

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286250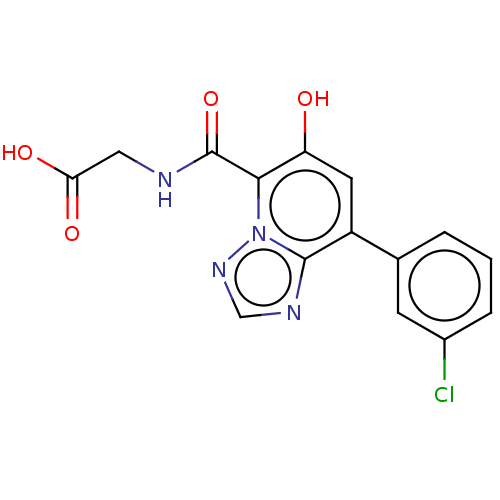 (CHEMBL4159751) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286228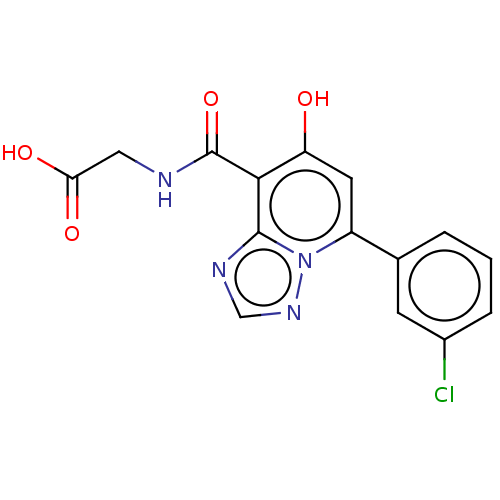 (CHEMBL4174643) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286232 (CHEMBL4173606) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286231 (CHEMBL4175056) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286071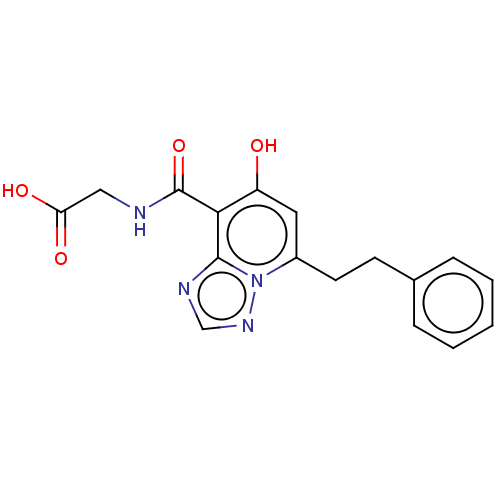 (CHEMBL4166742) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286068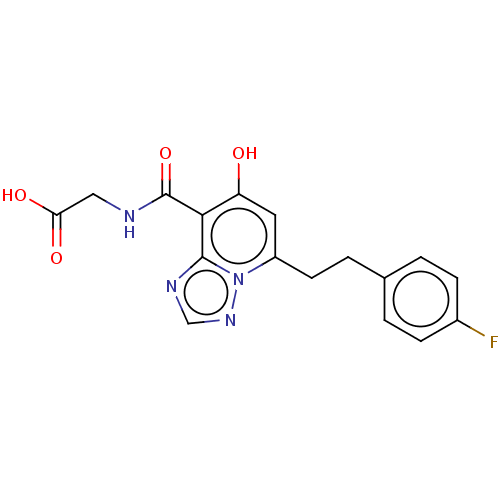 (CHEMBL4171201) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50193145 (2-(1-chloro-4-hydroxyisoquinoline-3-carboxamido)ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of PHD2 (181 to 426 residues) (unknown origin) using biotinylated CODD peptide as substrate preincubated for 15 mins followed by substrate... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286263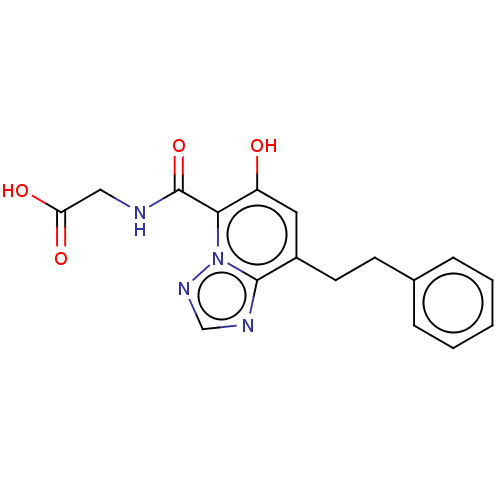 (CHEMBL4167657) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286233 (CHEMBL4175892) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286227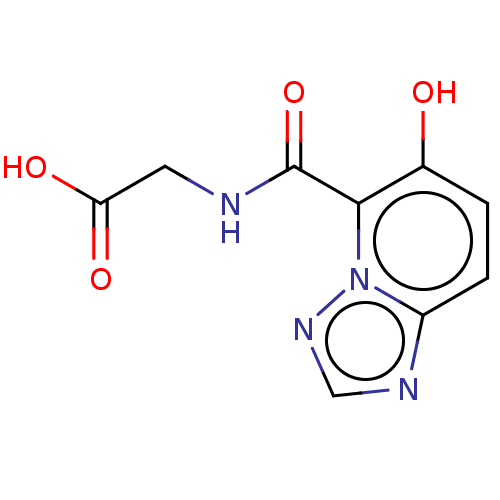 (CHEMBL4160770) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286224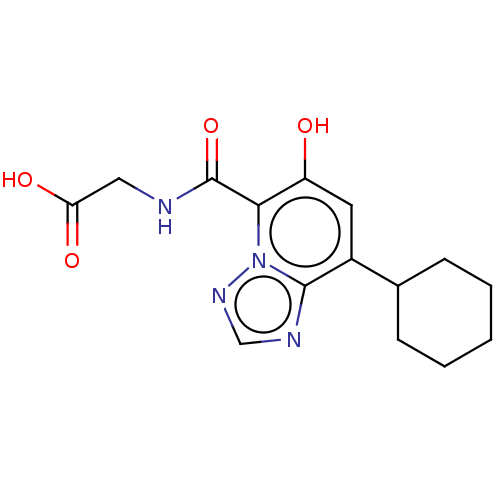 (CHEMBL4162891) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286230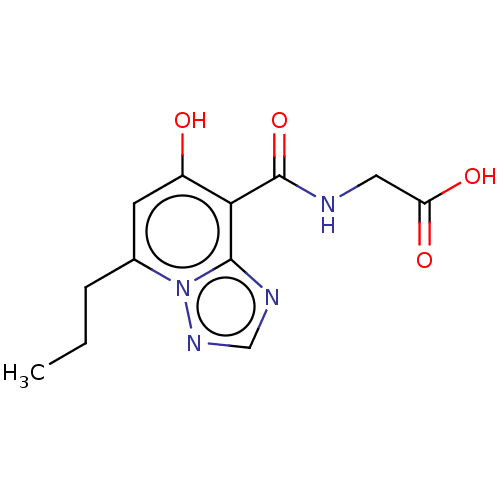 (CHEMBL4171371) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286072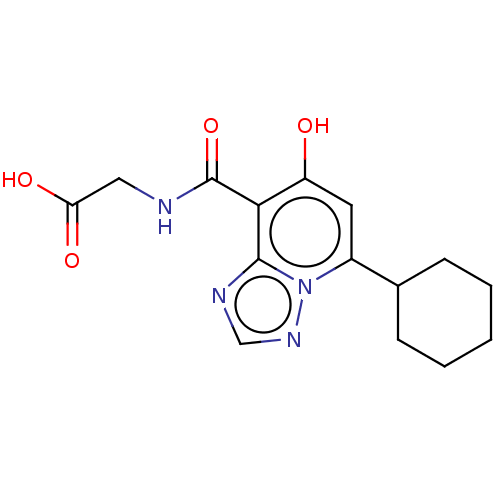 (CHEMBL4164403) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286069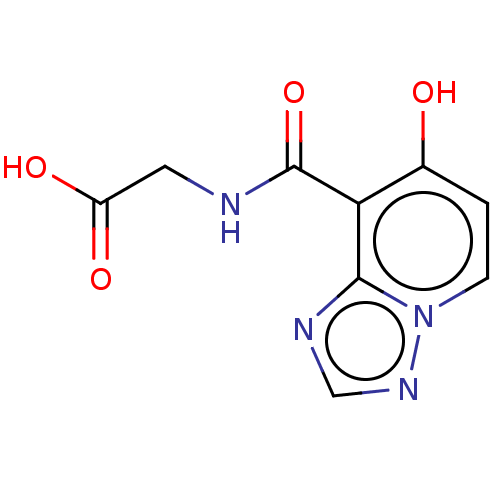 (CHEMBL4174815) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286248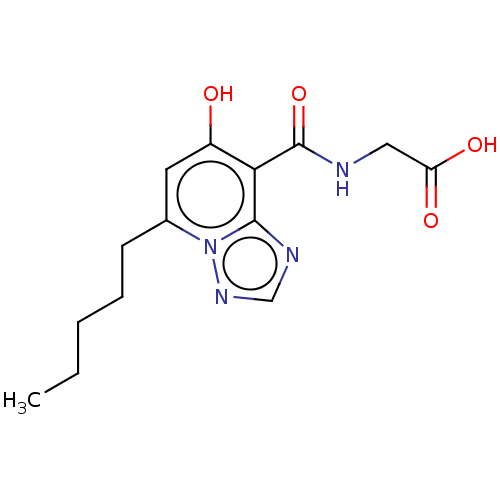 (CHEMBL4168030) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286247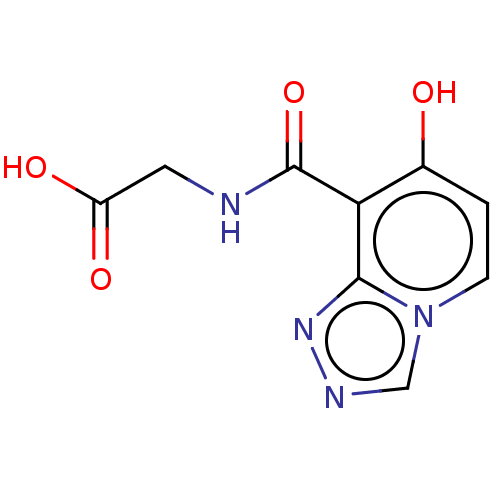 (CHEMBL4164159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286070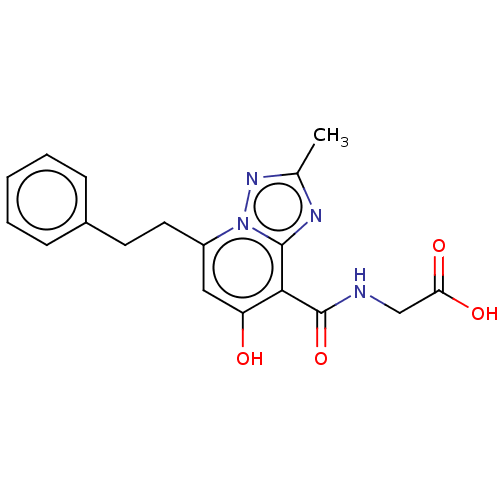 (CHEMBL4166512) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286245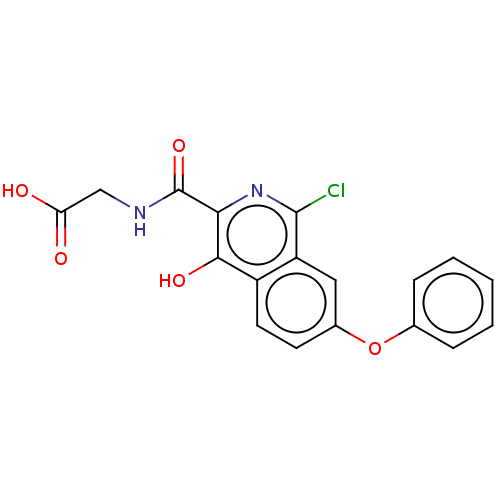 (CHEMBL4163070) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286251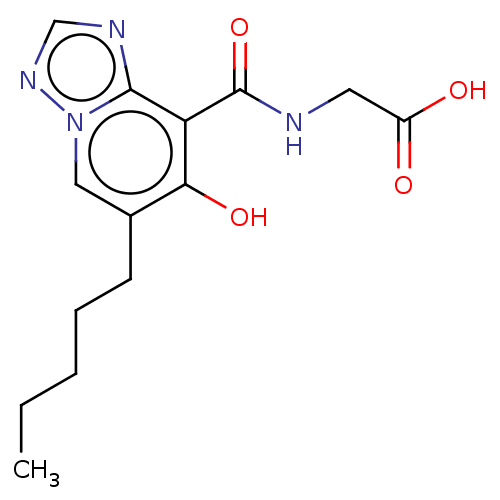 (CHEMBL4167844) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286246 (CHEMBL4159695) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286249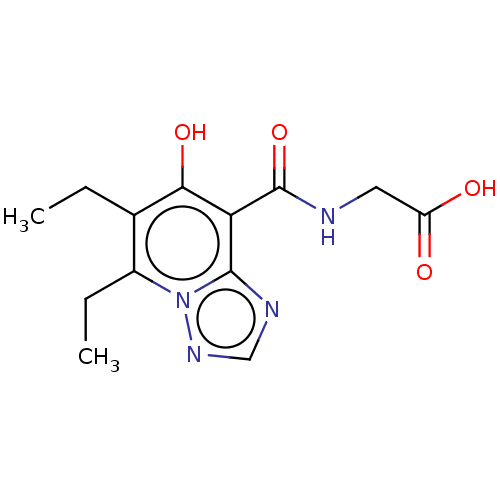 (CHEMBL4159929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50193145 (2-(1-chloro-4-hydroxyisoquinoline-3-carboxamido)ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50286229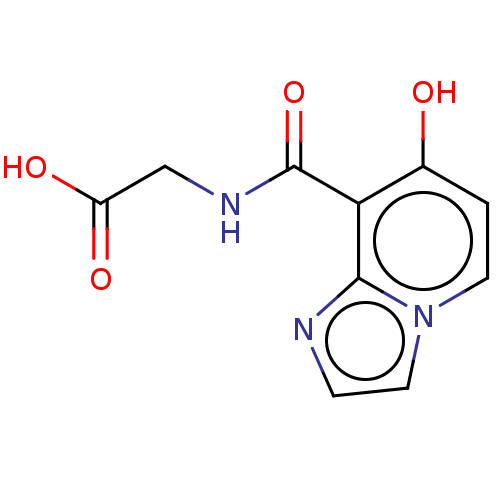 (CHEMBL4167612) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub... | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50286071 (CHEMBL4166742) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2A6 in human liver microsomes by LC-MS/MS analysis | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50286071 (CHEMBL4166742) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2B6 in human liver microsomes by LC-MS/MS analysis | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50286071 (CHEMBL4166742) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at holding potential of -80 mV by whole cell patch clamp method | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50286071 (CHEMBL4166742) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes by LC-MS/MS analysis | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50286071 (CHEMBL4166742) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes by LC-MS/MS analysis | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50286071 (CHEMBL4166742) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes by LC-MS/MS analysis | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50286071 (CHEMBL4166742) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midozolam as substrate by LC-MS/MS analysis | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50286071 (CHEMBL4166742) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes by LC-MS/MS analysis | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50286071 (CHEMBL4166742) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes using midazolam as substrate by LC-MS/MS analysis | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50286071 (CHEMBL4166742) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes by LC-MS/MS analysis | ACS Med Chem Lett 8: 1320-1325 (2017) Article DOI: 10.1021/acsmedchemlett.7b00404 BindingDB Entry DOI: 10.7270/Q21N83N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Nef (Human immunodeficiency virus type 1 (isolate BRU/L...) | BDBM288019 (US10087178, Example 1) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | 25 |
JAPAN TOBACCO INC. US Patent | Assay Description The medium (40 μL), a test substance (10 μL) diluted with the medium, and a 1×105 cells/mL MT-4 cell suspension (50 μL) wherein HIV-1 ... | US Patent US10087178 (2018) BindingDB Entry DOI: 10.7270/Q20C4XSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Nef (Human immunodeficiency virus type 1 (isolate BRU/L...) | BDBM288020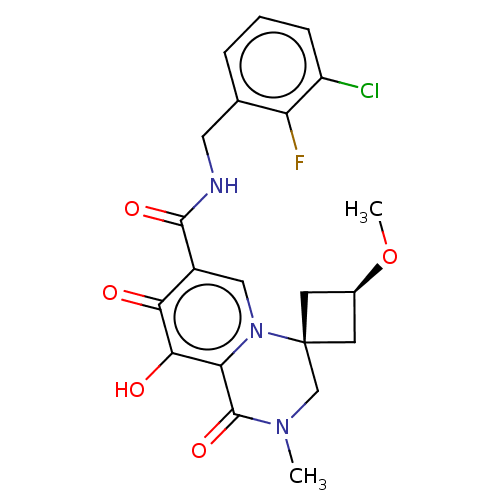 (US10087178, Example 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | 25 |
JAPAN TOBACCO INC. US Patent | Assay Description The medium (40 μL), a test substance (10 μL) diluted with the medium, and a 1×105 cells/mL MT-4 cell suspension (50 μL) wherein HIV-1 ... | US Patent US10087178 (2018) BindingDB Entry DOI: 10.7270/Q20C4XSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Nef (Human immunodeficiency virus type 1 (isolate BRU/L...) | BDBM288021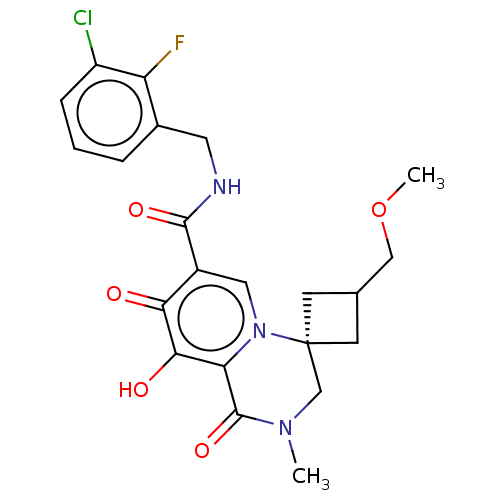 (US10087178, Example 3) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | 25 |
JAPAN TOBACCO INC. US Patent | Assay Description The medium (40 μL), a test substance (10 μL) diluted with the medium, and a 1×105 cells/mL MT-4 cell suspension (50 μL) wherein HIV-1 ... | US Patent US10087178 (2018) BindingDB Entry DOI: 10.7270/Q20C4XSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Nef (Human immunodeficiency virus type 1 (isolate BRU/L...) | BDBM288022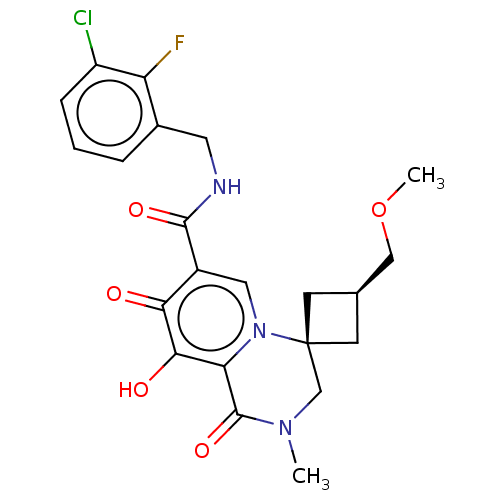 (US10087178, Example 4) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | 25 |
JAPAN TOBACCO INC. US Patent | Assay Description The medium (40 μL), a test substance (10 μL) diluted with the medium, and a 1×105 cells/mL MT-4 cell suspension (50 μL) wherein HIV-1 ... | US Patent US10087178 (2018) BindingDB Entry DOI: 10.7270/Q20C4XSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Nef (Human immunodeficiency virus type 1 (isolate BRU/L...) | BDBM288023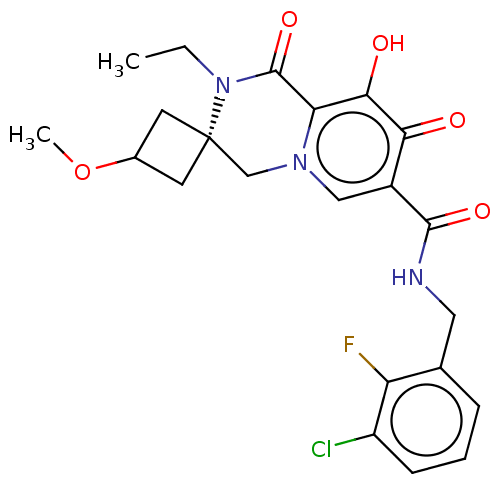 (US10087178, Example 5) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | 25 |
JAPAN TOBACCO INC. US Patent | Assay Description The medium (40 μL), a test substance (10 μL) diluted with the medium, and a 1×105 cells/mL MT-4 cell suspension (50 μL) wherein HIV-1 ... | US Patent US10087178 (2018) BindingDB Entry DOI: 10.7270/Q20C4XSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Nef (Human immunodeficiency virus type 1 (isolate BRU/L...) | BDBM288024 (US10087178, Example 6) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | 25 |
JAPAN TOBACCO INC. US Patent | Assay Description The medium (40 μL), a test substance (10 μL) diluted with the medium, and a 1×105 cells/mL MT-4 cell suspension (50 μL) wherein HIV-1 ... | US Patent US10087178 (2018) BindingDB Entry DOI: 10.7270/Q20C4XSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Nef (Human immunodeficiency virus type 1 (isolate BRU/L...) | BDBM288025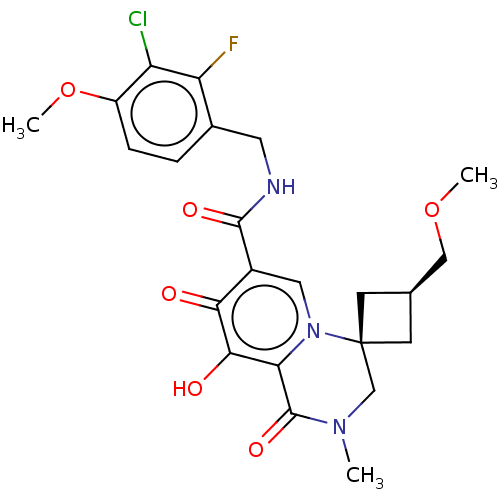 (US10087178, Example 7) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | 25 |
JAPAN TOBACCO INC. US Patent | Assay Description The medium (40 μL), a test substance (10 μL) diluted with the medium, and a 1×105 cells/mL MT-4 cell suspension (50 μL) wherein HIV-1 ... | US Patent US10087178 (2018) BindingDB Entry DOI: 10.7270/Q20C4XSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Nef (Human immunodeficiency virus type 1 (isolate BRU/L...) | BDBM288027 (US10087178, Example 8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 8.10 | n/a | n/a | n/a | 25 |
JAPAN TOBACCO INC. US Patent | Assay Description The medium (40 μL), a test substance (10 μL) diluted with the medium, and a 1×105 cells/mL MT-4 cell suspension (50 μL) wherein HIV-1 ... | US Patent US10087178 (2018) BindingDB Entry DOI: 10.7270/Q20C4XSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Nef (Human immunodeficiency virus type 1 (isolate BRU/L...) | BDBM288028 (US10087178, Example 9) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 5.60 | n/a | n/a | n/a | 25 |
JAPAN TOBACCO INC. US Patent | Assay Description The medium (40 μL), a test substance (10 μL) diluted with the medium, and a 1×105 cells/mL MT-4 cell suspension (50 μL) wherein HIV-1 ... | US Patent US10087178 (2018) BindingDB Entry DOI: 10.7270/Q20C4XSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Nef (Human immunodeficiency virus type 1 (isolate BRU/L...) | BDBM288029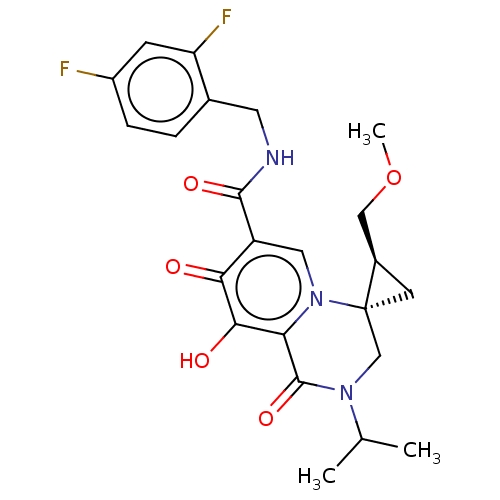 (US10087178, Example 10) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | 25 |
JAPAN TOBACCO INC. US Patent | Assay Description The medium (40 μL), a test substance (10 μL) diluted with the medium, and a 1×105 cells/mL MT-4 cell suspension (50 μL) wherein HIV-1 ... | US Patent US10087178 (2018) BindingDB Entry DOI: 10.7270/Q20C4XSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Nef (Human immunodeficiency virus type 1 (isolate BRU/L...) | BDBM288030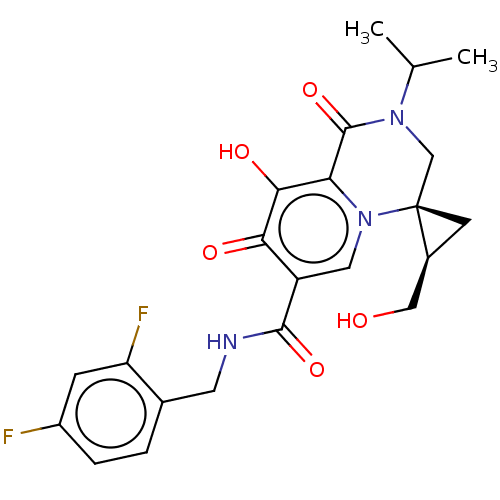 (US10087178, Example 11 | US10087178, Example 15) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 5.80 | n/a | n/a | n/a | 25 |
JAPAN TOBACCO INC. US Patent | Assay Description The medium (40 μL), a test substance (10 μL) diluted with the medium, and a 1×105 cells/mL MT-4 cell suspension (50 μL) wherein HIV-1 ... | US Patent US10087178 (2018) BindingDB Entry DOI: 10.7270/Q20C4XSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Nef (Human immunodeficiency virus type 1 (isolate BRU/L...) | BDBM288031 (US10087178, Example 12 | US10087178, Example 16) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | 25 |
JAPAN TOBACCO INC. US Patent | Assay Description The medium (40 μL), a test substance (10 μL) diluted with the medium, and a 1×105 cells/mL MT-4 cell suspension (50 μL) wherein HIV-1 ... | US Patent US10087178 (2018) BindingDB Entry DOI: 10.7270/Q20C4XSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Nef (Human immunodeficiency virus type 1 (isolate BRU/L...) | BDBM288032 (US10087178, Example 13) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 5.40 | n/a | n/a | n/a | 25 |
JAPAN TOBACCO INC. US Patent | Assay Description The medium (40 μL), a test substance (10 μL) diluted with the medium, and a 1×105 cells/mL MT-4 cell suspension (50 μL) wherein HIV-1 ... | US Patent US10087178 (2018) BindingDB Entry DOI: 10.7270/Q20C4XSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Nef (Human immunodeficiency virus type 1 (isolate BRU/L...) | BDBM288033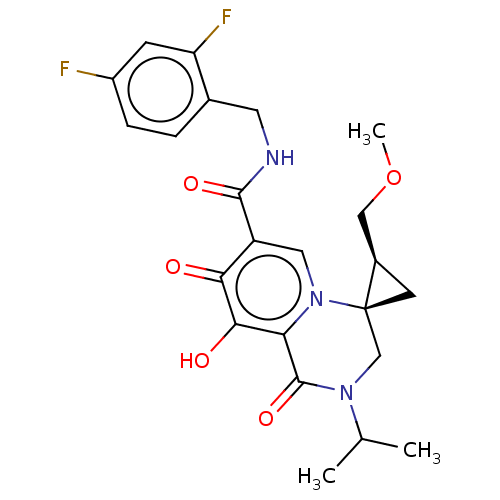 (US10087178, Example 14) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 9.10 | n/a | n/a | n/a | 25 |
JAPAN TOBACCO INC. US Patent | Assay Description The medium (40 μL), a test substance (10 μL) diluted with the medium, and a 1×105 cells/mL MT-4 cell suspension (50 μL) wherein HIV-1 ... | US Patent US10087178 (2018) BindingDB Entry DOI: 10.7270/Q20C4XSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Nef (Human immunodeficiency virus type 1 (isolate BRU/L...) | BDBM288030 (US10087178, Example 11 | US10087178, Example 15) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 7.20 | n/a | n/a | n/a | 25 |
JAPAN TOBACCO INC. US Patent | Assay Description The medium (40 μL), a test substance (10 μL) diluted with the medium, and a 1×105 cells/mL MT-4 cell suspension (50 μL) wherein HIV-1 ... | US Patent US10087178 (2018) BindingDB Entry DOI: 10.7270/Q20C4XSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Nef (Human immunodeficiency virus type 1 (isolate BRU/L...) | BDBM288031 (US10087178, Example 12 | US10087178, Example 16) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 6.10 | n/a | n/a | n/a | 25 |
JAPAN TOBACCO INC. US Patent | Assay Description The medium (40 μL), a test substance (10 μL) diluted with the medium, and a 1×105 cells/mL MT-4 cell suspension (50 μL) wherein HIV-1 ... | US Patent US10087178 (2018) BindingDB Entry DOI: 10.7270/Q20C4XSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Nef (Human immunodeficiency virus type 1 (isolate BRU/L...) | BDBM288036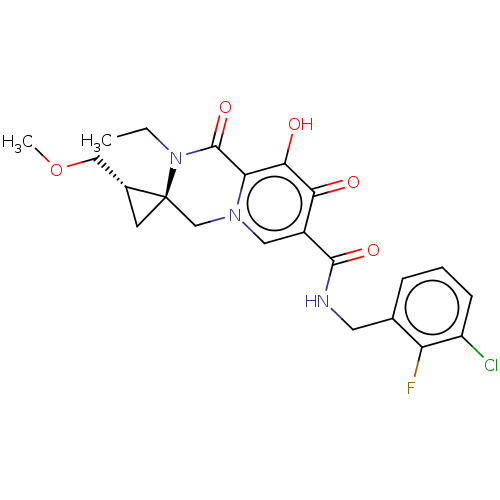 (US10087178, Example 17) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | 25 |
JAPAN TOBACCO INC. US Patent | Assay Description The medium (40 μL), a test substance (10 μL) diluted with the medium, and a 1×105 cells/mL MT-4 cell suspension (50 μL) wherein HIV-1 ... | US Patent US10087178 (2018) BindingDB Entry DOI: 10.7270/Q20C4XSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 218 total ) | Next | Last >> |