 Found 467 hits with Last Name = 'thompson' and Initial = 'je'
Found 467 hits with Last Name = 'thompson' and Initial = 'je' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM15241
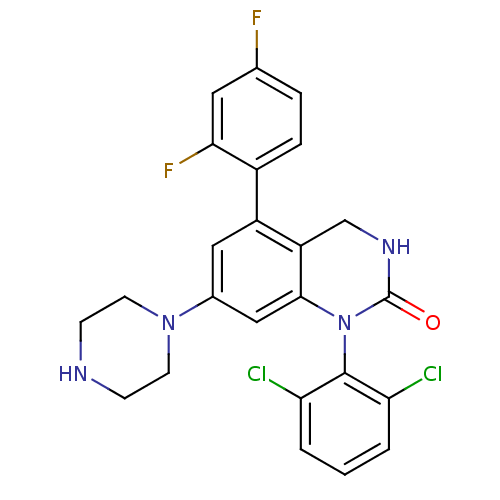
(1-(2,6-dichlorophenyl)-5-(2,4-difluorophenyl)-7-(p...)Show SMILES Fc1ccc(c(F)c1)-c1cc(cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl)N1CCNCC1 Show InChI InChI=1S/C24H20Cl2F2N4O/c25-19-2-1-3-20(26)23(19)32-22-12-15(31-8-6-29-7-9-31)11-17(18(22)13-30-24(32)33)16-5-4-14(27)10-21(16)28/h1-5,10-12,29H,6-9,13H2,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of labeled MK-499 from human ERG in HEK293 cells |
Bioorg Med Chem Lett 20: 2765-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.069
BindingDB Entry DOI: 10.7270/Q23T9HCC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50194461

(7-amino-5-(2,4-difluorophenyl)-1-(2,6-difluorophen...)Show SMILES Nc1cc2n(-c3c(F)cccc3F)c(=O)ccc2c(n1)-c1ccc(F)cc1F |(15,-16.28,;13.66,-17.04,;12.34,-16.27,;11.01,-17.03,;9.67,-16.27,;9.67,-14.73,;11.01,-13.96,;12.35,-14.72,;11.01,-12.4,;9.67,-11.64,;8.34,-12.41,;8.34,-13.96,;7,-14.73,;8.34,-17.03,;7.01,-16.26,;8.34,-18.57,;9.67,-19.35,;11,-18.57,;12.32,-19.35,;13.66,-18.59,;12.31,-20.89,;10.97,-21.64,;10.96,-23.18,;12.29,-23.96,;12.28,-25.51,;13.63,-23.19,;13.64,-21.66,;14.97,-20.89,)| Show InChI InChI=1S/C20H11F4N3O/c21-10-4-5-11(15(24)8-10)19-12-6-7-18(28)27(16(12)9-17(25)26-19)20-13(22)2-1-3-14(20)23/h1-9H,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of labeled MK-499 from human ERG in HEK293 cells |
Bioorg Med Chem Lett 20: 2765-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.069
BindingDB Entry DOI: 10.7270/Q23T9HCC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50317587
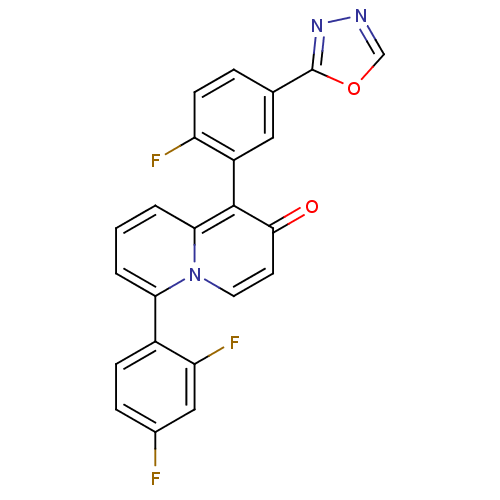
(6-(2,4-difluorophenyl)-1-(2-fluoro-5-(1,3,4-oxadia...)Show SMILES Fc1ccc(c(F)c1)-c1cccc2c(-c3cc(ccc3F)-c3nnco3)c(=O)ccn12 |(12.42,-9.58,;12.42,-8.04,;11.08,-7.27,;11.09,-5.73,;12.43,-4.96,;13.76,-5.73,;15.09,-4.95,;13.76,-7.26,;12.43,-3.42,;13.76,-2.65,;13.75,-1.11,;12.42,-.35,;11.1,-1.11,;9.77,-.33,;9.77,1.21,;11.11,1.97,;11.11,3.51,;9.77,4.29,;8.43,3.51,;8.44,1.97,;7.11,1.2,;12.44,4.28,;13.91,3.83,;14.8,5.09,;13.87,6.32,;12.42,5.83,;8.44,-1.11,;7.1,-.35,;8.44,-2.65,;9.77,-3.42,;11.1,-2.65,)| Show InChI InChI=1S/C23H12F3N3O2/c24-14-5-6-15(18(26)11-14)19-2-1-3-20-22(21(30)8-9-29(19)20)16-10-13(4-7-17(16)25)23-28-27-12-31-23/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of labeled MK-499 from human ERG in HEK293 cells |
Bioorg Med Chem Lett 20: 2765-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.069
BindingDB Entry DOI: 10.7270/Q23T9HCC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50317588
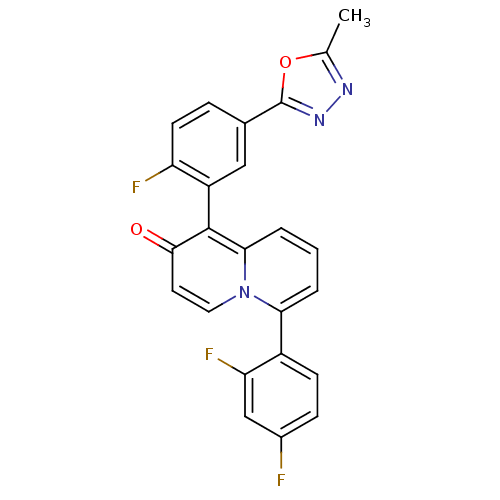
(6-(2,4-difluorophenyl)-1-(2-fluoro-5-(5-methyl-1,3...)Show SMILES Cc1nnc(o1)-c1ccc(F)c(c1)-c1c2cccc(-c3ccc(F)cc3F)n2ccc1=O |(31.09,6.57,;30.64,5.09,;31.57,3.86,;30.68,2.6,;29.21,3.05,;29.18,4.59,;27.88,2.28,;26.54,3.06,;25.2,2.28,;25.21,.74,;23.87,-.03,;26.54,-.02,;27.87,.74,;26.54,-1.56,;27.87,-2.34,;29.19,-1.58,;30.52,-2.34,;30.52,-3.88,;29.19,-4.65,;29.19,-6.19,;27.85,-6.96,;27.85,-8.5,;29.19,-9.27,;29.19,-10.81,;30.53,-8.49,;30.52,-6.95,;31.85,-6.18,;27.86,-3.88,;26.54,-4.65,;25.21,-3.88,;25.21,-2.34,;23.87,-1.58,)| Show InChI InChI=1S/C24H14F3N3O2/c1-13-28-29-24(32-13)14-5-8-18(26)17(11-14)23-21-4-2-3-20(30(21)10-9-22(23)31)16-7-6-15(25)12-19(16)27/h2-12H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of labeled MK-499 from human ERG in HEK293 cells |
Bioorg Med Chem Lett 20: 2765-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.069
BindingDB Entry DOI: 10.7270/Q23T9HCC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50317579

(6-(2,4-difluorophenyl)-1-(2,6-difluorophenyl)-2H-q...)Show SMILES Fc1ccc(c(F)c1)-c1cccc2c(-c3c(F)cccc3F)c(=O)ccn12 |(29.65,-6.7,;29.65,-5.16,;28.31,-4.39,;28.31,-2.85,;29.65,-2.08,;30.98,-2.84,;32.31,-2.07,;30.99,-4.38,;29.65,-.54,;30.98,.23,;30.98,1.77,;29.65,2.53,;28.33,1.77,;27,2.55,;27,4.09,;28.33,4.85,;29.67,4.08,;28.33,6.39,;27,7.17,;25.66,6.39,;25.67,4.85,;24.33,4.08,;25.67,1.77,;24.33,2.53,;25.67,.23,;27,-.54,;28.32,.23,)| Show InChI InChI=1S/C21H11F4NO/c22-12-7-8-13(16(25)11-12)17-5-2-6-18-21(19(27)9-10-26(17)18)20-14(23)3-1-4-15(20)24/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of labeled MK-499 from human ERG in HEK293 cells |
Bioorg Med Chem Lett 20: 2765-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.069
BindingDB Entry DOI: 10.7270/Q23T9HCC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175747
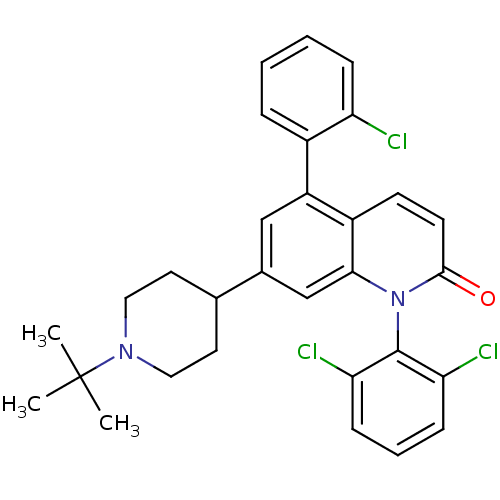
(7-(1-tert-butylpiperidin-4-yl)-5-(2-chlorophenyl)-...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccccc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(19.17,-37.34,;17.84,-38.12,;18.62,-39.45,;17.07,-36.79,;16.51,-38.89,;16.51,-40.43,;15.18,-41.2,;13.84,-40.43,;13.83,-38.89,;15.17,-38.12,;12.51,-41.21,;12.51,-42.76,;11.17,-43.53,;11.18,-45.07,;9.84,-45.84,;9.84,-47.37,;11.17,-48.15,;12.51,-47.37,;12.51,-45.83,;13.84,-45.06,;9.84,-42.76,;8.51,-43.54,;7.17,-42.76,;7.17,-41.22,;5.84,-40.45,;8.51,-40.45,;8.51,-38.92,;7.18,-38.15,;5.85,-38.92,;7.18,-36.61,;8.52,-35.84,;9.85,-36.61,;9.85,-38.15,;11.18,-38.92,;9.84,-41.22,;11.17,-40.45,)| Show InChI InChI=1S/C30H29Cl3N2O/c1-30(2,3)34-15-13-19(14-16-34)20-17-23(21-7-4-5-8-24(21)31)22-11-12-28(36)35(27(22)18-20)29-25(32)9-6-10-26(29)33/h4-12,17-19H,13-16H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175745
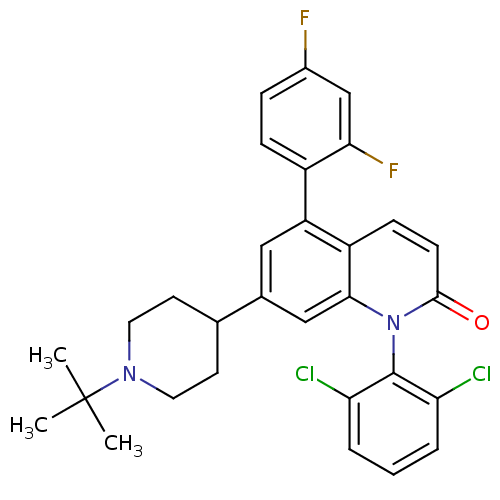
(7-(1-tert-butylpiperidin-4-yl)-1-(2,6-dichlorophen...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccc(F)cc2F)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(5.3,.54,;3.97,-.23,;4.75,-1.56,;3.2,1.1,;2.64,-1,;2.64,-2.54,;1.31,-3.31,;-.03,-2.54,;-.04,-1.01,;1.3,-.23,;-1.36,-3.31,;-1.35,-4.86,;-2.69,-5.64,;-2.69,-7.17,;-4.03,-7.94,;-4.03,-9.48,;-2.69,-10.25,;-2.69,-11.79,;-1.35,-9.47,;-1.36,-7.94,;-.03,-7.16,;-4.03,-4.86,;-5.36,-5.63,;-6.69,-4.85,;-6.69,-3.31,;-8.02,-2.53,;-5.34,-2.55,;-5.33,-1.01,;-6.66,-.24,;-8,-1,;-6.66,1.3,;-5.31,2.07,;-3.98,1.29,;-3.99,-.25,;-2.67,-1.03,;-4.02,-3.32,;-2.69,-2.55,)| Show InChI InChI=1S/C30H28Cl2F2N2O/c1-30(2,3)35-13-11-18(12-14-35)19-15-23(21-8-7-20(33)17-26(21)34)22-9-10-28(37)36(27(22)16-19)29-24(31)5-4-6-25(29)32/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM17060
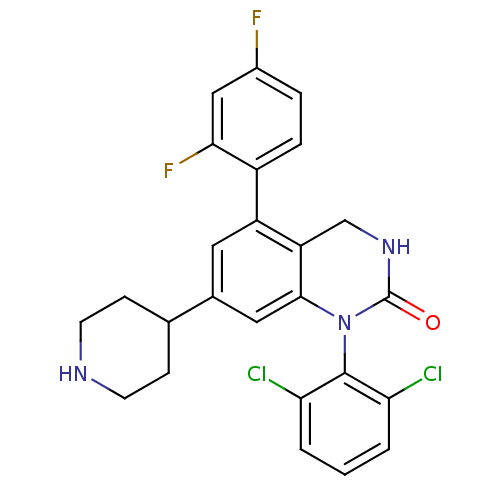
(1-(2,6-dichlorophenyl)-5-(2,4-difluorophenyl)-7-(p...)Show SMILES Fc1ccc(c(F)c1)-c1cc(cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl)C1CCNCC1 Show InChI InChI=1S/C25H21Cl2F2N3O/c26-20-2-1-3-21(27)24(20)32-23-11-15(14-6-8-30-9-7-14)10-18(19(23)13-31-25(32)33)17-5-4-16(28)12-22(17)29/h1-5,10-12,14,30H,6-9,13H2,(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with p38 enzyme, and substrates in the presence ATP/[gamma-33P] ATP. After the rea... |
Bioorg Med Chem Lett 16: 4400-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.045
BindingDB Entry DOI: 10.7270/Q2NS0S56 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50222624
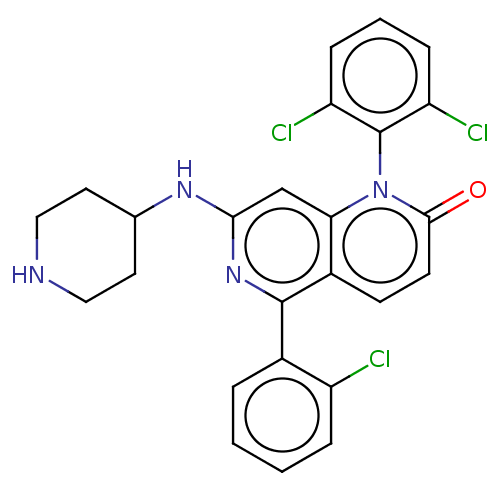
(CHEMBL357598)Show SMILES Clc1ccccc1-c1nc(NC2CCNCC2)cc2n(-c3c(Cl)cccc3Cl)c(=O)ccc12 |(10.11,-3.62,;8.78,-4.39,;8.78,-5.93,;7.43,-6.7,;6.1,-5.93,;6.11,-4.39,;7.45,-3.62,;7.47,-2.1,;8.8,-1.33,;8.8,.21,;10.13,.97,;11.46,.2,;12.79,.97,;14.12,.19,;14.12,-1.36,;12.79,-2.12,;11.46,-1.34,;7.47,.98,;6.14,.23,;4.81,.98,;4.81,2.52,;6.14,3.29,;7.47,2.52,;6.14,4.85,;4.81,5.62,;3.48,4.83,;3.48,3.29,;2.15,2.54,;3.48,.23,;2.13,1,;3.46,-1.31,;4.79,-2.08,;6.14,-1.31,)| Show InChI InChI=1S/C25H21Cl3N4O/c26-18-5-2-1-4-16(18)24-17-8-9-23(33)32(25-19(27)6-3-7-20(25)28)21(17)14-22(31-24)30-15-10-12-29-13-11-15/h1-9,14-15,29H,10-13H2,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of p38 MAP kinase |
Bioorg Med Chem Lett 13: 467-70 (2003)
BindingDB Entry DOI: 10.7270/Q22F7QM4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50222627
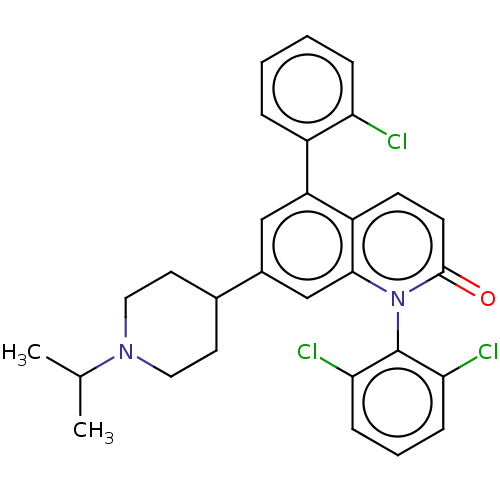
(CHEMBL356125)Show SMILES CC(C)N1CCC(CC1)c1cc(-c2ccccc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(15.97,6.5,;15.96,4.96,;17.29,4.19,;14.61,4.2,;14.61,2.66,;13.28,1.91,;11.95,2.68,;11.95,4.22,;13.28,4.97,;10.62,1.91,;10.62,.37,;9.27,-.4,;9.27,-1.94,;7.92,-2.69,;7.92,-4.23,;9.25,-5.02,;10.59,-4.25,;10.59,-2.71,;11.92,-1.92,;7.94,.37,;6.61,-.4,;5.28,.39,;5.28,1.91,;3.95,2.68,;6.63,2.68,;6.63,4.22,;7.96,4.99,;9.29,4.22,;7.96,6.53,;6.63,7.32,;5.3,6.53,;5.3,4.99,;3.95,4.24,;7.96,1.91,;9.29,2.68,)| Show InChI InChI=1S/C29H27Cl3N2O/c1-18(2)33-14-12-19(13-15-33)20-16-23(21-6-3-4-7-24(21)30)22-10-11-28(35)34(27(22)17-20)29-25(31)8-5-9-26(29)32/h3-11,16-19H,12-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of p38 MAP kinase |
Bioorg Med Chem Lett 13: 467-70 (2003)
BindingDB Entry DOI: 10.7270/Q22F7QM4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM17061
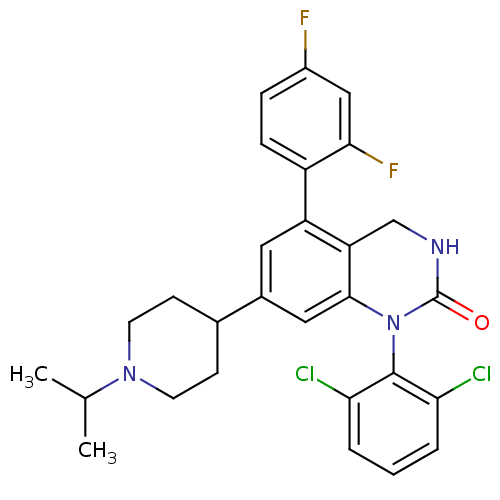
(1-(2,6-dichlorophenyl)-5-(2,4-difluorophenyl)-7-[1...)Show SMILES CC(C)N1CCC(CC1)c1cc2N(C(=O)NCc2c(c1)-c1ccc(F)cc1F)c1c(Cl)cccc1Cl Show InChI InChI=1S/C28H27Cl2F2N3O/c1-16(2)34-10-8-17(9-11-34)18-12-21(20-7-6-19(31)14-25(20)32)22-15-33-28(36)35(26(22)13-18)27-23(29)4-3-5-24(27)30/h3-7,12-14,16-17H,8-11,15H2,1-2H3,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with p38 enzyme, and substrates in the presence ATP/[gamma-33P] ATP. After the rea... |
Bioorg Med Chem Lett 16: 4400-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.045
BindingDB Entry DOI: 10.7270/Q2NS0S56 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175762
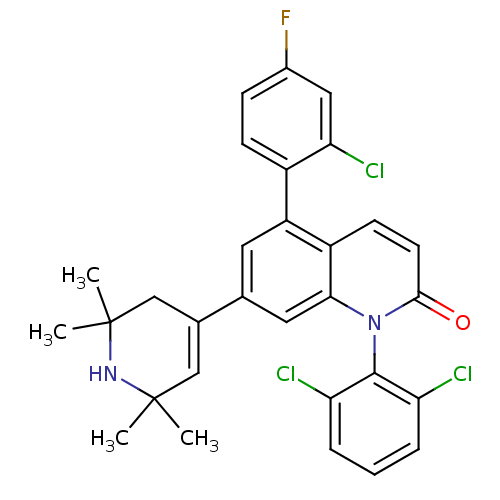
(5-(2-chloro-4-fluorophenyl)-1-(2,6-dichlorophenyl)...)Show SMILES CC1(C)CC(=CC(C)(C)N1)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |c:4,(2.04,.58,;2.06,2.12,;3.39,2.89,;.73,1.35,;-.61,2.12,;-.61,3.66,;.72,4.43,;2.05,5.21,;-.61,5.2,;2.06,3.66,;-1.94,1.34,;-1.94,-.21,;-3.27,-.98,;-3.27,-2.51,;-4.61,-3.28,;-4.61,-4.82,;-3.27,-5.59,;-3.27,-7.13,;-1.94,-4.81,;-1.94,-3.28,;-.61,-2.5,;-4.61,-.21,;-5.94,-.98,;-7.28,-.21,;-7.28,1.34,;-8.61,2.11,;-5.94,2.1,;-5.94,3.64,;-7.27,4.4,;-8.6,3.63,;-7.27,5.94,;-5.93,6.72,;-4.6,5.94,;-4.6,4.41,;-3.27,3.63,;-4.61,1.34,;-3.28,2.11,)| Show InChI InChI=1S/C30H26Cl3FN2O/c1-29(2)15-18(16-30(3,4)35-29)17-12-22(20-9-8-19(34)14-25(20)33)21-10-11-27(37)36(26(21)13-17)28-23(31)6-5-7-24(28)32/h5-15,35H,16H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175758
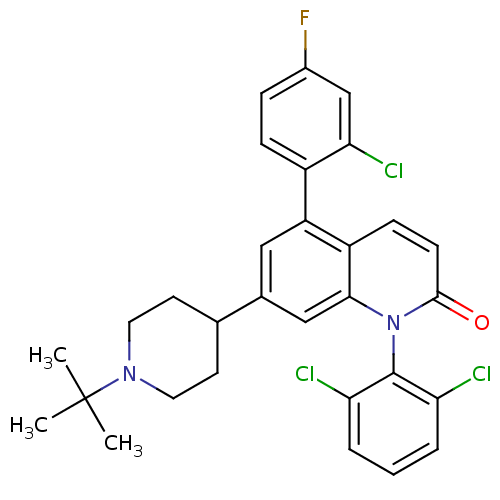
(7-(1-tert-butylpiperidin-4-yl)-5-(2-chloro-4-fluor...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(36.13,-13.37,;34.8,-14.15,;35.58,-15.48,;34.03,-12.81,;33.47,-14.92,;33.47,-16.46,;32.14,-17.22,;30.8,-16.46,;30.79,-14.92,;32.13,-14.15,;29.47,-17.23,;29.47,-18.78,;28.13,-19.55,;28.14,-21.09,;26.8,-21.86,;26.8,-23.4,;28.13,-24.17,;28.14,-25.71,;29.47,-23.39,;29.47,-21.85,;30.8,-21.08,;26.8,-18.78,;25.47,-19.56,;24.13,-18.79,;24.13,-17.24,;22.8,-16.47,;25.47,-16.47,;25.47,-14.94,;24.14,-14.17,;22.81,-14.95,;24.14,-12.64,;25.48,-11.86,;26.81,-12.63,;26.81,-14.17,;28.14,-14.94,;26.8,-17.24,;28.13,-16.47,)| Show InChI InChI=1S/C30H28Cl3FN2O/c1-30(2,3)35-13-11-18(12-14-35)19-15-23(21-8-7-20(34)17-26(21)33)22-9-10-28(37)36(27(22)16-19)29-24(31)5-4-6-25(29)32/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of TNF alpha release in THP1 cells |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175760
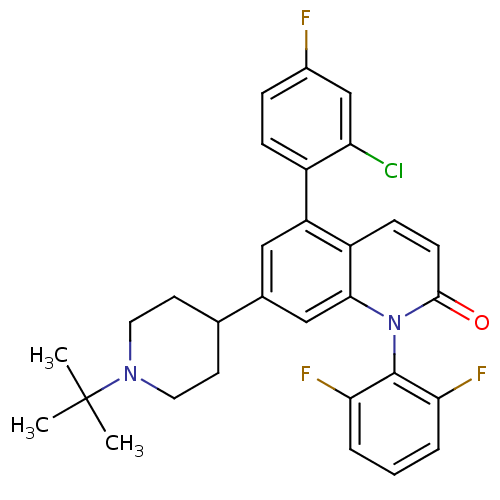
(7-(1-tert-butylpiperidin-4-yl)-5-(2-chloro-4-fluor...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(F)cccc3F)c2c1 |(36.23,-35.35,;34.9,-36.13,;35.68,-37.46,;34.13,-34.8,;33.57,-36.9,;33.57,-38.44,;32.24,-39.21,;30.9,-38.44,;30.9,-36.9,;32.23,-36.13,;29.57,-39.22,;29.57,-40.77,;28.24,-41.54,;28.24,-43.08,;26.9,-43.84,;26.9,-45.38,;28.24,-46.15,;28.24,-47.69,;29.57,-45.38,;29.57,-43.84,;30.9,-43.07,;26.9,-40.77,;25.57,-41.55,;24.23,-40.77,;24.23,-39.23,;22.9,-38.46,;25.57,-38.46,;25.57,-36.92,;24.24,-36.16,;22.91,-36.93,;24.24,-34.62,;25.58,-33.85,;26.91,-34.62,;26.91,-36.16,;28.24,-36.93,;26.9,-39.23,;28.23,-38.46,)| Show InChI InChI=1S/C30H28ClF3N2O/c1-30(2,3)35-13-11-18(12-14-35)19-15-23(21-8-7-20(32)17-24(21)31)22-9-10-28(37)36(27(22)16-19)29-25(33)5-4-6-26(29)34/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175758

(7-(1-tert-butylpiperidin-4-yl)-5-(2-chloro-4-fluor...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(36.13,-13.37,;34.8,-14.15,;35.58,-15.48,;34.03,-12.81,;33.47,-14.92,;33.47,-16.46,;32.14,-17.22,;30.8,-16.46,;30.79,-14.92,;32.13,-14.15,;29.47,-17.23,;29.47,-18.78,;28.13,-19.55,;28.14,-21.09,;26.8,-21.86,;26.8,-23.4,;28.13,-24.17,;28.14,-25.71,;29.47,-23.39,;29.47,-21.85,;30.8,-21.08,;26.8,-18.78,;25.47,-19.56,;24.13,-18.79,;24.13,-17.24,;22.8,-16.47,;25.47,-16.47,;25.47,-14.94,;24.14,-14.17,;22.81,-14.95,;24.14,-12.64,;25.48,-11.86,;26.81,-12.63,;26.81,-14.17,;28.14,-14.94,;26.8,-17.24,;28.13,-16.47,)| Show InChI InChI=1S/C30H28Cl3FN2O/c1-30(2,3)35-13-11-18(12-14-35)19-15-23(21-8-7-20(34)17-26(21)33)22-9-10-28(37)36(27(22)16-19)29-24(31)5-4-6-25(29)32/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50421140
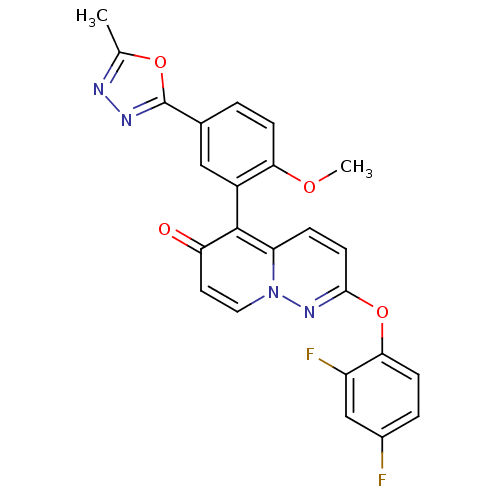
(CHEMBL2088591)Show SMILES COc1ccc(cc1-c1c2ccc(Oc3ccc(F)cc3F)nn2ccc1=O)-c1nnc(C)o1 |(33.77,-46.54,;35.1,-47.32,;36.43,-46.55,;36.44,-45,;37.77,-44.23,;39.1,-45,;39.11,-46.55,;37.77,-47.32,;37.77,-48.85,;39.1,-49.62,;40.42,-48.85,;41.75,-49.61,;41.75,-51.14,;43.09,-51.91,;43.1,-53.45,;41.76,-54.22,;41.77,-55.76,;43.1,-56.53,;43.11,-58.07,;44.44,-55.74,;44.43,-54.21,;45.76,-53.43,;40.43,-51.91,;39.1,-51.15,;37.77,-51.93,;36.43,-51.16,;36.43,-49.62,;35.1,-48.85,;40.36,-44.1,;41.82,-44.58,;42.73,-43.34,;41.83,-42.09,;42.31,-40.63,;40.36,-42.56,)| Show InChI InChI=1S/C24H16F2N4O4/c1-13-27-28-24(33-13)14-3-6-20(32-2)16(11-14)23-18-5-8-22(29-30(18)10-9-19(23)31)34-21-7-4-15(25)12-17(21)26/h3-12H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38apha assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175761
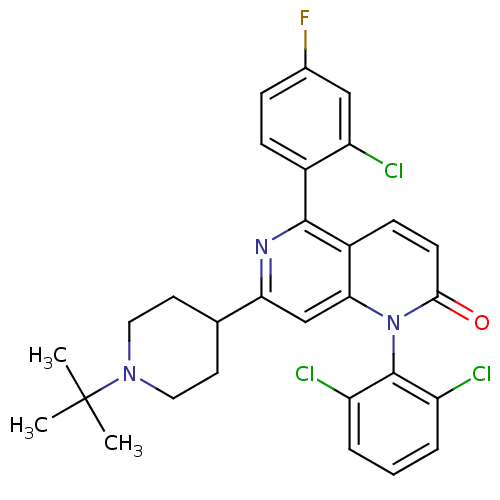
(7-(1-tert-butylpiperidin-4-yl)-5-(2-chloro-4-fluor...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc2n(-c3c(Cl)cccc3Cl)c(=O)ccc2c(n1)-c1ccc(F)cc1Cl |(3.3,5.4,;1.97,4.62,;2.74,3.29,;1.2,5.95,;.64,3.85,;.63,2.31,;-.69,1.54,;-2.04,2.31,;-2.04,3.85,;-.7,4.62,;-3.37,1.53,;-4.7,2.3,;-6.03,1.52,;-7.36,2.29,;-7.36,3.83,;-8.69,4.59,;-10.02,3.82,;-8.7,6.13,;-7.36,6.9,;-6.02,6.13,;-6.02,4.59,;-4.69,3.82,;-8.7,1.52,;-10.04,2.29,;-8.7,-.02,;-7.36,-.8,;-6.03,-.02,;-4.7,-.79,;-3.36,-.02,;-4.7,-2.33,;-6.03,-3.1,;-6.03,-4.63,;-4.7,-5.41,;-4.7,-6.95,;-3.36,-4.63,;-3.37,-3.09,;-2.03,-2.32,)| Show InChI InChI=1S/C29H27Cl3FN3O/c1-29(2,3)35-13-11-17(12-14-35)24-16-25-20(27(34-24)19-8-7-18(33)15-23(19)32)9-10-26(37)36(25)28-21(30)5-4-6-22(28)31/h4-10,15-17H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50222629

(CHEMBL142187)Show SMILES CN1C2CCC1CC(C2)Nc1cc2N(C(=O)NCc2c(c1)-c1ccccc1Cl)c1c(Cl)cccc1Cl |THB:9:7:1:3.4| Show InChI InChI=1S/C28H27Cl3N4O/c1-34-18-9-10-19(34)12-16(11-18)33-17-13-21(20-5-2-3-6-23(20)29)22-15-32-28(36)35(26(22)14-17)27-24(30)7-4-8-25(27)31/h2-8,13-14,16,18-19,33H,9-12,15H2,1H3,(H,32,36) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of p38 MAP kinase |
Bioorg Med Chem Lett 13: 467-70 (2003)
BindingDB Entry DOI: 10.7270/Q22F7QM4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50222628

(CHEMBL142697)Show SMILES CN1C2CCC1CC(C2)Nc1cc2n(-c3c(Cl)cccc3Cl)c(=O)ccc2c(n1)-c1ccccc1Cl |THB:9:7:1:3.4,(5,-1.84,;4,-.68,;2.5,-.29,;2.5,1.25,;3.83,2.03,;3.79,.89,;2.31,.48,;.98,1.25,;.96,-.29,;-.35,2.02,;-1.7,1.26,;-3.03,2.03,;-4.36,1.28,;-5.69,2.03,;-5.69,3.57,;-4.34,4.36,;-3.01,3.57,;-4.34,5.9,;-5.69,6.67,;-7.02,5.88,;-7.02,4.36,;-8.35,3.59,;-7.02,1.28,;-8.35,2.05,;-7.02,-.26,;-5.69,-1.03,;-4.36,-.26,;-3.03,-1.05,;-1.7,-.28,;-3.03,-2.57,;-4.38,-3.34,;-4.39,-4.88,;-3.06,-5.65,;-1.72,-4.88,;-1.72,-3.34,;-.39,-2.57,)| Show InChI InChI=1S/C28H25Cl3N4O/c1-34-17-9-10-18(34)14-16(13-17)32-25-15-24-20(27(33-25)19-5-2-3-6-21(19)29)11-12-26(36)35(24)28-22(30)7-4-8-23(28)31/h2-8,11-12,15-18H,9-10,13-14H2,1H3,(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of p38 MAP kinase |
Bioorg Med Chem Lett 13: 467-70 (2003)
BindingDB Entry DOI: 10.7270/Q22F7QM4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175760

(7-(1-tert-butylpiperidin-4-yl)-5-(2-chloro-4-fluor...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(F)cccc3F)c2c1 |(36.23,-35.35,;34.9,-36.13,;35.68,-37.46,;34.13,-34.8,;33.57,-36.9,;33.57,-38.44,;32.24,-39.21,;30.9,-38.44,;30.9,-36.9,;32.23,-36.13,;29.57,-39.22,;29.57,-40.77,;28.24,-41.54,;28.24,-43.08,;26.9,-43.84,;26.9,-45.38,;28.24,-46.15,;28.24,-47.69,;29.57,-45.38,;29.57,-43.84,;30.9,-43.07,;26.9,-40.77,;25.57,-41.55,;24.23,-40.77,;24.23,-39.23,;22.9,-38.46,;25.57,-38.46,;25.57,-36.92,;24.24,-36.16,;22.91,-36.93,;24.24,-34.62,;25.58,-33.85,;26.91,-34.62,;26.91,-36.16,;28.24,-36.93,;26.9,-39.23,;28.23,-38.46,)| Show InChI InChI=1S/C30H28ClF3N2O/c1-30(2,3)35-13-11-18(12-14-35)19-15-23(21-8-7-20(32)17-24(21)31)22-9-10-28(37)36(27(22)16-19)29-25(33)5-4-6-26(29)34/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of TNF alpha release in THP1 cells |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175742

(7-(8-aza-bicyclo[3.2.1]octan-3-yl)-5-(2-chloro-4-f...)Show SMILES Fc1ccc(c(Cl)c1)-c1cc(cc2n(-c3c(Cl)cccc3Cl)c(=O)ccc12)C1CC2CCC(C1)N2 |TLB:10:27:34:30.31,(12.26,-6.73,;12.26,-5.19,;10.92,-4.42,;10.93,-2.88,;12.26,-2.12,;13.59,-2.88,;14.92,-2.11,;13.6,-4.42,;12.26,-.58,;13.59,.19,;13.59,1.74,;12.25,2.5,;10.92,1.73,;9.59,2.5,;9.6,4.03,;8.27,4.8,;6.94,4.03,;8.26,6.33,;9.6,7.11,;10.93,6.34,;10.93,4.8,;12.26,4.03,;8.26,1.73,;6.92,2.5,;8.26,.19,;9.6,-.59,;10.92,.19,;14.92,2.51,;15.86,3.37,;16.12,5.19,;14.93,6.36,;15.46,5.1,;16.7,4.31,;16.49,2.56,;17.68,5.23,)| Show InChI InChI=1S/C28H22Cl3FN2O/c29-23-2-1-3-24(30)28(23)34-26-13-16(15-10-18-5-6-19(11-15)33-18)12-22(21(26)8-9-27(34)35)20-7-4-17(32)14-25(20)31/h1-4,7-9,12-15,18-19,33H,5-6,10-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM17081
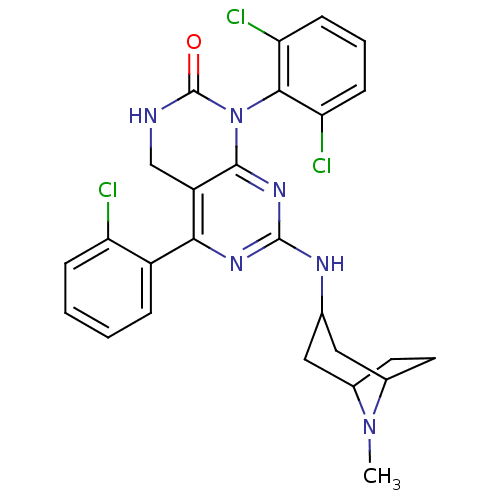
(5-(2-chlorophenyl)-1-(2,6-dichlorophenyl)-7-({8-me...)Show SMILES CN1C2CCC1CC(C2)Nc1nc2N(C(=O)NCc2c(n1)-c1ccccc1Cl)c1c(Cl)cccc1Cl Show InChI InChI=1S/C26H25Cl3N6O/c1-34-15-9-10-16(34)12-14(11-15)31-25-32-22(17-5-2-3-6-19(17)27)18-13-30-26(36)35(24(18)33-25)23-20(28)7-4-8-21(23)29/h2-8,14-16H,9-13H2,1H3,(H,30,36)(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with p38 enzyme, and substrates in the presence ATP/[gamma-33P] ATP. After the rea... |
Bioorg Med Chem Lett 16: 4400-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.045
BindingDB Entry DOI: 10.7270/Q2NS0S56 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50122391

(5-(2-Chloro-phenyl)-1-(2,6-dichloro-phenyl)-7-(pip...)Show SMILES Clc1ccccc1-c1cc(NC2CCNCC2)cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl Show InChI InChI=1S/C25H23Cl3N4O/c26-20-5-2-1-4-17(20)18-12-16(31-15-8-10-29-11-9-15)13-23-19(18)14-30-25(33)32(23)24-21(27)6-3-7-22(24)28/h1-7,12-13,15,29,31H,8-11,14H2,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of p38 MAP kinase |
Bioorg Med Chem Lett 13: 467-70 (2003)
BindingDB Entry DOI: 10.7270/Q22F7QM4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM17074
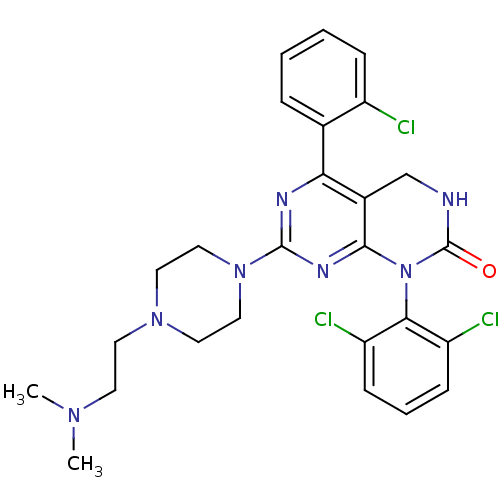
(5-(2-chlorophenyl)-1-(2,6-dichlorophenyl)-7-{4-[2-...)Show SMILES CN(C)CCN1CCN(CC1)c1nc2N(C(=O)NCc2c(n1)-c1ccccc1Cl)c1c(Cl)cccc1Cl Show InChI InChI=1S/C26H28Cl3N7O/c1-33(2)10-11-34-12-14-35(15-13-34)25-31-22(17-6-3-4-7-19(17)27)18-16-30-26(37)36(24(18)32-25)23-20(28)8-5-9-21(23)29/h3-9H,10-16H2,1-2H3,(H,30,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with p38 enzyme, and substrates in the presence ATP/[gamma-33P] ATP. After the rea... |
Bioorg Med Chem Lett 16: 4400-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.045
BindingDB Entry DOI: 10.7270/Q2NS0S56 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175751

(7-(1-tert-butylpiperidin-4-yl)-1-(2,6-dichlorophen...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc2n(-c3c(Cl)cccc3Cl)c(=O)ccc2c(n1)-c1ccc(F)cc1F |(18.46,6,;17.14,5.23,;17.91,3.89,;16.36,6.56,;15.8,4.46,;15.8,2.92,;14.47,2.15,;13.13,2.91,;13.13,4.45,;14.47,5.22,;11.8,2.14,;10.47,2.9,;9.13,2.13,;7.8,2.9,;7.8,4.43,;6.48,5.2,;5.14,4.43,;6.47,6.73,;7.81,7.52,;9.14,6.74,;9.14,5.2,;10.47,4.43,;6.46,2.13,;5.13,2.9,;6.47,.59,;7.8,-.19,;9.13,.59,;10.47,-.18,;11.81,.59,;10.47,-1.72,;9.13,-2.49,;9.13,-4.03,;10.47,-4.8,;10.47,-6.34,;11.81,-4.02,;11.8,-2.48,;13.13,-1.71,)| Show InChI InChI=1S/C29H27Cl2F2N3O/c1-29(2,3)35-13-11-17(12-14-35)24-16-25-20(27(34-24)19-8-7-18(32)15-23(19)33)9-10-26(37)36(25)28-21(30)5-4-6-22(28)31/h4-10,15-17H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50476040

(CHEMBL219796)Show SMILES CNc1cc2n(-c3c(F)cccc3F)c(=O)ccc2c(n1)-c1ccc(F)cc1F |(30.74,-17.07,;29.41,-16.3,;28.07,-17.06,;26.74,-16.29,;25.42,-17.05,;24.08,-16.29,;24.08,-14.75,;25.42,-13.98,;26.75,-14.74,;25.41,-12.43,;24.07,-11.66,;22.75,-12.43,;22.74,-13.98,;21.41,-14.75,;22.75,-17.05,;21.41,-16.28,;22.74,-18.59,;24.07,-19.37,;25.4,-18.59,;26.73,-19.37,;28.06,-18.61,;26.72,-20.91,;25.38,-21.66,;25.36,-23.2,;26.69,-23.98,;26.69,-25.52,;28.04,-23.21,;28.04,-21.68,;29.38,-20.91,)| Show InChI InChI=1S/C21H13F4N3O/c1-26-18-10-17-13(20(27-18)12-6-5-11(22)9-16(12)25)7-8-19(29)28(17)21-14(23)3-2-4-15(21)24/h2-10H,1H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of p38 |
Bioorg Med Chem Lett 16: 5468-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.084
BindingDB Entry DOI: 10.7270/Q2HT2S35 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175750

(5-(2-chloro-4-fluorophenyl)-1-(2,6-dichlorophenyl)...)Show SMILES CC(C)N1C2CCC1CC(C2)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |THB:11:9:3:5.6,(3.56,-16.01,;2.34,-15.07,;2.54,-13.55,;.92,-15.66,;-.64,-15.7,;-1.83,-14.54,;-1.3,-15.8,;-.06,-16.59,;-.27,-18.33,;-1.84,-18.38,;-.9,-17.52,;-3.17,-19.15,;-3.17,-20.7,;-4.5,-21.48,;-4.5,-23.01,;-5.84,-23.78,;-5.84,-25.32,;-4.5,-26.09,;-4.5,-27.63,;-3.17,-25.31,;-3.17,-23.78,;-1.84,-23,;-5.84,-20.71,;-7.17,-21.48,;-8.51,-20.71,;-8.51,-19.16,;-9.84,-18.39,;-7.17,-18.39,;-7.17,-16.86,;-8.5,-16.1,;-9.83,-16.87,;-8.5,-14.56,;-7.16,-13.78,;-5.83,-14.55,;-5.83,-16.09,;-4.5,-16.87,;-5.84,-19.16,;-4.51,-18.39,)| Show InChI InChI=1S/C31H28Cl3FN2O/c1-17(2)36-21-7-8-22(36)13-18(12-21)19-14-25(23-9-6-20(35)16-28(23)34)24-10-11-30(38)37(29(24)15-19)31-26(32)4-3-5-27(31)33/h3-6,9-11,14-18,21-22H,7-8,12-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50421137
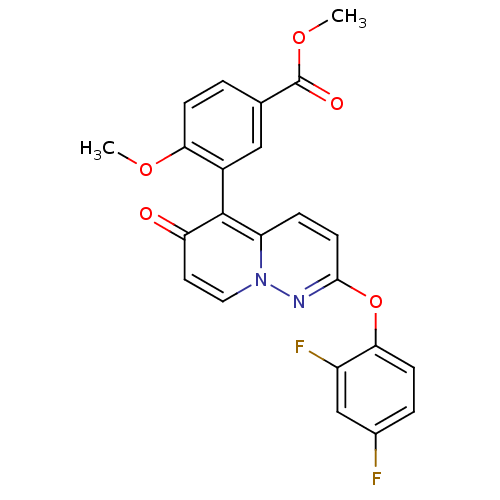
(CHEMBL2088588)Show SMILES COC(=O)c1ccc(OC)c(c1)-c1c2ccc(Oc3ccc(F)cc3F)nn2ccc1=O |(7.25,-42.41,;5.92,-43.19,;4.58,-42.42,;4.58,-40.88,;3.25,-43.2,;1.92,-42.43,;.59,-43.21,;.59,-44.75,;-.75,-45.52,;-2.08,-44.75,;1.92,-45.52,;3.26,-44.75,;1.92,-47.06,;3.25,-47.82,;4.57,-47.05,;5.9,-47.81,;5.91,-49.35,;7.24,-50.11,;7.25,-51.65,;5.91,-52.42,;5.92,-53.96,;7.26,-54.73,;7.26,-56.27,;8.59,-53.95,;8.58,-52.41,;9.91,-51.63,;4.58,-50.12,;3.25,-49.35,;1.92,-50.14,;.59,-49.36,;.59,-47.83,;-.75,-47.05,)| Show InChI InChI=1S/C23H16F2N2O5/c1-30-19-6-3-13(23(29)31-2)11-15(19)22-17-5-8-21(26-27(17)10-9-18(22)28)32-20-7-4-14(24)12-16(20)25/h3-12H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38apha assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50421141
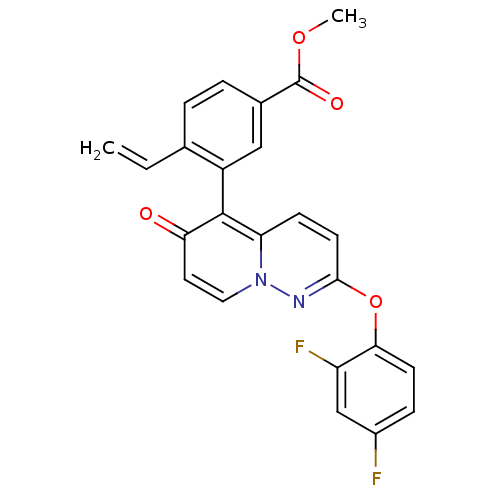
(CHEMBL2088578)Show SMILES COC(=O)c1ccc(C=C)c(c1)-c1c2ccc(Oc3ccc(F)cc3F)nn2ccc1=O |(46,-11.48,;44.67,-12.26,;43.33,-11.49,;43.33,-9.95,;42,-12.27,;40.67,-11.5,;39.34,-12.27,;39.34,-13.82,;38,-14.59,;36.67,-13.82,;40.67,-14.59,;42.01,-13.82,;40.67,-16.12,;42,-16.89,;43.32,-16.12,;44.65,-16.88,;44.65,-18.42,;45.99,-19.18,;46,-20.72,;44.66,-21.49,;44.67,-23.03,;46.01,-23.8,;46.01,-25.34,;47.34,-23.01,;47.33,-21.48,;48.66,-20.7,;43.33,-19.18,;42,-18.42,;40.67,-19.2,;39.33,-18.43,;39.34,-16.89,;38,-16.12,)| Show InChI InChI=1S/C24H16F2N2O4/c1-3-14-4-5-15(24(30)31-2)12-17(14)23-19-7-9-22(27-28(19)11-10-20(23)29)32-21-8-6-16(25)13-18(21)26/h3-13H,1H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38apha assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50122389
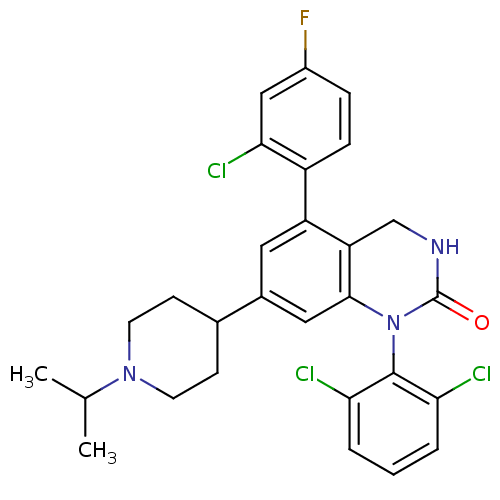
(5-(2-Chloro-4-fluoro-phenyl)-1-(2,6-dichloro-pheny...)Show SMILES CC(C)N1CCC(CC1)c1cc2N(C(=O)NCc2c(c1)-c1ccc(F)cc1Cl)c1c(Cl)cccc1Cl Show InChI InChI=1S/C28H27Cl3FN3O/c1-16(2)34-10-8-17(9-11-34)18-12-21(20-7-6-19(32)14-25(20)31)22-15-33-28(36)35(26(22)13-18)27-23(29)4-3-5-24(27)30/h3-7,12-14,16-17H,8-11,15H2,1-2H3,(H,33,36) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of p38 MAP kinase |
Bioorg Med Chem Lett 13: 467-70 (2003)
BindingDB Entry DOI: 10.7270/Q22F7QM4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM17082
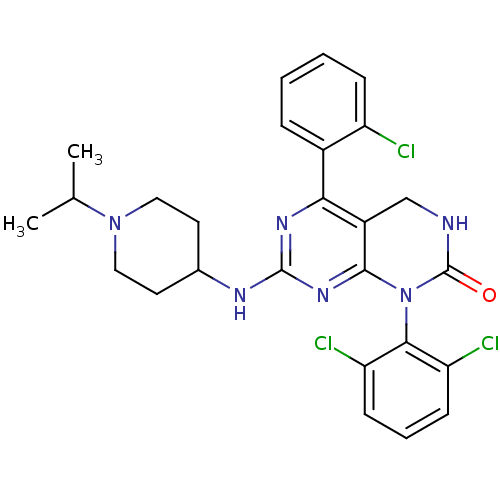
(5-(2-chlorophenyl)-1-(2,6-dichlorophenyl)-7-{[1-(p...)Show SMILES CC(C)N1CCC(CC1)Nc1nc2N(C(=O)NCc2c(n1)-c1ccccc1Cl)c1c(Cl)cccc1Cl Show InChI InChI=1S/C26H27Cl3N6O/c1-15(2)34-12-10-16(11-13-34)31-25-32-22(17-6-3-4-7-19(17)27)18-14-30-26(36)35(24(18)33-25)23-20(28)8-5-9-21(23)29/h3-9,15-16H,10-14H2,1-2H3,(H,30,36)(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with p38 enzyme, and substrates in the presence ATP/[gamma-33P] ATP. After the rea... |
Bioorg Med Chem Lett 16: 4400-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.045
BindingDB Entry DOI: 10.7270/Q2NS0S56 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50194461

(7-amino-5-(2,4-difluorophenyl)-1-(2,6-difluorophen...)Show SMILES Nc1cc2n(-c3c(F)cccc3F)c(=O)ccc2c(n1)-c1ccc(F)cc1F |(15,-16.28,;13.66,-17.04,;12.34,-16.27,;11.01,-17.03,;9.67,-16.27,;9.67,-14.73,;11.01,-13.96,;12.35,-14.72,;11.01,-12.4,;9.67,-11.64,;8.34,-12.41,;8.34,-13.96,;7,-14.73,;8.34,-17.03,;7.01,-16.26,;8.34,-18.57,;9.67,-19.35,;11,-18.57,;12.32,-19.35,;13.66,-18.59,;12.31,-20.89,;10.97,-21.64,;10.96,-23.18,;12.29,-23.96,;12.28,-25.51,;13.63,-23.19,;13.64,-21.66,;14.97,-20.89,)| Show InChI InChI=1S/C20H11F4N3O/c21-10-4-5-11(15(24)8-10)19-12-6-7-18(28)27(16(12)9-17(25)26-19)20-13(22)2-1-3-14(20)23/h1-9H,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38alpha after 3 hrs by SPA method |
Bioorg Med Chem Lett 20: 2765-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.069
BindingDB Entry DOI: 10.7270/Q23T9HCC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175748
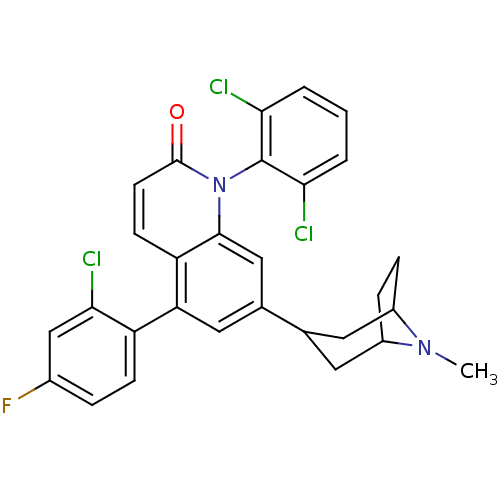
(5-(2-chloro-4-fluorophenyl)-1-(2,6-dichlorophenyl)...)Show SMILES CN1C2CCC1CC(C2)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |THB:9:7:1:3.4,(34.88,5.72,;33.46,5.13,;31.9,5.09,;30.71,6.25,;31.25,4.99,;32.49,4.2,;32.27,2.46,;30.7,2.41,;31.64,3.27,;29.37,1.64,;29.38,.09,;28.04,-.68,;28.05,-2.22,;26.71,-2.98,;26.71,-4.52,;28.04,-5.29,;28.04,-6.83,;29.38,-4.52,;29.37,-2.98,;30.7,-2.21,;26.71,.09,;25.38,-.69,;24.04,.09,;24.04,1.63,;22.71,2.4,;25.38,2.4,;25.38,3.93,;24.05,4.7,;22.72,3.93,;24.05,6.23,;25.39,7.01,;26.72,6.24,;26.72,4.7,;28.05,3.93,;26.71,1.63,;28.04,2.4,)| Show InChI InChI=1S/C29H24Cl3FN2O/c1-34-19-6-7-20(34)12-16(11-19)17-13-23(21-8-5-18(33)15-26(21)32)22-9-10-28(36)35(27(22)14-17)29-24(30)3-2-4-25(29)31/h2-5,8-10,13-16,19-20H,6-7,11-12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175751

(7-(1-tert-butylpiperidin-4-yl)-1-(2,6-dichlorophen...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc2n(-c3c(Cl)cccc3Cl)c(=O)ccc2c(n1)-c1ccc(F)cc1F |(18.46,6,;17.14,5.23,;17.91,3.89,;16.36,6.56,;15.8,4.46,;15.8,2.92,;14.47,2.15,;13.13,2.91,;13.13,4.45,;14.47,5.22,;11.8,2.14,;10.47,2.9,;9.13,2.13,;7.8,2.9,;7.8,4.43,;6.48,5.2,;5.14,4.43,;6.47,6.73,;7.81,7.52,;9.14,6.74,;9.14,5.2,;10.47,4.43,;6.46,2.13,;5.13,2.9,;6.47,.59,;7.8,-.19,;9.13,.59,;10.47,-.18,;11.81,.59,;10.47,-1.72,;9.13,-2.49,;9.13,-4.03,;10.47,-4.8,;10.47,-6.34,;11.81,-4.02,;11.8,-2.48,;13.13,-1.71,)| Show InChI InChI=1S/C29H27Cl2F2N3O/c1-29(2,3)35-13-11-17(12-14-35)24-16-25-20(27(34-24)19-8-7-18(32)15-23(19)33)9-10-26(37)36(25)28-21(30)5-4-6-22(28)31/h4-10,15-17H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of TNF alpha release in THP1 cells |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50194461

(7-amino-5-(2,4-difluorophenyl)-1-(2,6-difluorophen...)Show SMILES Nc1cc2n(-c3c(F)cccc3F)c(=O)ccc2c(n1)-c1ccc(F)cc1F |(15,-16.28,;13.66,-17.04,;12.34,-16.27,;11.01,-17.03,;9.67,-16.27,;9.67,-14.73,;11.01,-13.96,;12.35,-14.72,;11.01,-12.4,;9.67,-11.64,;8.34,-12.41,;8.34,-13.96,;7,-14.73,;8.34,-17.03,;7.01,-16.26,;8.34,-18.57,;9.67,-19.35,;11,-18.57,;12.32,-19.35,;13.66,-18.59,;12.31,-20.89,;10.97,-21.64,;10.96,-23.18,;12.29,-23.96,;12.28,-25.51,;13.63,-23.19,;13.64,-21.66,;14.97,-20.89,)| Show InChI InChI=1S/C20H11F4N3O/c21-10-4-5-11(15(24)8-10)19-12-6-7-18(28)27(16(12)9-17(25)26-19)20-13(22)2-1-3-14(20)23/h1-9H,(H2,25,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of p38 |
Bioorg Med Chem Lett 16: 5468-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.084
BindingDB Entry DOI: 10.7270/Q2HT2S35 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM17083
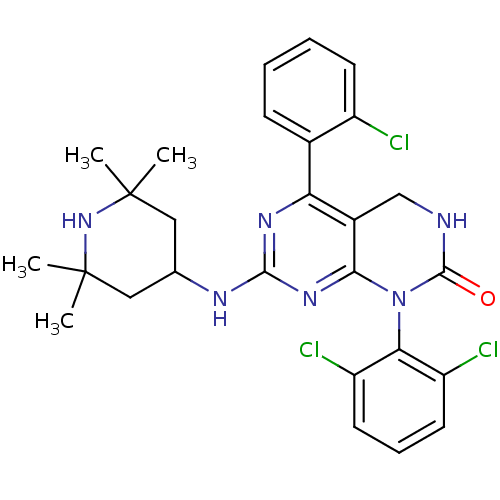
(5-(2-chlorophenyl)-1-(2,6-dichlorophenyl)-7-[(2,2,...)Show SMILES CC1(C)CC(CC(C)(C)N1)Nc1nc2N(C(=O)NCc2c(n1)-c1ccccc1Cl)c1c(Cl)cccc1Cl Show InChI InChI=1S/C27H29Cl3N6O/c1-26(2)12-15(13-27(3,4)35-26)32-24-33-21(16-8-5-6-9-18(16)28)17-14-31-25(37)36(23(17)34-24)22-19(29)10-7-11-20(22)30/h5-11,15,35H,12-14H2,1-4H3,(H,31,37)(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with p38 enzyme, and substrates in the presence ATP/[gamma-33P] ATP. After the rea... |
Bioorg Med Chem Lett 16: 4400-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.045
BindingDB Entry DOI: 10.7270/Q2NS0S56 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175744

(7-(1-tert-butyl-1,2,3,6-tetrahydropyridin-4-yl)-5-...)Show SMILES CC(C)(C)N1CCC(=CC1)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |c:7,(18.29,-14.38,;16.96,-15.16,;17.74,-16.49,;16.19,-13.83,;15.63,-15.93,;15.63,-17.47,;14.3,-18.24,;12.96,-17.47,;12.96,-15.93,;14.29,-15.16,;11.63,-18.24,;11.63,-19.8,;10.3,-20.57,;10.3,-22.1,;8.96,-22.87,;8.96,-24.41,;10.3,-25.18,;10.3,-26.72,;11.63,-24.4,;11.63,-22.87,;12.96,-22.09,;8.96,-19.8,;7.63,-20.57,;6.29,-19.8,;6.29,-18.25,;4.96,-17.48,;7.63,-17.48,;7.63,-15.95,;6.3,-15.19,;4.97,-15.96,;6.3,-13.65,;7.64,-12.87,;8.97,-13.64,;8.97,-15.18,;10.3,-15.96,;8.96,-18.25,;10.29,-17.48,)| Show InChI InChI=1S/C30H26Cl3FN2O/c1-30(2,3)35-13-11-18(12-14-35)19-15-23(21-8-7-20(34)17-26(21)33)22-9-10-28(37)36(27(22)16-19)29-24(31)5-4-6-25(29)32/h4-11,15-17H,12-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of TNF alpha release in THP1 cells |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175749
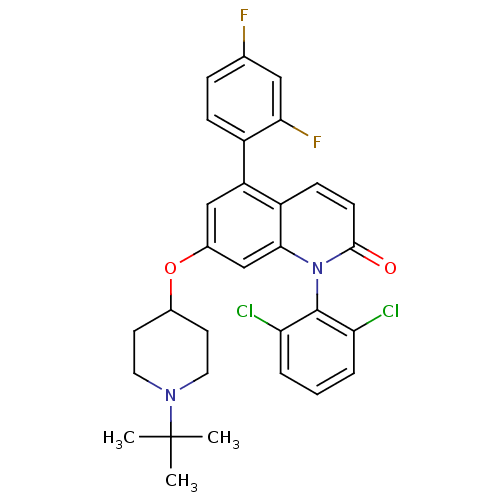
(7-(1-tert-butylpiperidin-4-yloxy)-1-(2,6-dichlorop...)Show SMILES CC(C)(C)N1CCC(CC1)Oc1cc(-c2ccc(F)cc2F)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(31.06,-19.78,;29.74,-19.02,;28.97,-20.35,;30.51,-17.69,;28.4,-18.25,;27.07,-19.02,;25.74,-18.25,;25.73,-16.71,;27.06,-15.93,;28.4,-16.71,;24.39,-15.94,;23.06,-16.72,;23.07,-18.27,;21.73,-19.04,;21.73,-20.57,;20.4,-21.34,;20.4,-22.88,;21.73,-23.65,;21.73,-25.19,;23.07,-22.87,;23.06,-21.34,;24.4,-20.57,;20.4,-18.27,;19.07,-19.05,;17.73,-18.27,;17.73,-16.73,;16.4,-15.95,;19.07,-15.96,;19.07,-14.42,;17.74,-13.66,;16.41,-14.43,;17.74,-12.12,;19.07,-11.35,;20.41,-12.12,;20.41,-13.66,;21.74,-14.43,;20.4,-16.72,;21.73,-15.95,)| Show InChI InChI=1S/C30H28Cl2F2N2O2/c1-30(2,3)35-13-11-19(12-14-35)38-20-16-23(21-8-7-18(33)15-26(21)34)22-9-10-28(37)36(27(22)17-20)29-24(31)5-4-6-25(29)32/h4-10,15-17,19H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of TNF alpha release in THP1 cells |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50222626

(CHEMBL142289)Show SMILES CC(C)N1CCC(CC1)c1cc2N(C(=O)CCc2c(c1)-c1ccc(F)cc1Cl)c1c(Cl)cccc1Cl Show InChI InChI=1S/C29H28Cl3FN2O/c1-17(2)34-12-10-18(11-13-34)19-14-23(21-7-6-20(33)16-26(21)32)22-8-9-28(36)35(27(22)15-19)29-24(30)4-3-5-25(29)31/h3-7,14-18H,8-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of p38 MAP kinase |
Bioorg Med Chem Lett 13: 467-70 (2003)
BindingDB Entry DOI: 10.7270/Q22F7QM4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50222619

(CHEMBL343682)Show SMILES CC1(C)CC(CC(C)(C)N1)Nc1cc2n(-c3c(Cl)cccc3Cl)c(=O)ccc2c(n1)-c1ccccc1Cl |(4.02,2.72,;4.04,1.18,;5.12,.09,;2.69,1.96,;1.38,1.19,;1.36,-.36,;2.69,-1.13,;1.35,-1.89,;4.02,-1.9,;4.02,-.36,;.03,1.96,;-1.3,1.19,;-2.63,1.96,;-3.96,1.21,;-5.29,1.96,;-5.29,3.5,;-3.94,4.27,;-2.61,3.5,;-3.94,5.83,;-5.29,6.6,;-6.62,5.81,;-6.62,4.27,;-7.95,3.52,;-6.62,1.21,;-7.97,1.98,;-6.63,-.33,;-5.3,-1.1,;-3.96,-.33,;-2.63,-1.12,;-1.3,-.35,;-2.64,-2.64,;-3.98,-3.41,;-3.99,-4.95,;-2.66,-5.72,;-1.32,-4.95,;-1.32,-3.41,;.01,-2.64,)| Show InChI InChI=1S/C29H29Cl3N4O/c1-28(2)15-17(16-29(3,4)35-28)33-24-14-23-19(26(34-24)18-8-5-6-9-20(18)30)12-13-25(37)36(23)27-21(31)10-7-11-22(27)32/h5-14,17,35H,15-16H2,1-4H3,(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of p38 MAP kinase |
Bioorg Med Chem Lett 13: 467-70 (2003)
BindingDB Entry DOI: 10.7270/Q22F7QM4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM17068
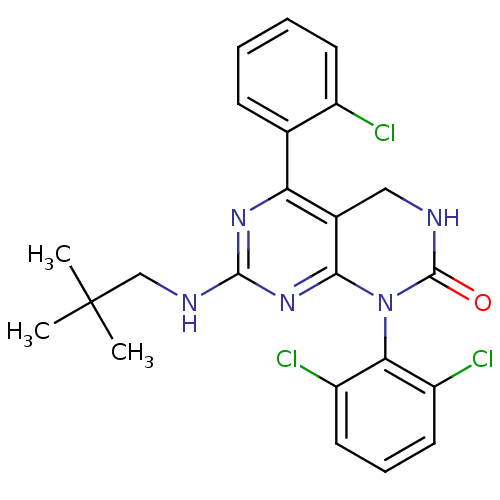
(5-(2-chlorophenyl)-1-(2,6-dichlorophenyl)-7-[(2,2-...)Show SMILES CC(C)(C)CNc1nc2N(C(=O)NCc2c(n1)-c1ccccc1Cl)c1c(Cl)cccc1Cl Show InChI InChI=1S/C23H22Cl3N5O/c1-23(2,3)12-28-21-29-18(13-7-4-5-8-15(13)24)14-11-27-22(32)31(20(14)30-21)19-16(25)9-6-10-17(19)26/h4-10H,11-12H2,1-3H3,(H,27,32)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with p38 enzyme, and substrates in the presence ATP/[gamma-33P] ATP. After the rea... |
Bioorg Med Chem Lett 16: 4400-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.045
BindingDB Entry DOI: 10.7270/Q2NS0S56 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175748

(5-(2-chloro-4-fluorophenyl)-1-(2,6-dichlorophenyl)...)Show SMILES CN1C2CCC1CC(C2)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |THB:9:7:1:3.4,(34.88,5.72,;33.46,5.13,;31.9,5.09,;30.71,6.25,;31.25,4.99,;32.49,4.2,;32.27,2.46,;30.7,2.41,;31.64,3.27,;29.37,1.64,;29.38,.09,;28.04,-.68,;28.05,-2.22,;26.71,-2.98,;26.71,-4.52,;28.04,-5.29,;28.04,-6.83,;29.38,-4.52,;29.37,-2.98,;30.7,-2.21,;26.71,.09,;25.38,-.69,;24.04,.09,;24.04,1.63,;22.71,2.4,;25.38,2.4,;25.38,3.93,;24.05,4.7,;22.72,3.93,;24.05,6.23,;25.39,7.01,;26.72,6.24,;26.72,4.7,;28.05,3.93,;26.71,1.63,;28.04,2.4,)| Show InChI InChI=1S/C29H24Cl3FN2O/c1-34-19-6-7-20(34)12-16(11-19)17-13-23(21-8-5-18(33)15-26(21)32)22-9-10-28(36)35(27(22)14-17)29-24(30)3-2-4-25(29)31/h2-5,8-10,13-16,19-20H,6-7,11-12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of TNF alpha release in THP1 cells |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175755
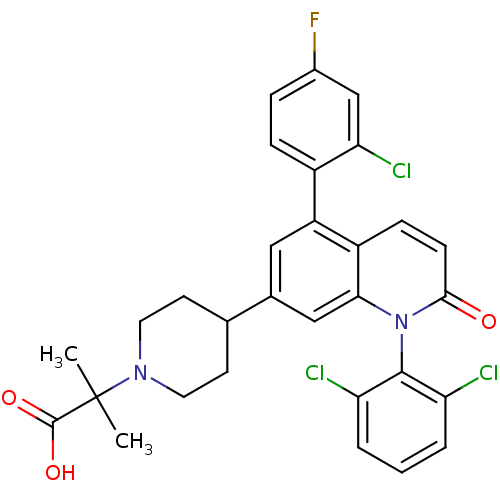
(2-(4-(5-(2-chloro-4-fluorophenyl)-1-(2,6-dichlorop...)Show SMILES CC(C)(N1CCC(CC1)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1)C(O)=O |(34.29,-15.36,;33.51,-14.03,;32.74,-12.7,;32.18,-14.8,;32.18,-16.34,;30.85,-17.11,;29.51,-16.34,;29.51,-14.8,;30.84,-14.03,;28.18,-17.12,;28.18,-18.67,;26.85,-19.44,;26.85,-20.98,;25.51,-21.74,;25.51,-23.28,;26.85,-24.05,;26.85,-25.59,;28.18,-23.28,;28.18,-21.74,;29.51,-20.97,;25.51,-18.67,;24.18,-19.45,;22.84,-18.67,;22.84,-17.13,;21.51,-16.35,;24.18,-16.36,;24.18,-14.82,;22.85,-14.06,;21.52,-14.83,;22.85,-12.52,;24.19,-11.75,;25.52,-12.52,;25.52,-14.06,;26.85,-14.83,;25.51,-17.12,;26.84,-16.35,;34.84,-13.25,;36.18,-14.01,;34.83,-11.71,)| Show InChI InChI=1S/C30H26Cl3FN2O3/c1-30(2,29(38)39)35-12-10-17(11-13-35)18-14-22(20-7-6-19(34)16-25(20)33)21-8-9-27(37)36(26(21)15-18)28-23(31)4-3-5-24(28)32/h3-9,14-17H,10-13H2,1-2H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175742

(7-(8-aza-bicyclo[3.2.1]octan-3-yl)-5-(2-chloro-4-f...)Show SMILES Fc1ccc(c(Cl)c1)-c1cc(cc2n(-c3c(Cl)cccc3Cl)c(=O)ccc12)C1CC2CCC(C1)N2 |TLB:10:27:34:30.31,(12.26,-6.73,;12.26,-5.19,;10.92,-4.42,;10.93,-2.88,;12.26,-2.12,;13.59,-2.88,;14.92,-2.11,;13.6,-4.42,;12.26,-.58,;13.59,.19,;13.59,1.74,;12.25,2.5,;10.92,1.73,;9.59,2.5,;9.6,4.03,;8.27,4.8,;6.94,4.03,;8.26,6.33,;9.6,7.11,;10.93,6.34,;10.93,4.8,;12.26,4.03,;8.26,1.73,;6.92,2.5,;8.26,.19,;9.6,-.59,;10.92,.19,;14.92,2.51,;15.86,3.37,;16.12,5.19,;14.93,6.36,;15.46,5.1,;16.7,4.31,;16.49,2.56,;17.68,5.23,)| Show InChI InChI=1S/C28H22Cl3FN2O/c29-23-2-1-3-24(30)28(23)34-26-13-16(15-10-18-5-6-19(11-15)33-18)12-22(21(26)8-9-27(34)35)20-7-4-17(32)14-25(20)31/h1-4,7-9,12-15,18-19,33H,5-6,10-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of TNF alpha release in THP1 cells |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175750

(5-(2-chloro-4-fluorophenyl)-1-(2,6-dichlorophenyl)...)Show SMILES CC(C)N1C2CCC1CC(C2)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |THB:11:9:3:5.6,(3.56,-16.01,;2.34,-15.07,;2.54,-13.55,;.92,-15.66,;-.64,-15.7,;-1.83,-14.54,;-1.3,-15.8,;-.06,-16.59,;-.27,-18.33,;-1.84,-18.38,;-.9,-17.52,;-3.17,-19.15,;-3.17,-20.7,;-4.5,-21.48,;-4.5,-23.01,;-5.84,-23.78,;-5.84,-25.32,;-4.5,-26.09,;-4.5,-27.63,;-3.17,-25.31,;-3.17,-23.78,;-1.84,-23,;-5.84,-20.71,;-7.17,-21.48,;-8.51,-20.71,;-8.51,-19.16,;-9.84,-18.39,;-7.17,-18.39,;-7.17,-16.86,;-8.5,-16.1,;-9.83,-16.87,;-8.5,-14.56,;-7.16,-13.78,;-5.83,-14.55,;-5.83,-16.09,;-4.5,-16.87,;-5.84,-19.16,;-4.51,-18.39,)| Show InChI InChI=1S/C31H28Cl3FN2O/c1-17(2)36-21-7-8-22(36)13-18(12-21)19-14-25(23-9-6-20(35)16-28(23)34)24-10-11-30(38)37(29(24)15-19)31-26(32)4-3-5-27(31)33/h3-6,9-11,14-18,21-22H,7-8,12-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of TNF alpha release in THP1 cells |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM26198
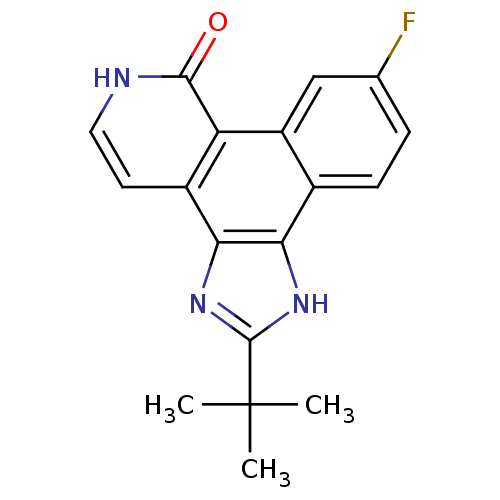
(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Tyrosine kinase 2 kinase |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase Jak 2 |
Bioorg Med Chem Lett 12: 1219-23 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D67 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175747

(7-(1-tert-butylpiperidin-4-yl)-5-(2-chlorophenyl)-...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccccc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(19.17,-37.34,;17.84,-38.12,;18.62,-39.45,;17.07,-36.79,;16.51,-38.89,;16.51,-40.43,;15.18,-41.2,;13.84,-40.43,;13.83,-38.89,;15.17,-38.12,;12.51,-41.21,;12.51,-42.76,;11.17,-43.53,;11.18,-45.07,;9.84,-45.84,;9.84,-47.37,;11.17,-48.15,;12.51,-47.37,;12.51,-45.83,;13.84,-45.06,;9.84,-42.76,;8.51,-43.54,;7.17,-42.76,;7.17,-41.22,;5.84,-40.45,;8.51,-40.45,;8.51,-38.92,;7.18,-38.15,;5.85,-38.92,;7.18,-36.61,;8.52,-35.84,;9.85,-36.61,;9.85,-38.15,;11.18,-38.92,;9.84,-41.22,;11.17,-40.45,)| Show InChI InChI=1S/C30H29Cl3N2O/c1-30(2,3)34-15-13-19(14-16-34)20-17-23(21-7-4-5-8-24(21)31)22-11-12-28(36)35(27(22)18-20)29-25(32)9-6-10-26(29)33/h4-12,17-19H,13-16H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of TNF alpha release in THP1 cells |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175760

(7-(1-tert-butylpiperidin-4-yl)-5-(2-chloro-4-fluor...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(F)cccc3F)c2c1 |(36.23,-35.35,;34.9,-36.13,;35.68,-37.46,;34.13,-34.8,;33.57,-36.9,;33.57,-38.44,;32.24,-39.21,;30.9,-38.44,;30.9,-36.9,;32.23,-36.13,;29.57,-39.22,;29.57,-40.77,;28.24,-41.54,;28.24,-43.08,;26.9,-43.84,;26.9,-45.38,;28.24,-46.15,;28.24,-47.69,;29.57,-45.38,;29.57,-43.84,;30.9,-43.07,;26.9,-40.77,;25.57,-41.55,;24.23,-40.77,;24.23,-39.23,;22.9,-38.46,;25.57,-38.46,;25.57,-36.92,;24.24,-36.16,;22.91,-36.93,;24.24,-34.62,;25.58,-33.85,;26.91,-34.62,;26.91,-36.16,;28.24,-36.93,;26.9,-39.23,;28.23,-38.46,)| Show InChI InChI=1S/C30H28ClF3N2O/c1-30(2,3)35-13-11-18(12-14-35)19-15-23(21-8-7-20(32)17-24(21)31)22-9-10-28(37)36(27(22)16-19)29-25(33)5-4-6-26(29)34/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of LPS stimulated TNF alpha release in whole blood |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM17075
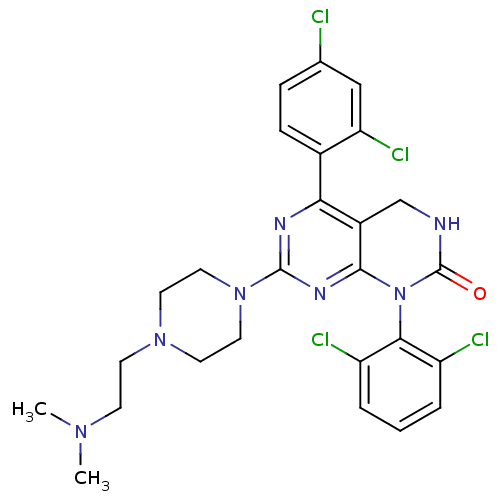
(5-(2,4-dichlorophenyl)-1-(2,6-dichlorophenyl)-7-{4...)Show SMILES CN(C)CCN1CCN(CC1)c1nc2N(C(=O)NCc2c(n1)-c1ccc(Cl)cc1Cl)c1c(Cl)cccc1Cl Show InChI InChI=1S/C26H27Cl4N7O/c1-34(2)8-9-35-10-12-36(13-11-35)25-32-22(17-7-6-16(27)14-21(17)30)18-15-31-26(38)37(24(18)33-25)23-19(28)4-3-5-20(23)29/h3-7,14H,8-13,15H2,1-2H3,(H,31,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with p38 enzyme, and substrates in the presence ATP/[gamma-33P] ATP. After the rea... |
Bioorg Med Chem Lett 16: 4400-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.045
BindingDB Entry DOI: 10.7270/Q2NS0S56 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data