

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM181015 (US9133199, 254) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Cyclacel Limited US Patent | Assay Description CDC25C (2 ug/well) with PLKl (1 ug/well) in 20 mM Tris/HCl buffer pH 7.5, supplemented with 25 mM beta-glycerophosphate, 5 mM EGTA, 1 mM DTT and 1 mM... | US Patent US9133199 (2015) BindingDB Entry DOI: 10.7270/Q21C1VNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM181016 (US9133199, I-253) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Cyclacel Limited US Patent | Assay Description CDC25C (2 ug/well) with PLKl (1 ug/well) in 20 mM Tris/HCl buffer pH 7.5, supplemented with 25 mM beta-glycerophosphate, 5 mM EGTA, 1 mM DTT and 1 mM... | US Patent US9133199 (2015) BindingDB Entry DOI: 10.7270/Q21C1VNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoshikimate 1-carboxyvinyltransferase (Escherichia coli (strain K12)) | BDBM50123088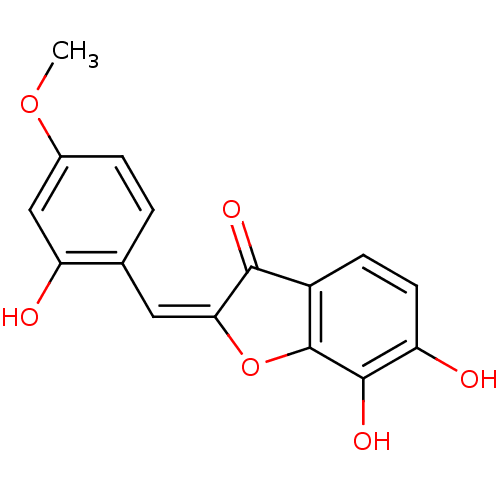 (6,7-Dihydroxy-2-[1-(2-hydroxy-4-methoxy-phenyl)-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Competitive inhibitory activity of the compound was determined with respect to EPSP (5-enolpyruvylshikimate- 3-phosphate) synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoshikimate 1-carboxyvinyltransferase (Escherichia coli (strain K12)) | BDBM50123114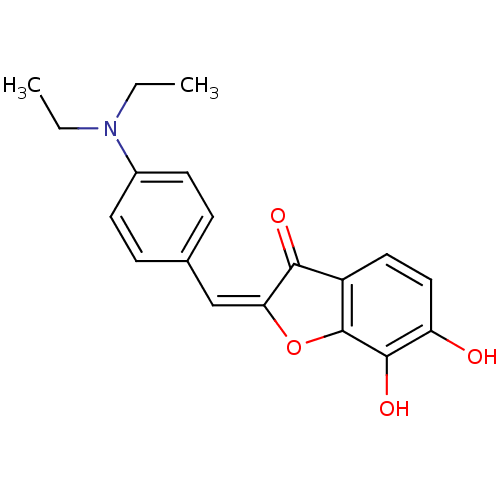 (2-[1-(4-Diethylamino-phenyl)-meth-(E)-ylidene]-6,7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Competitive inhibitory activity of the compound was determined with respect to EPSP (5-enolpyruvylshikimate- 3-phosphate) synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50463653 (CHEMBL4241707) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of aurora B (unknown origin) | Bioorg Med Chem Lett 28: 3025-3030 (2018) Article DOI: 10.1016/j.bmcl.2018.08.005 BindingDB Entry DOI: 10.7270/Q2WH2SNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50463653 (CHEMBL4241707) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of CB1 (unknown origin) | Bioorg Med Chem Lett 28: 3025-3030 (2018) Article DOI: 10.1016/j.bmcl.2018.08.005 BindingDB Entry DOI: 10.7270/Q2WH2SNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 15 (Homo sapiens (Human)) | BDBM50548060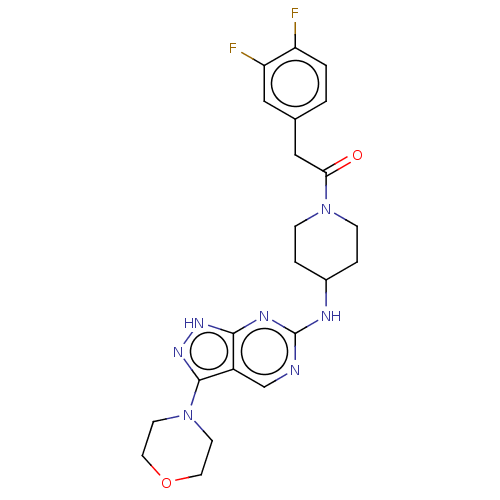 (CHEMBL4762609) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERK8 (2 to 544 residues) incubated for 5 mins in presence of [gamma33P]ATP by scintillation counting analysis | Citation and Details Article DOI: 10.1039/d0md00203h BindingDB Entry DOI: 10.7270/Q2QJ7MWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50384600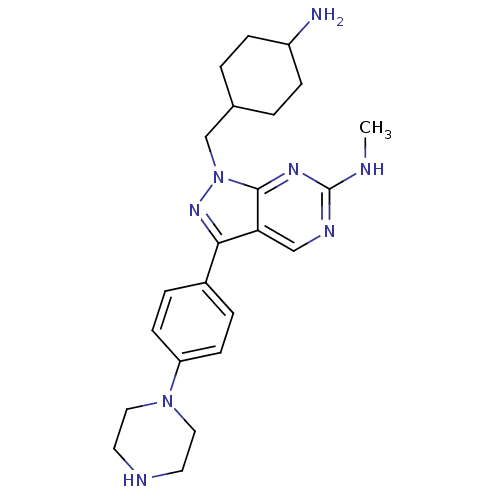 (CHEMBL2036792 | US9744172, Compound UNC00000563A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of CDK2 (unknown origin) | J Med Chem 62: 1180-1202 (2019) Article DOI: 10.1021/acs.jmedchem.8b01218 BindingDB Entry DOI: 10.7270/Q2ZC867R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM50384600 (CHEMBL2036792 | US9744172, Compound UNC00000563A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of CDK4 (unknown origin) | J Med Chem 62: 1180-1202 (2019) Article DOI: 10.1021/acs.jmedchem.8b01218 BindingDB Entry DOI: 10.7270/Q2ZC867R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123120 (6,7-Dihydroxy-2-[1-(2-hydroxy-4-pentyloxy-phenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123099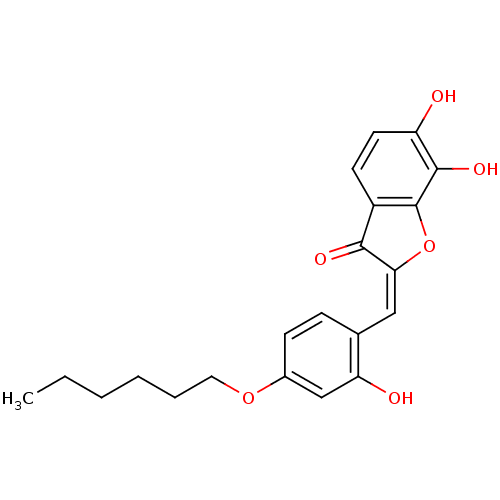 (2-[1-(4-Hexyloxy-2-hydroxy-phenyl)-meth-(E)-yliden...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123103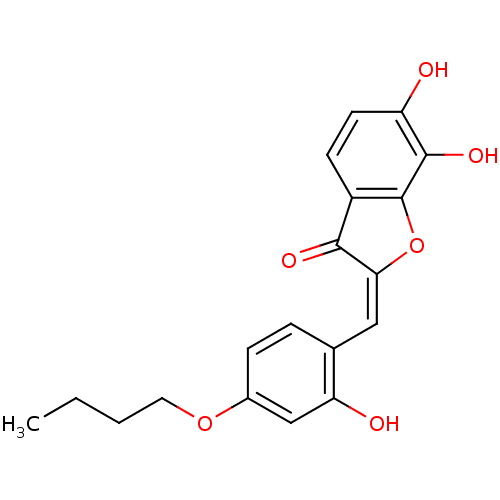 (2-[1-(4-Butoxy-2-hydroxy-phenyl)-meth-(E)-ylidene]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123101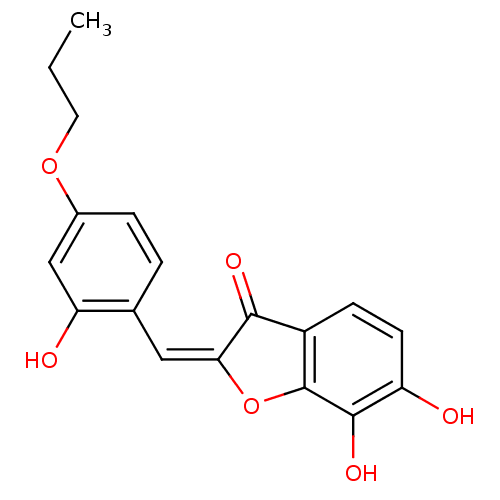 (6,7-Dihydroxy-2-[1-(2-hydroxy-4-propoxy-phenyl)-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11 (Homo sapiens (Human)) | BDBM50548060 (CHEMBL4762609) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human p38beta MAPK (1 to 364 residues) incubated for 5 mins in presence of [gamma33P]ATP by scintillation counting analysis | Citation and Details Article DOI: 10.1039/d0md00203h BindingDB Entry DOI: 10.7270/Q2QJ7MWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123096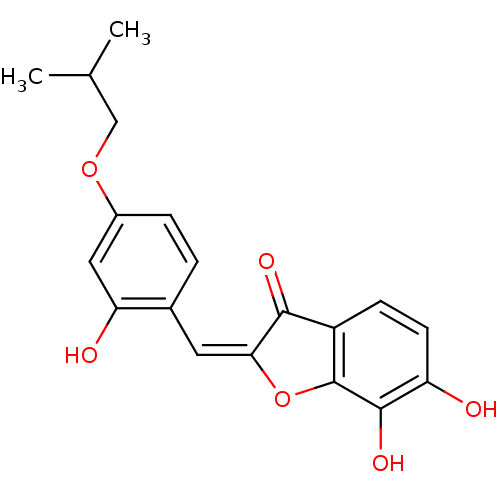 (6,7-Dihydroxy-2-[1-(2-hydroxy-4-isobutoxy-phenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123093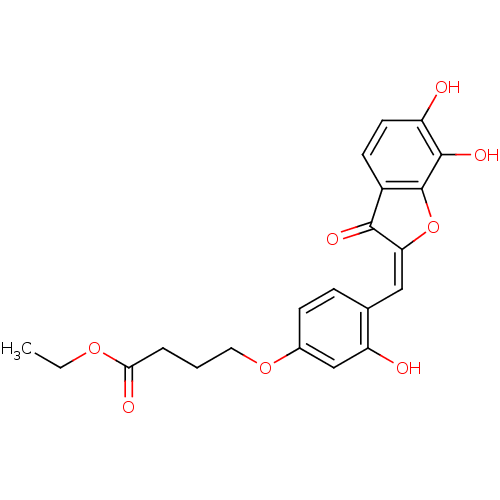 (4-{4-[6,7-Dihydroxy-3-oxo-3H-benzofuran-(2E)-ylide...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123118 (6,7-Dihydroxy-2-[1-(2-hydroxy-phenyl)-meth-(E)-yli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123087 (6,7-Dihydroxy-2-[1-(2-hydroxy-3-methoxy-phenyl)-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123090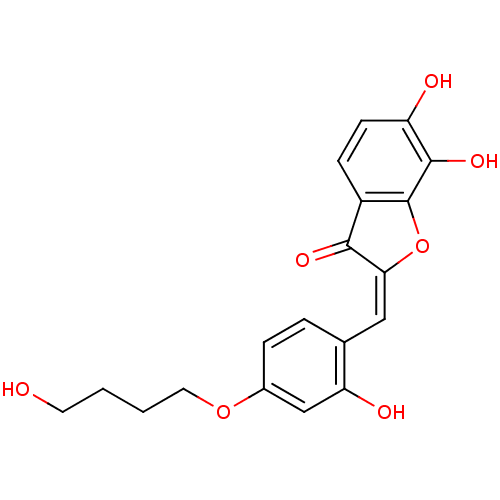 (6,7-Dihydroxy-2-[1-[2-hydroxy-4-(4-hydroxy-butoxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123104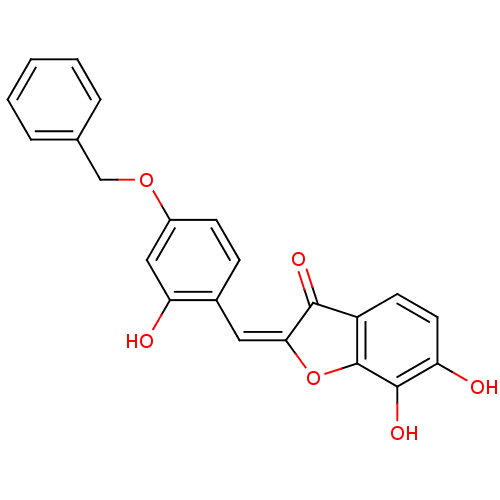 (2-[1-(4-Benzyloxy-2-hydroxy-phenyl)-meth-(E)-ylide...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123108 (6,7-Dihydroxy-2-[1-(2-hydroxy-4-isopropoxy-phenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123085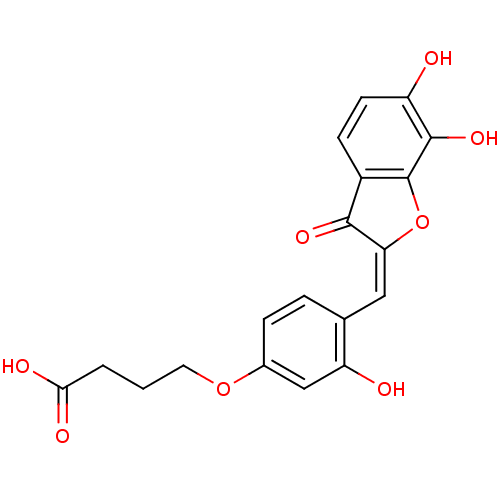 (4-{4-[6,7-Dihydroxy-3-oxo-3H-benzofuran-(2E)-ylide...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123111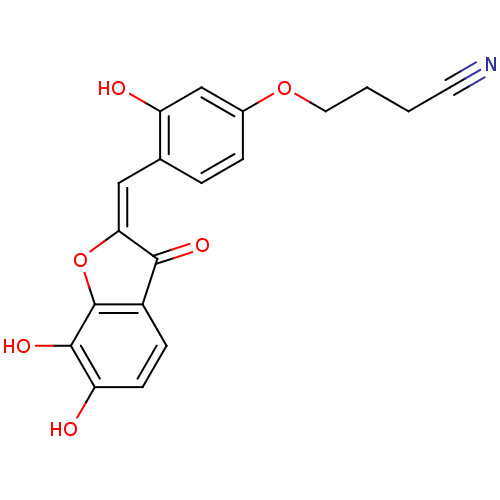 (4-{4-[6,7-Dihydroxy-3-oxo-3H-benzofuran-(2E)-ylide...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123124 (2-[1-(4-Butoxy-phenyl)-meth-(E)-ylidene]-6,7-dihyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123121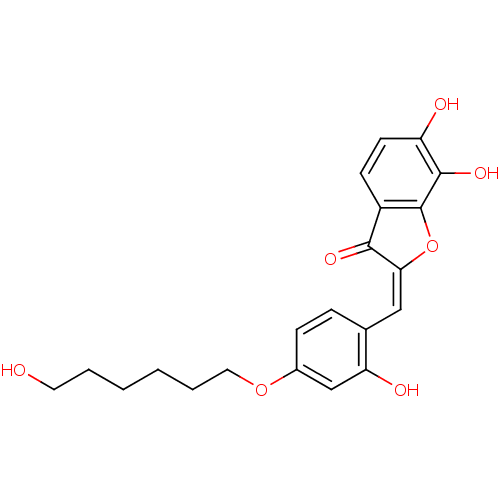 (6,7-Dihydroxy-2-[1-[2-hydroxy-4-(6-hydroxy-hexylox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123088 (6,7-Dihydroxy-2-[1-(2-hydroxy-4-methoxy-phenyl)-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123098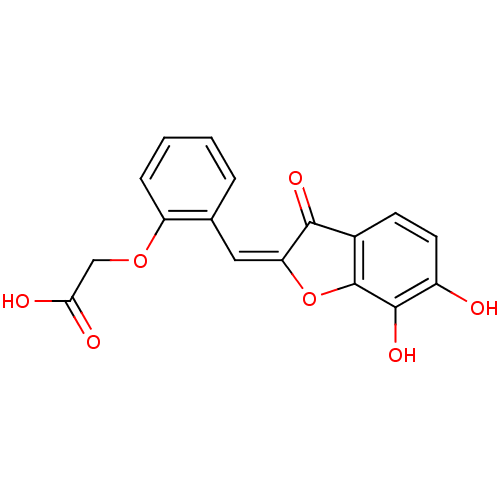 (CHEMBL134581 | {2-[6,7-Dihydroxy-3-oxo-3H-benzofur...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123089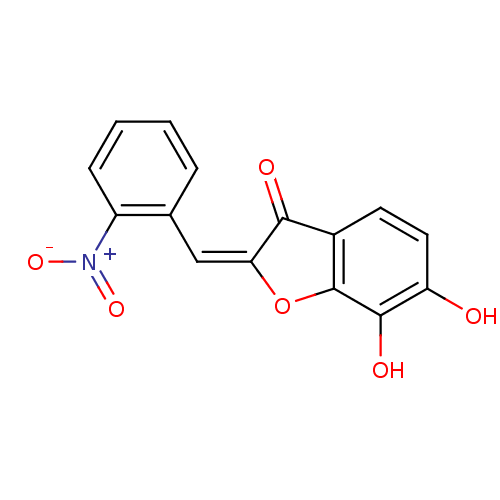 (6,7-Dihydroxy-2-[1-(2-nitro-phenyl)-meth-(E)-ylide...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123109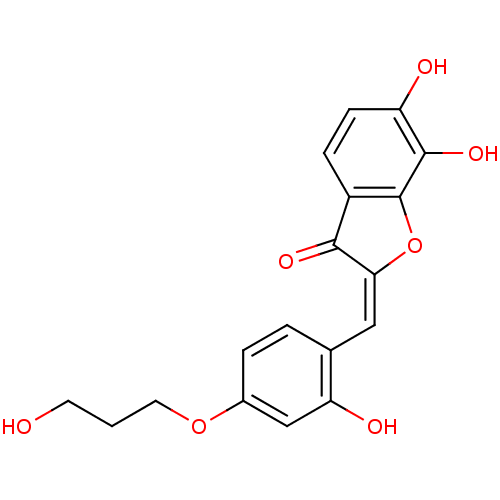 (6,7-Dihydroxy-2-[1-[2-hydroxy-4-(3-hydroxy-propoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123116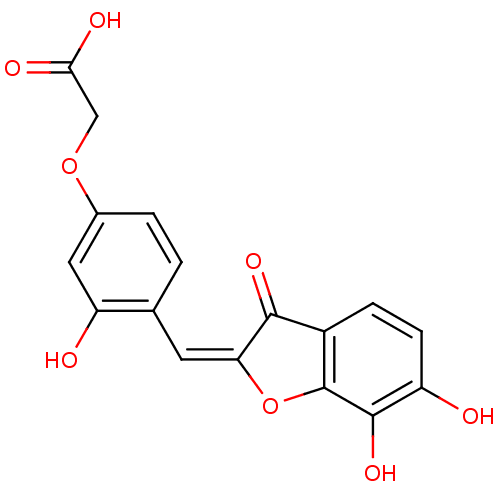 (CHEMBL135330 | {4-[6,7-Dihydroxy-3-oxo-3H-benzofur...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123113 (2-[1-{4-[Ethyl-(2-hydroxy-ethyl)-amino]-2-methyl-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123106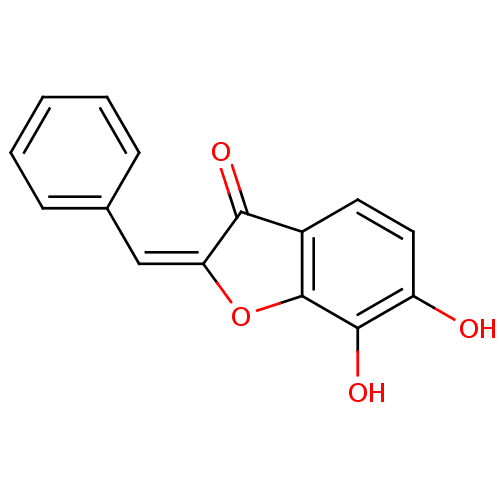 (6,7-Dihydroxy-2-[1-phenyl-meth-(E)-ylidene]-benzof...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50463653 (CHEMBL4241707) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 28: 3025-3030 (2018) Article DOI: 10.1016/j.bmcl.2018.08.005 BindingDB Entry DOI: 10.7270/Q2WH2SNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50542902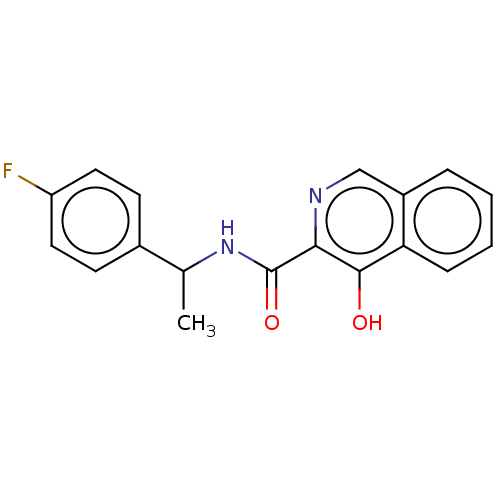 (CHEMBL4645906) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of MAOA (unknown origin) | J Med Chem 63: 9523-9539 (2020) Article DOI: 10.1021/acs.jmedchem.0c00705 BindingDB Entry DOI: 10.7270/Q2PK0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123095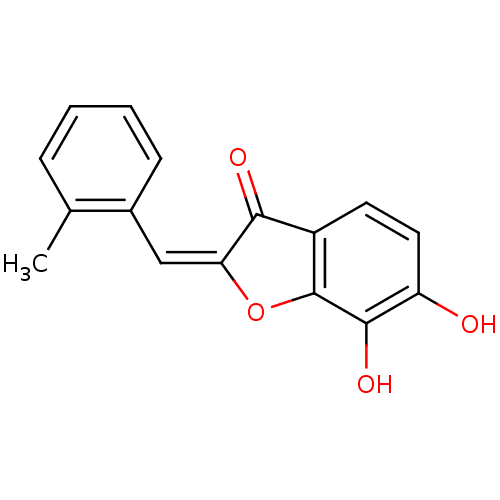 (6,7-Dihydroxy-2-[1-o-tolyl-meth-(E)-ylidene]-benzo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123100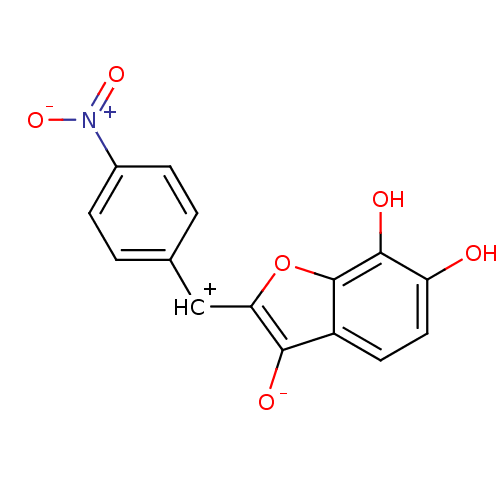 (6,7-Dihydroxy-2-[1-(4-nitro-phenyl)-meth-(E)-ylide...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123123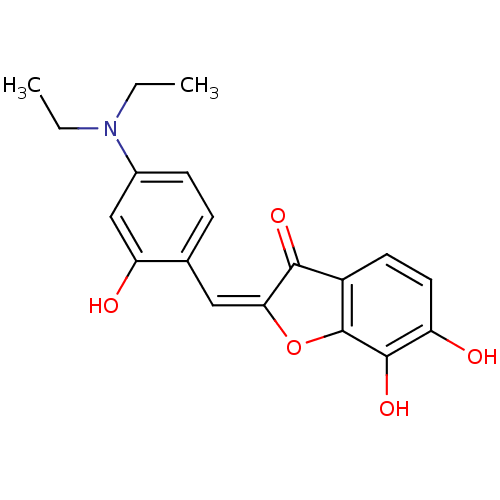 (2-[1-(4-Diethylamino-2-hydroxy-phenyl)-meth-(E)-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50463654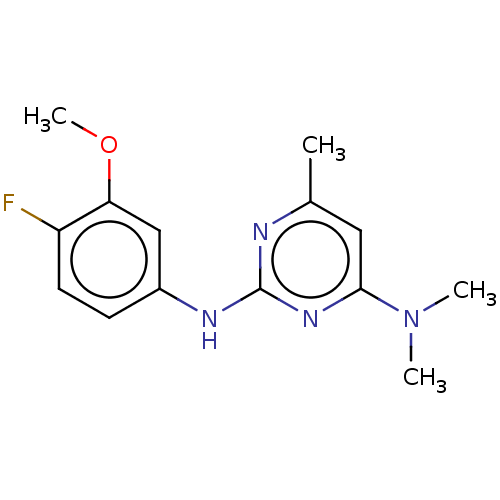 (CHEMBL4241692) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 28: 3025-3030 (2018) Article DOI: 10.1016/j.bmcl.2018.08.005 BindingDB Entry DOI: 10.7270/Q2WH2SNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123112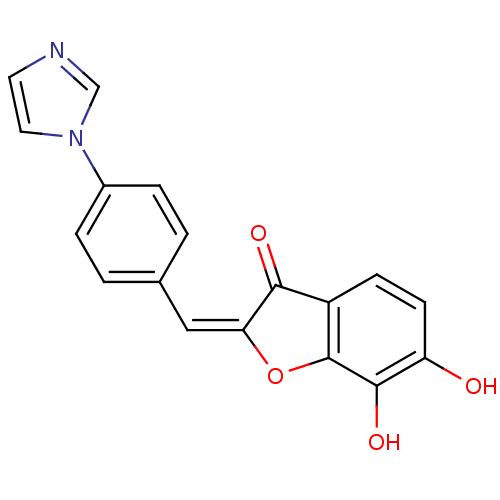 (6,7-Dihydroxy-2-[1-(4-imidazol-1-yl-phenyl)-meth-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123086 (2-[1-(2-Chloro-phenyl)-meth-(E)-ylidene]-6,7-dihyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123115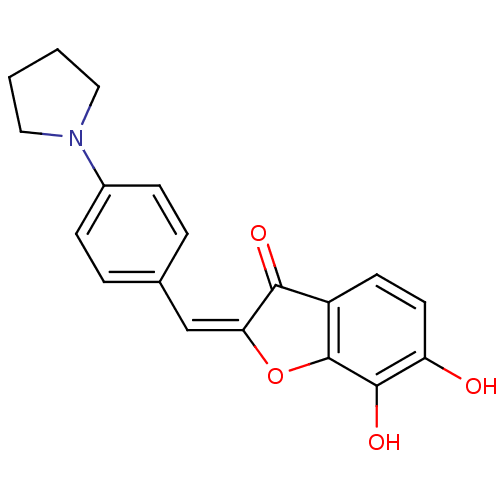 (6,7-Dihydroxy-2-[1-(4-pyrrolidin-1-yl-phenyl)-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123097 (2-[1-(4-Ethoxy-phenyl)-meth-(E)-ylidene]-6,7-dihyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50517286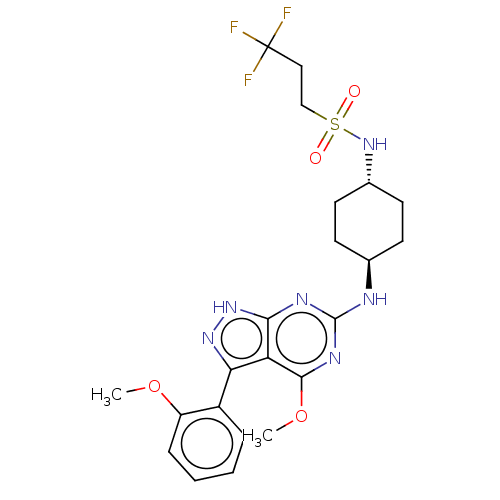 (CHEMBL4516798) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 20 mins followed by substrate addition and measured afte... | J Med Chem 62: 1180-1202 (2019) Article DOI: 10.1021/acs.jmedchem.8b01218 BindingDB Entry DOI: 10.7270/Q2ZC867R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123125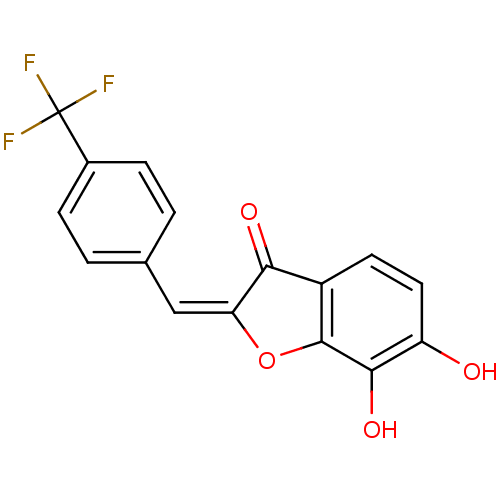 (6,7-Dihydroxy-2-[1-(4-trifluoromethyl-phenyl)-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123114 (2-[1-(4-Diethylamino-phenyl)-meth-(E)-ylidene]-6,7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM50463655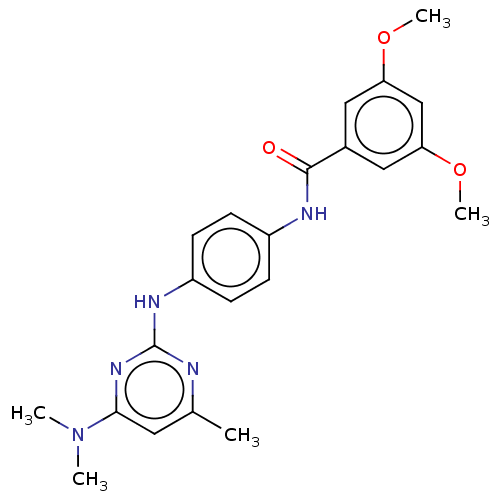 (CHEMBL546608 | TCMDC-124726) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi CYP51 | Bioorg Med Chem Lett 28: 3025-3030 (2018) Article DOI: 10.1016/j.bmcl.2018.08.005 BindingDB Entry DOI: 10.7270/Q2WH2SNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123110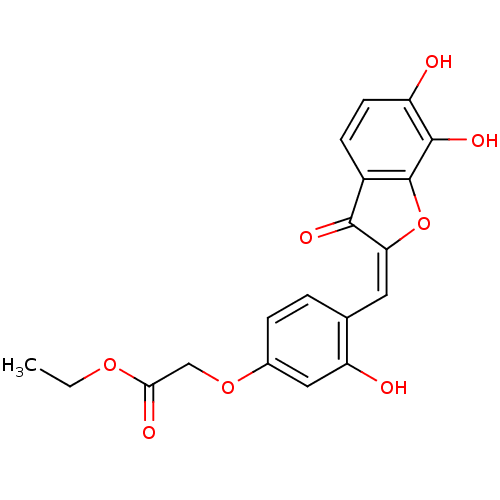 (CHEMBL434823 | {4-[6,7-Dihydroxy-3-oxo-3H-benzofur...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123102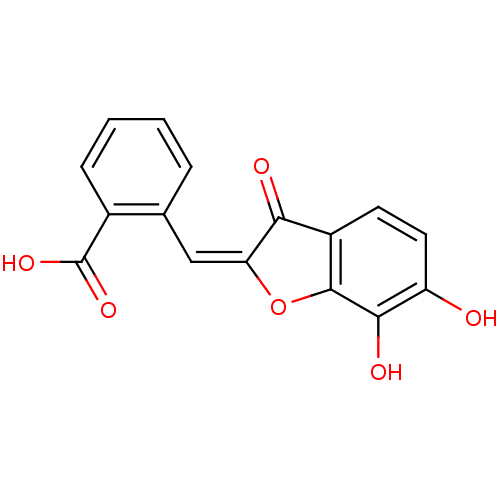 (2-[6,7-Dihydroxy-3-oxo-3H-benzofuran-(2E)-ylidenem...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123122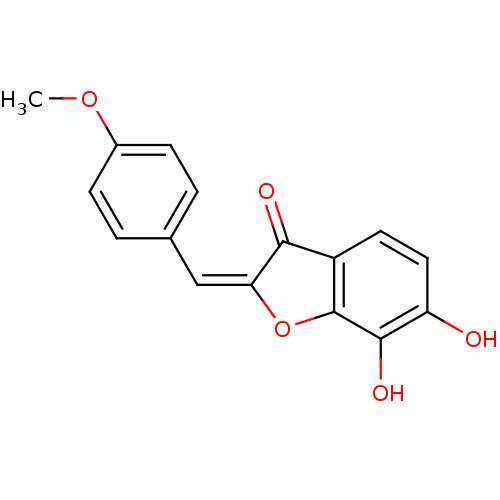 (6,7-Dihydroxy-2-[1-(4-methoxy-phenyl)-meth-(E)-yli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate synthase (Streptococcus pneumoniae) | BDBM50123119 (6,7-Dihydroxy-2-[1-(4-hydroxy-phenyl)-meth-(E)-yli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PanTherix Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against Streptococcus pneumoniae chorismate synthase | Bioorg Med Chem Lett 13: 423-6 (2003) BindingDB Entry DOI: 10.7270/Q2TH8M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 62 total ) | Next | Last >> |