

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM50142887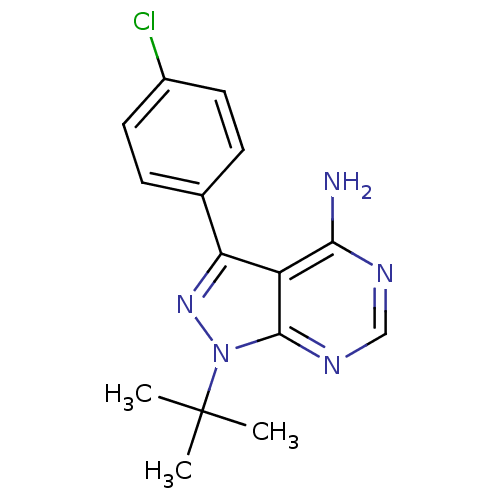 (1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM50433806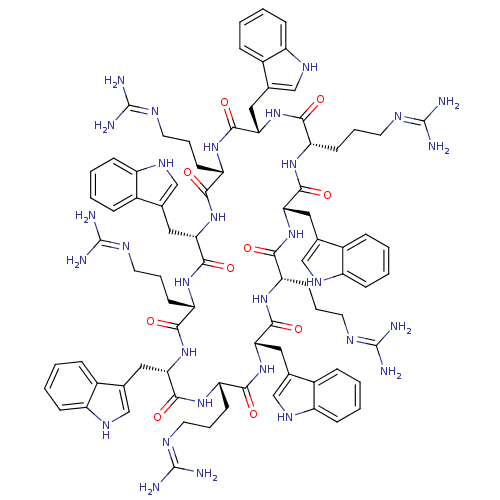 (CHEMBL2382016) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island Curated by ChEMBL | Assay Description Inhibition of GST-fused Csk (unknown origin) expressed in Escherichia coli using polyE4Y as substrate after 20 mins by scintillation counting analysi... | Bioorg Med Chem Lett 23: 3230-4 (2013) Article DOI: 10.1016/j.bmcl.2013.03.124 BindingDB Entry DOI: 10.7270/Q2JW8G8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50433806 (CHEMBL2382016) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island Curated by ChEMBL | Assay Description Inhibition of GST-fused Abl (unknown origin) expressed in Escherichia coli using CrkL as substrate after 20 mins by scintillation counting analysis i... | Bioorg Med Chem Lett 23: 3230-4 (2013) Article DOI: 10.1016/j.bmcl.2013.03.124 BindingDB Entry DOI: 10.7270/Q2JW8G8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM110206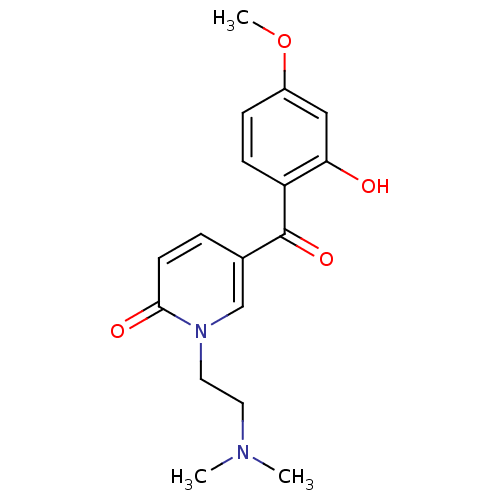 (1-[2-(Dimethylamino)ethyl]-5-(2-hydroxy-4-methoxyb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM110208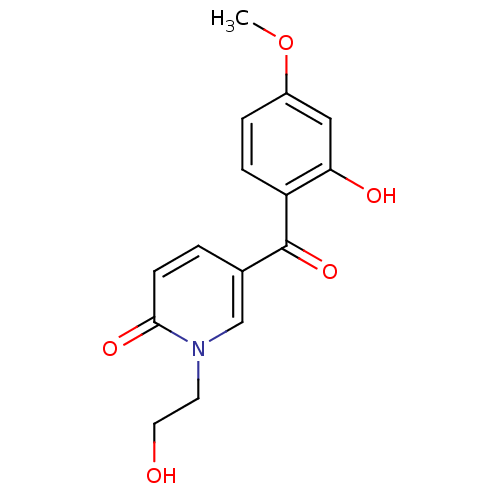 (5-(2-Hydroxy-4-methoxybenzoyl)-1-(2-hydroxyethyl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.99E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM110215 ((E)-1-Cyclohexyl-5-[(cyclohexylimino)(2-hydroxyphe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM110199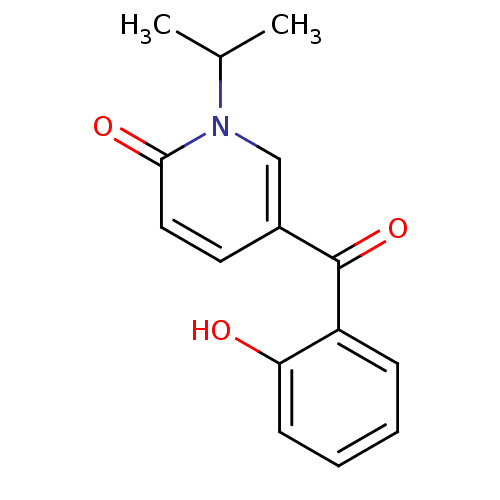 (5-(2-Hydroxybenzoyl)-1-isopropylpyridin-2(1H)-one ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.18E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM110213 (t-Butyl [2-{5-(2-hydroxy-5-methoxybenzoyl)-2-oxopy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM110209 (5-(2,5-Dihydroxybenzoyl)-1-hexylpyridin-2(1H)-one ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.21E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM110211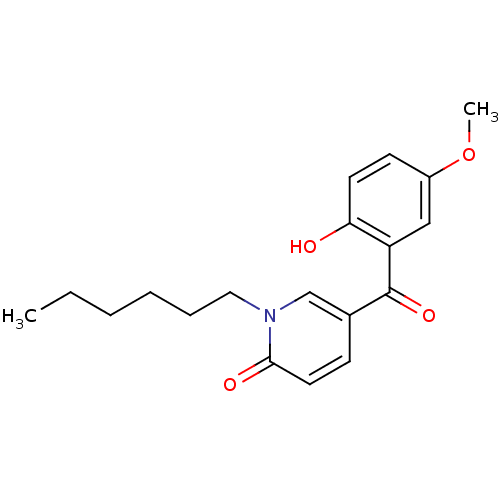 (1-Hexyl-5-(2-hydroxy-5-methoxybenzoyl)pyridin-2(1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.35E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM110212 (1-Cyclohexyl-5-(2-hydroxy-5-methoxybenzoyl)pyridin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.48E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM110200 (1-Cyclohexyl-5-(2-hydroxybenzoyl)pyridin-2(1H)-one...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.54E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM110210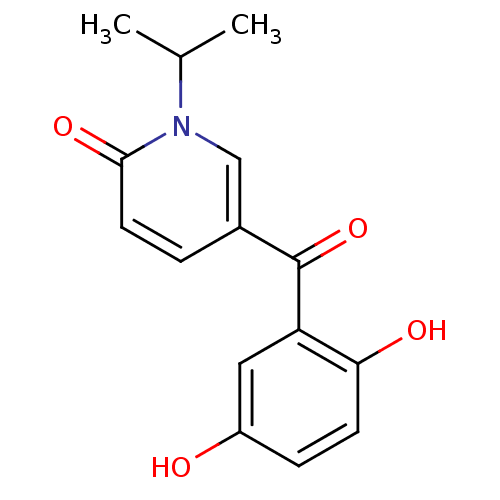 (5-(2,5-Dihydroxybenzoyl)-1-isopropylpyridin-2(1H)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.61E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM110207 (t-Butyl [2-{5-(2-hydroxy-4-methoxybenzoyl)-2-oxopy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.76E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM110202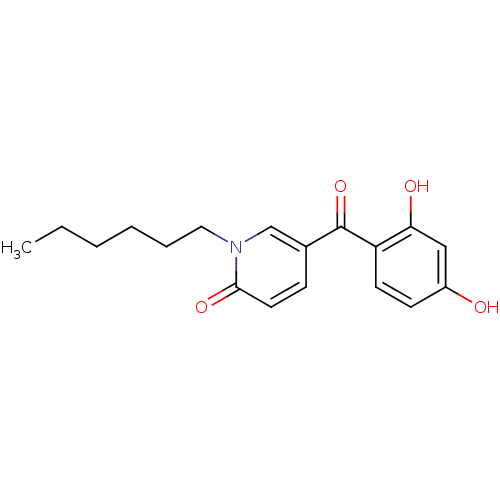 (5-(2,4-Dihydroxybenzoyl)-1-hexylpyridin-2(1H)-one ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.77E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM110198 (1-Hexyl-5-(2-hydroxybenzoyl)pyridin-2(1H)-one (28)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.81E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM110201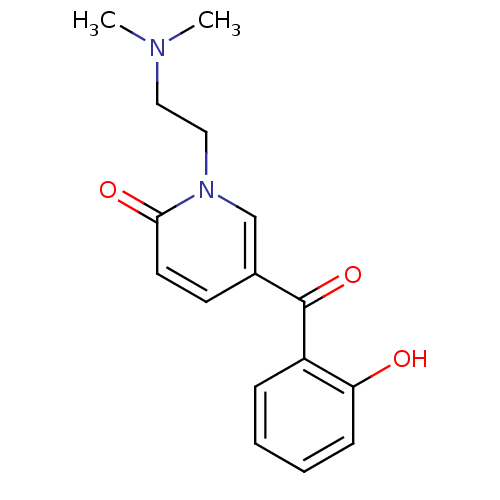 (1-[2-(Dimethylamino)ethyl]-5-(2-hydroxybenzoyl)pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.82E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM110204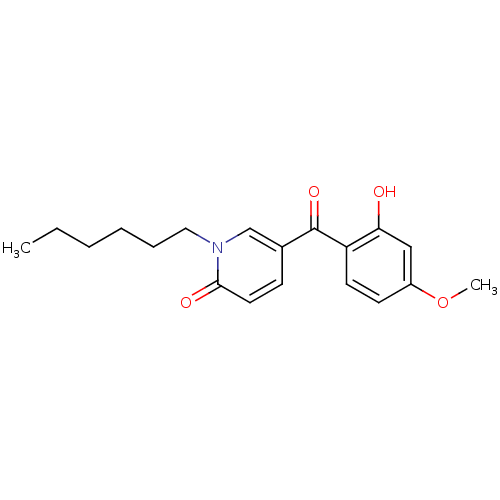 (1-Hexyl-5-(2-hydroxy-4-methoxybenzoyl)pyridin-2(1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.41E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM110214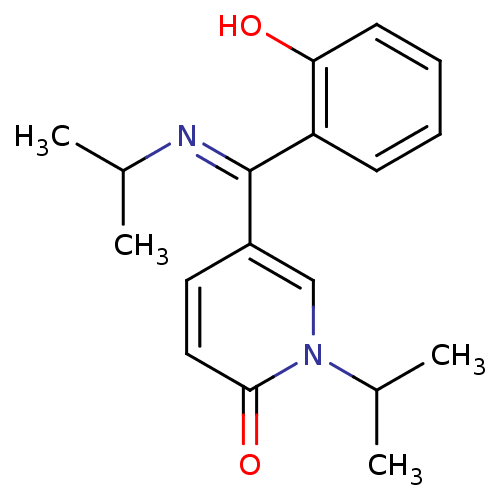 ((E)-5-[(2-Hydroxyphenyl)(isopropylimino)methyl]-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.79E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM110205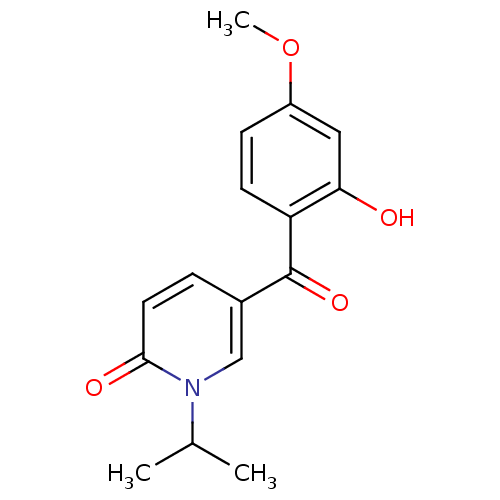 (5-(2-Hydroxy-4-methoxybenzoyl)-1-isopropylpyridin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM110203 (t-Butyl [2-{5-(2,4-dihydroxybenzoyl)-2-oxopyridin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.78E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Delhi, Delhi 110007, India | Assay Description The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM110206 (1-[2-(Dimethylamino)ethyl]-5-(2-hydroxy-4-methoxyb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Delhi, Delhi 110007, India | Assay Description EGFR was incubated with 8 mM MOPS (pH 7.0), 0.2 mM EDTA, 10 mM MnCl2, 0.1 mg/mL poly(Glu, Tyr) 4:1. MAPK1 was incubated with 25 mM Tris (pH 7.5), 0.0... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM110206 (1-[2-(Dimethylamino)ethyl]-5-(2-hydroxy-4-methoxyb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Delhi, Delhi 110007, India | Assay Description EGFR was incubated with 8 mM MOPS (pH 7.0), 0.2 mM EDTA, 10 mM MnCl2, 0.1 mg/mL poly(Glu, Tyr) 4:1. MAPK1 was incubated with 25 mM Tris (pH 7.5), 0.0... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-973] (Homo sapiens (Human)) | BDBM110206 (1-[2-(Dimethylamino)ethyl]-5-(2-hydroxy-4-methoxyb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Delhi, Delhi 110007, India | Assay Description EGFR was incubated with 8 mM MOPS (pH 7.0), 0.2 mM EDTA, 10 mM MnCl2, 0.1 mg/mL poly(Glu, Tyr) 4:1. MAPK1 was incubated with 25 mM Tris (pH 7.5), 0.0... | Bioorg Chem 53: 75-82 (2014) Article DOI: 10.1016/j.bioorg.2014.02.001 BindingDB Entry DOI: 10.7270/Q2HX1BBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||