CCC(CC2)Oc2ccc(Cl)c(Cl)c2)CC1 Show InChI InChI=1S/C24H28Cl2N2O3/c25-21-6-5-19(15-22(21)26)31-18-9-11-27(12-10-18)16-17-7-13-28(14-8-17)23-4-2-1-3-20(23)24(29)30/h1-6,15,17-18H,7-14,16H2,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR3 expressed in CHOK1 cells by radioligand displacement assay |
Bioorg Med Chem Lett 22: 6694-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.103
BindingDB Entry DOI: 10.7270/Q2Z320SD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394112
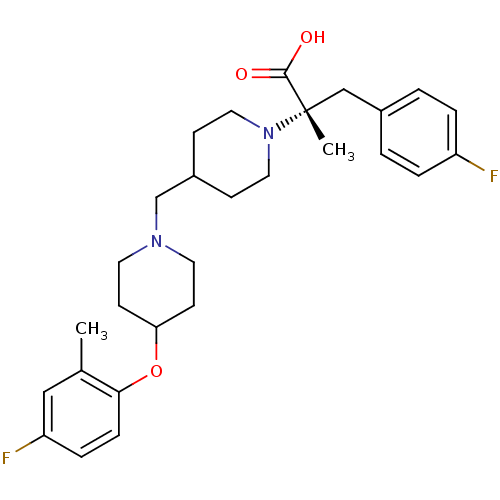
(CHEMBL2158793)Show SMILES Cc1cc(F)ccc1OC1CCN(CC2CCN(CC2)[C@@](C)(Cc2ccc(F)cc2)C(O)=O)CC1 |r| Show InChI InChI=1S/C28H36F2N2O3/c1-20-17-24(30)7-8-26(20)35-25-11-13-31(14-12-25)19-22-9-15-32(16-10-22)28(2,27(33)34)18-21-3-5-23(29)6-4-21/h3-8,17,22,25H,9-16,18-19H2,1-2H3,(H,33,34)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR3 expressed in CHOK1 cells by radioligand displacement assay |
Bioorg Med Chem Lett 22: 6694-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.103
BindingDB Entry DOI: 10.7270/Q2Z320SD |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186127
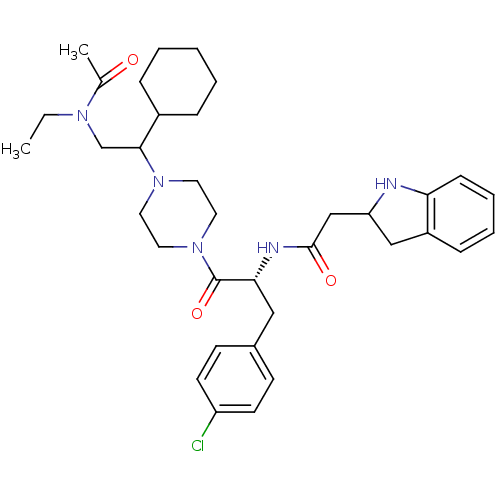
(CHEMBL378408 | N-[(R)-2-{4-[2-(acetyl-ethyl-amino)...)Show SMILES CCN(CC(C1CCCCC1)N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)CC1Cc2ccccc2N1)C(C)=O Show InChI InChI=1S/C35H48ClN5O3/c1-3-39(25(2)42)24-33(27-9-5-4-6-10-27)40-17-19-41(20-18-40)35(44)32(21-26-13-15-29(36)16-14-26)38-34(43)23-30-22-28-11-7-8-12-31(28)37-30/h7-8,11-16,27,30,32-33,37H,3-6,9-10,17-24H2,1-2H3,(H,38,43)/t30?,32-,33?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125]I-NDP-alpha-MSH binding to human MC4R transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 3449-53 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.002
BindingDB Entry DOI: 10.7270/Q2ST7PFS |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288672

(CHEMBL113362 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES COc1cccc(CC[C@H](CP(O)(=O)CCCCN2C(=O)c3ccccc3C2=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)c1 Show InChI InChI=1S/C36H44N3O7P/c1-25(2)22-32(34(41)37-28-13-5-4-6-14-28)38-33(40)27(19-18-26-12-11-15-29(23-26)46-3)24-47(44,45)21-10-9-20-39-35(42)30-16-7-8-17-31(30)36(39)43/h4-8,11-17,23,25,27,32H,9-10,18-22,24H2,1-3H3,(H,37,41)(H,38,40)(H,44,45)/t27-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50285557
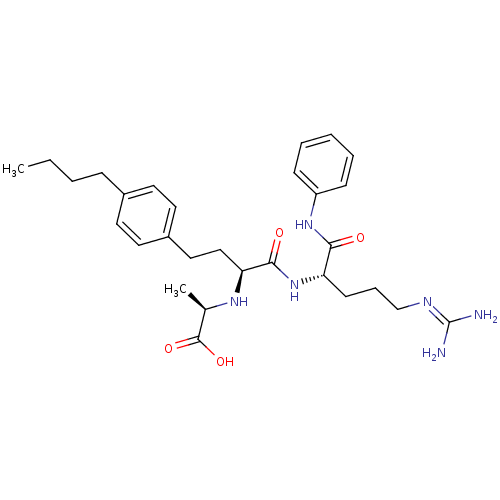
((R)-2-[(S)-3-(4-butyl-phenyl)-1-((S)-4-guanidino-1...)Show SMILES [#6]-[#6]-[#6]-[#6]-c1ccc(-[#6]-[#6]-[#6@H](-[#7]-[#6@H](-[#6])-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c2ccccc2)cc1 Show InChI InChI=1S/C29H42N6O4/c1-3-4-9-21-13-15-22(16-14-21)17-18-25(33-20(2)28(38)39)27(37)35-24(12-8-19-32-29(30)31)26(36)34-23-10-6-5-7-11-23/h5-7,10-11,13-16,20,24-25,33H,3-4,8-9,12,17-19H2,1-2H3,(H,34,36)(H,35,37)(H,38,39)(H4,30,31,32)/t20-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase-A (MMP-2) |
Bioorg Med Chem Lett 5: 2441-2446 (1995)
Article DOI: 10.1016/0960-894X(95)00425-S
BindingDB Entry DOI: 10.7270/Q2D21XK6 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288673

(CHEMBL264455 | {4-[((S)-1-Benzoyl-pyrrolidine-2-ca...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@@H]1CCCN1C(=O)c1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C39H51N4O6P/c1-29(2)27-34(37(45)41-33-19-10-5-11-20-33)42-36(44)32(23-22-30-15-6-3-7-16-30)28-50(48,49)26-13-12-24-40-38(46)35-21-14-25-43(35)39(47)31-17-8-4-9-18-31/h3-11,15-20,29,32,34-35H,12-14,21-28H2,1-2H3,(H,40,46)(H,41,45)(H,42,44)(H,48,49)/t32-,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288683
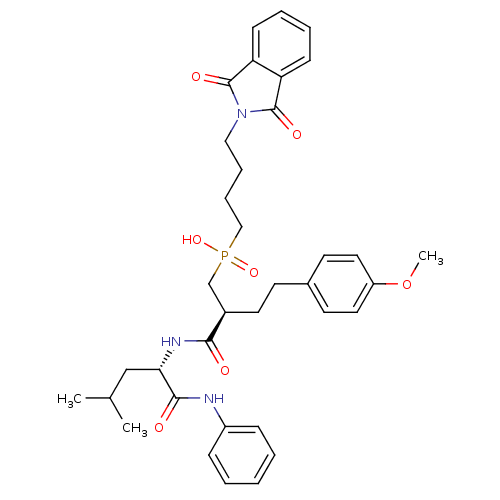
(CHEMBL109438 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES COc1ccc(CC[C@H](CP(O)(=O)CCCCN2C(=O)c3ccccc3C2=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C36H44N3O7P/c1-25(2)23-32(34(41)37-28-11-5-4-6-12-28)38-33(40)27(18-15-26-16-19-29(46-3)20-17-26)24-47(44,45)22-10-9-21-39-35(42)30-13-7-8-14-31(30)36(39)43/h4-8,11-14,16-17,19-20,25,27,32H,9-10,15,18,21-24H2,1-3H3,(H,37,41)(H,38,40)(H,44,45)/t27-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288672

(CHEMBL113362 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES COc1cccc(CC[C@H](CP(O)(=O)CCCCN2C(=O)c3ccccc3C2=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)c1 Show InChI InChI=1S/C36H44N3O7P/c1-25(2)22-32(34(41)37-28-13-5-4-6-14-28)38-33(40)27(19-18-26-12-11-15-29(23-26)46-3)24-47(44,45)21-10-9-20-39-35(42)30-16-7-8-17-31(30)36(39)43/h4-8,11-17,23,25,27,32H,9-10,18-22,24H2,1-3H3,(H,37,41)(H,38,40)(H,44,45)/t27-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50558766
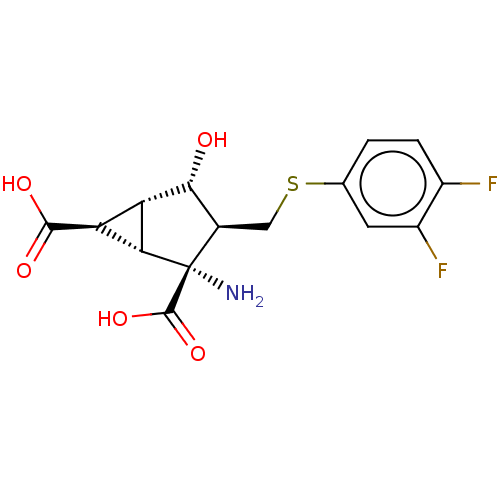
(CHEMBL4751065)Show SMILES Cl.[H][C@]12[C@H](C(O)=O)[C@@]1([H])[C@@](N)([C@H](CSc1ccc(F)c(F)c1)[C@H]2O)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LY459477 from recombinant human mGlu3 receptor expressed in hamster AV12 cell membranes co-expressing rat EAAT1 incubated for 90... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2611419 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50204261
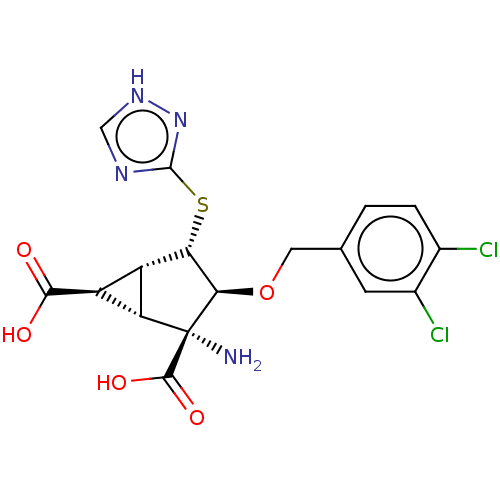
(CHEMBL3969063)Show SMILES [H][C@]12[C@H](C(O)=O)[C@@]1([H])[C@@](N)([C@H](OCc1ccc(Cl)c(Cl)c1)[C@H]2Sc1nc[nH]n1)C(O)=O |r| Show InChI InChI=1S/C17H16Cl2N4O5S/c18-7-2-1-6(3-8(7)19)4-28-13-12(29-16-21-5-22-23-16)9-10(14(24)25)11(9)17(13,20)15(26)27/h1-3,5,9-13H,4,20H2,(H,24,25)(H,26,27)(H,21,22,23)/t9-,10-,11-,12-,13+,17+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LY459477 from human mGlu3 receptor expressed in hamster AV12 cell membranes co-expressing human EAAT1 after 90 mins by liquid sc... |
Bioorg Med Chem Lett 26: 5663-5668 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.067
BindingDB Entry DOI: 10.7270/Q2JS9SDT |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50285567
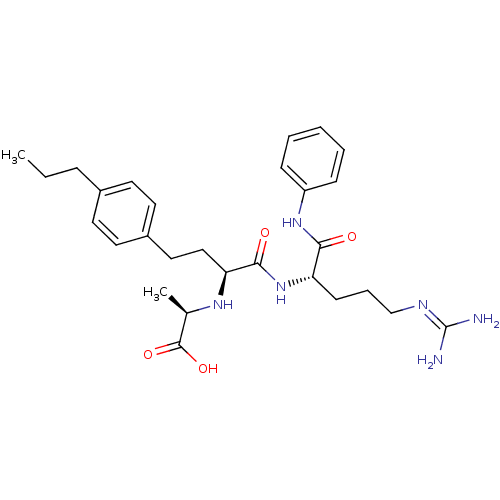
((R)-2-[(S)-1-((S)-4-guanidino-1-phenylcarbamoyl-bu...)Show SMILES [#6]-[#6]-[#6]-c1ccc(-[#6]-[#6]-[#6@H](-[#7]-[#6@H](-[#6])-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c2ccccc2)cc1 Show InChI InChI=1S/C28H40N6O4/c1-3-8-20-12-14-21(15-13-20)16-17-24(32-19(2)27(37)38)26(36)34-23(11-7-18-31-28(29)30)25(35)33-22-9-5-4-6-10-22/h4-6,9-10,12-15,19,23-24,32H,3,7-8,11,16-18H2,1-2H3,(H,33,35)(H,34,36)(H,37,38)(H4,29,30,31)/t19-,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase-A (MMP-2) |
Bioorg Med Chem Lett 5: 2441-2446 (1995)
Article DOI: 10.1016/0960-894X(95)00425-S
BindingDB Entry DOI: 10.7270/Q2D21XK6 |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50494356
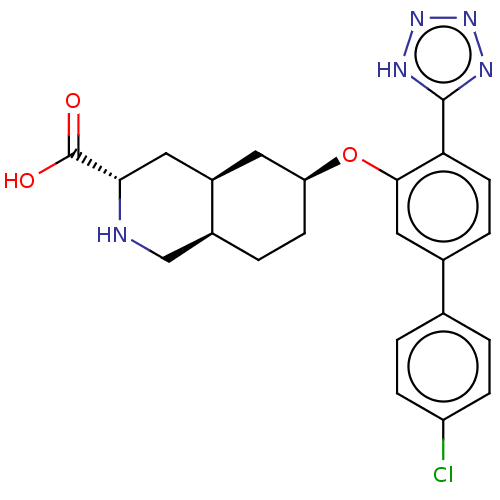
(CHEMBL3088070)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cc(ccc1-c1nnn[nH]1)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H24ClN5O3.ClH/c24-17-5-1-13(2-6-17)14-4-8-19(22-26-28-29-27-22)21(11-14)32-18-7-3-15-12-25-20(23(30)31)10-16(15)9-18;/h1-2,4-6,8,11,15-16,18,20,25H,3,7,9-10,12H2,(H,30,31)(H,26,27,28,29);1H/t15-,16+,18-,20-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]AMPA from homomeric recombinant GluA2 receptor (unknown origin) |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186127

(CHEMBL378408 | N-[(R)-2-{4-[2-(acetyl-ethyl-amino)...)Show SMILES CCN(CC(C1CCCCC1)N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)CC1Cc2ccccc2N1)C(C)=O Show InChI InChI=1S/C35H48ClN5O3/c1-3-39(25(2)42)24-33(27-9-5-4-6-10-27)40-17-19-41(20-18-40)35(44)32(21-26-13-15-29(36)16-14-26)38-34(43)23-30-22-28-11-7-8-12-31(28)37-30/h7-8,11-16,27,30,32-33,37H,3-6,9-10,17-24H2,1-2H3,(H,38,43)/t30?,32-,33?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125]I-NDP-alpha-MSH binding to human MC4R transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 3449-53 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.002
BindingDB Entry DOI: 10.7270/Q2ST7PFS |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186127

(CHEMBL378408 | N-[(R)-2-{4-[2-(acetyl-ethyl-amino)...)Show SMILES CCN(CC(C1CCCCC1)N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)CC1Cc2ccccc2N1)C(C)=O Show InChI InChI=1S/C35H48ClN5O3/c1-3-39(25(2)42)24-33(27-9-5-4-6-10-27)40-17-19-41(20-18-40)35(44)32(21-26-13-15-29(36)16-14-26)38-34(43)23-30-22-28-11-7-8-12-31(28)37-30/h7-8,11-16,27,30,32-33,37H,3-6,9-10,17-24H2,1-2H3,(H,38,43)/t30?,32-,33?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125]I-NDP-alpha-MSH binding to human MC4R transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 3449-53 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.002
BindingDB Entry DOI: 10.7270/Q2ST7PFS |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394161
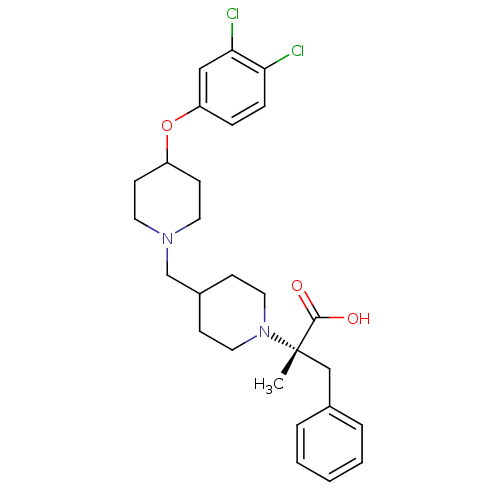
(CHEMBL2158786)Show SMILES C[C@@](Cc1ccccc1)(N1CCC(CN2CCC(CC2)Oc2ccc(Cl)c(Cl)c2)CC1)C(O)=O |r| Show InChI InChI=1S/C27H34Cl2N2O3/c1-27(26(32)33,18-20-5-3-2-4-6-20)31-15-9-21(10-16-31)19-30-13-11-22(12-14-30)34-23-7-8-24(28)25(29)17-23/h2-8,17,21-22H,9-16,18-19H2,1H3,(H,32,33)/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR3 expressed in CHOK1 cells by radioligand displacement assay |
Bioorg Med Chem Lett 22: 6694-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.103
BindingDB Entry DOI: 10.7270/Q2Z320SD |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50057090
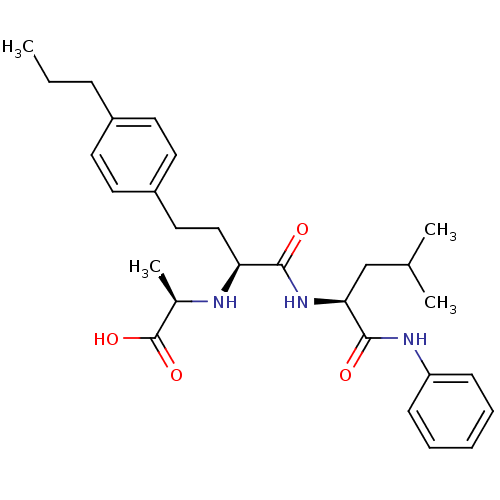
((R)-2-((S)-1-((S)-4-methyl-1-oxo-1-(phenylamino)pe...)Show SMILES CCCc1ccc(CC[C@H](N[C@H](C)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C28H39N3O4/c1-5-9-21-12-14-22(15-13-21)16-17-24(29-20(4)28(34)35)26(32)31-25(18-19(2)3)27(33)30-23-10-7-6-8-11-23/h6-8,10-15,19-20,24-25,29H,5,9,16-18H2,1-4H3,(H,30,33)(H,31,32)(H,34,35)/t20-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase-A (MMP-2) |
Bioorg Med Chem Lett 5: 2441-2446 (1995)
Article DOI: 10.1016/0960-894X(95)00425-S
BindingDB Entry DOI: 10.7270/Q2D21XK6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394125
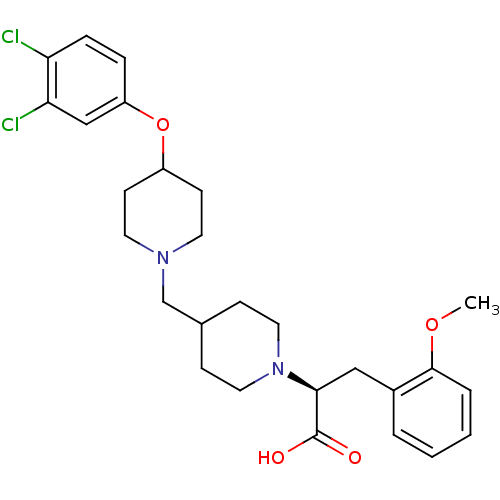
(CHEMBL2158775)Show SMILES COc1ccccc1C[C@H](N1CCC(CN2CCC(CC2)Oc2ccc(Cl)c(Cl)c2)CC1)C(O)=O |r| Show InChI InChI=1S/C27H34Cl2N2O4/c1-34-26-5-3-2-4-20(26)16-25(27(32)33)31-14-8-19(9-15-31)18-30-12-10-21(11-13-30)35-22-6-7-23(28)24(29)17-22/h2-7,17,19,21,25H,8-16,18H2,1H3,(H,32,33)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR3 expressed in CHOK1 cells by radioligand displacement assay |
Bioorg Med Chem Lett 22: 6694-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.103
BindingDB Entry DOI: 10.7270/Q2Z320SD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data