 Found 96 hits with Last Name = 'todderud' and Initial = 'g'
Found 96 hits with Last Name = 'todderud' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Steroid 17-alpha-hydroxylase/17,20 lyase
(Macaca fascicularis) | BDBM50156282
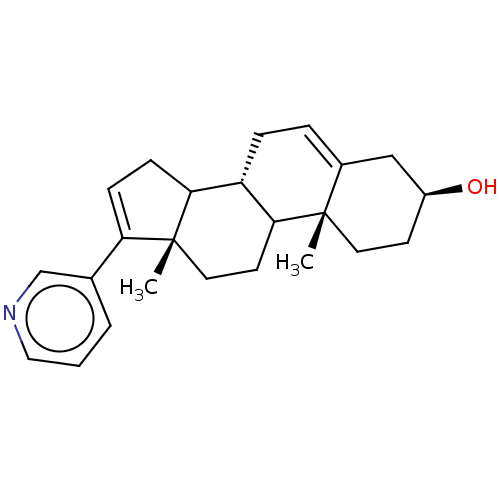
(CHEMBL3780847)Show SMILES [H][C@@]12CC=C3C[C@@H](O)CC[C@]3(C)C1CC[C@@]1(C)C2CC=C1c1cccnc1 |r,c:22,t:3| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21?,22?,23-,24+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP17A1 in cynomolgus monkey using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity assay in presence of... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50156232
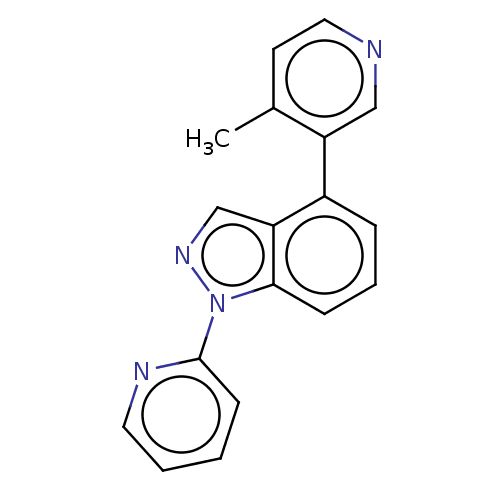
(CHEMBL3782020)Show InChI InChI=1S/C18H14N4/c1-13-8-10-19-11-15(13)14-5-4-6-17-16(14)12-21-22(17)18-7-2-3-9-20-18/h2-12H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Macaca fascicularis) | BDBM50391846

(CHEMBL2147041 | US9133160, 31)Show InChI InChI=1S/C15H12F3N3/c1-10-5-6-19-7-12(10)11-3-2-4-13-14(11)20-9-21(13)8-15(16,17)18/h2-7,9H,8H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP17A1 in cynomolgus monkey adrenal microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity a... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Macaca fascicularis) | BDBM50156282

(CHEMBL3780847)Show SMILES [H][C@@]12CC=C3C[C@@H](O)CC[C@]3(C)C1CC[C@@]1(C)C2CC=C1c1cccnc1 |r,c:22,t:3| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21?,22?,23-,24+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP17A1 in cynomolgus monkey adrenal microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity a... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50156233
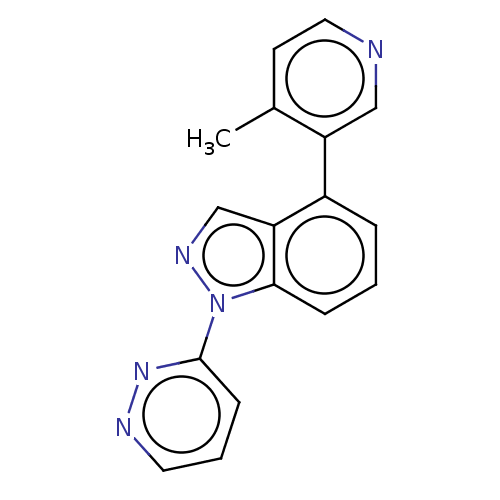
(CHEMBL3780658)Show InChI InChI=1S/C17H13N5/c1-12-7-9-18-10-14(12)13-4-2-5-16-15(13)11-20-22(16)17-6-3-8-19-21-17/h2-11H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50156281
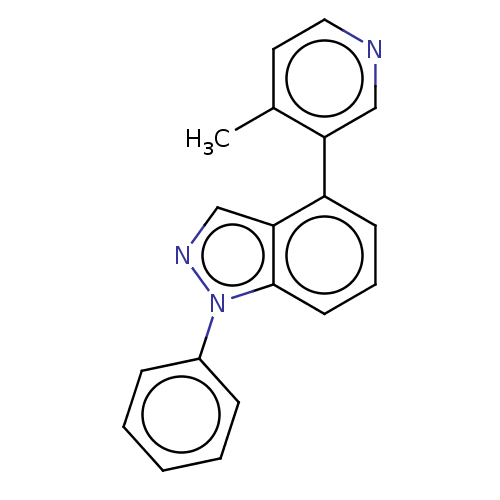
(CHEMBL3780743)Show InChI InChI=1S/C19H15N3/c1-14-10-11-20-12-17(14)16-8-5-9-19-18(16)13-21-22(19)15-6-3-2-4-7-15/h2-13H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Macaca fascicularis) | BDBM50391846

(CHEMBL2147041 | US9133160, 31)Show InChI InChI=1S/C15H12F3N3/c1-10-5-6-19-7-12(10)11-3-2-4-13-14(11)20-9-21(13)8-15(16,17)18/h2-7,9H,8H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP17A1 in cynomolgus monkey using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity assay in presence of... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50156235
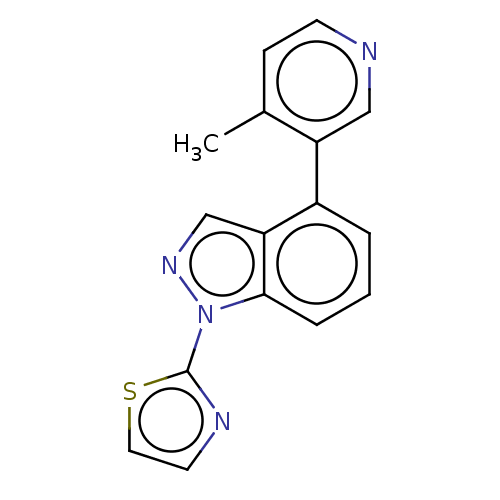
(CHEMBL3780266)Show InChI InChI=1S/C16H12N4S/c1-11-5-6-17-9-13(11)12-3-2-4-15-14(12)10-19-20(15)16-18-7-8-21-16/h2-10H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50156231

(CHEMBL3781112)Show InChI InChI=1S/C19H14FN3/c1-13-9-10-21-11-17(13)16-3-2-4-19-18(16)12-22-23(19)15-7-5-14(20)6-8-15/h2-12H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50156236
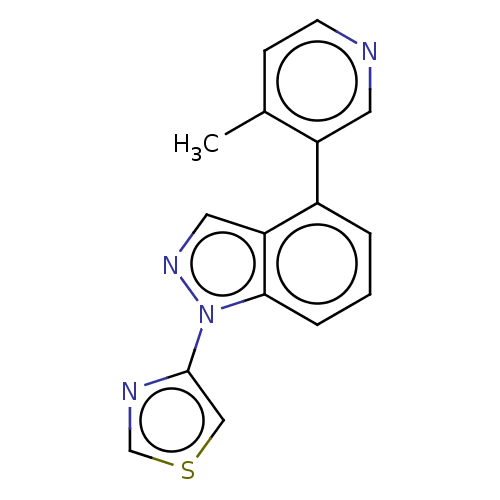
(CHEMBL3780048)Show InChI InChI=1S/C16H12N4S/c1-11-5-6-17-7-13(11)12-3-2-4-15-14(12)8-19-20(15)16-9-21-10-18-16/h2-10H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50156234
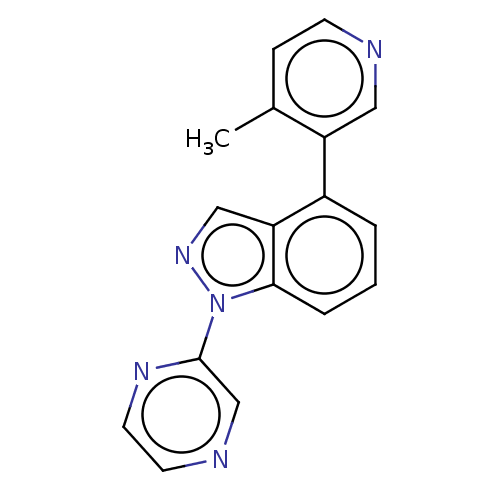
(CHEMBL3780226)Show InChI InChI=1S/C17H13N5/c1-12-5-6-18-9-14(12)13-3-2-4-16-15(13)10-21-22(16)17-11-19-7-8-20-17/h2-11H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50391839
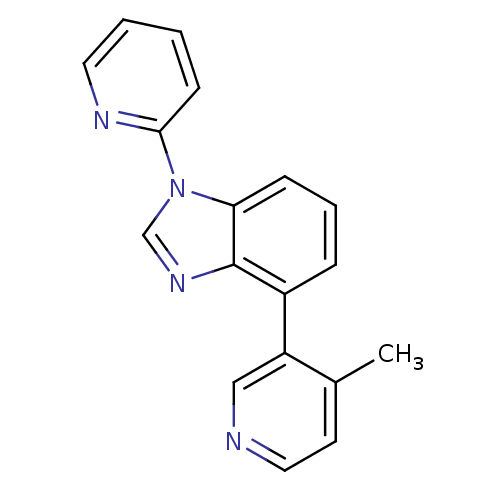
(CHEMBL2147034 | US9133160, 2)Show InChI InChI=1S/C18H14N4/c1-13-8-10-19-11-15(13)14-5-4-6-16-18(14)21-12-22(16)17-7-2-3-9-20-17/h2-12H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50156238
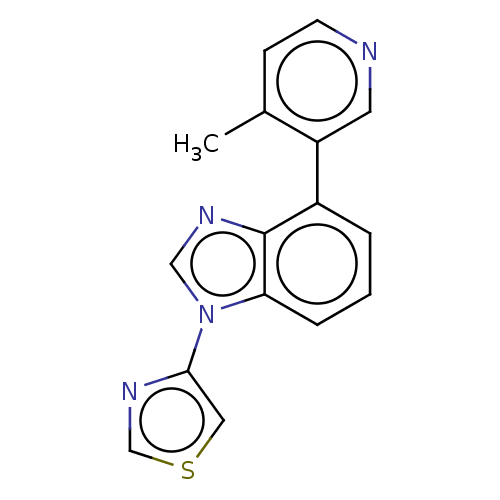
(CHEMBL3781910)Show InChI InChI=1S/C16H12N4S/c1-11-5-6-17-7-13(11)12-3-2-4-14-16(12)18-9-20(14)15-8-21-10-19-15/h2-10H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50156237
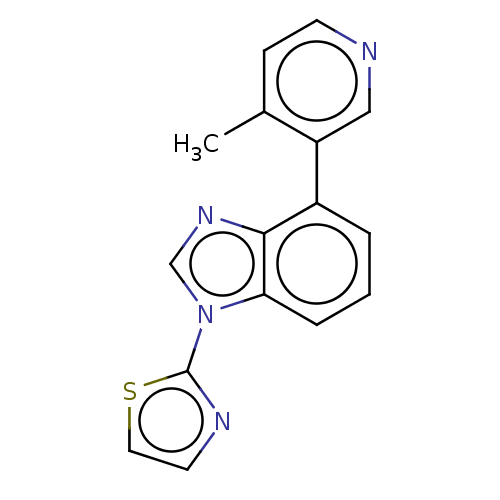
(CHEMBL3781487)Show InChI InChI=1S/C16H12N4S/c1-11-5-6-17-9-13(11)12-3-2-4-14-15(12)19-10-20(14)16-18-7-8-21-16/h2-10H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM30185
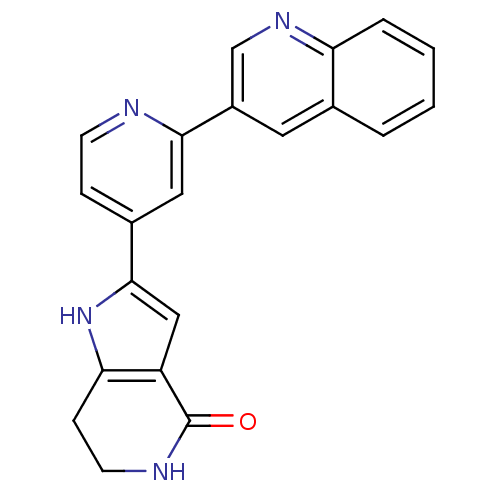
(CHEMBL226403 | Pyrrolopyridine, 16)Show SMILES O=C1NCCc2[nH]c(cc12)-c1ccnc(c1)-c1cnc2ccccc2c1 Show InChI InChI=1S/C21H16N4O/c26-21-16-11-20(25-18(16)6-8-23-21)14-5-7-22-19(10-14)15-9-13-3-1-2-4-17(13)24-12-15/h1-5,7,9-12,25H,6,8H2,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 expressed in Escherichia coli BL21(DE3) after 60 mins |
J Med Chem 51: 6225-9 (2008)
Article DOI: 10.1021/jm800747w
BindingDB Entry DOI: 10.7270/Q2JM29GJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218834
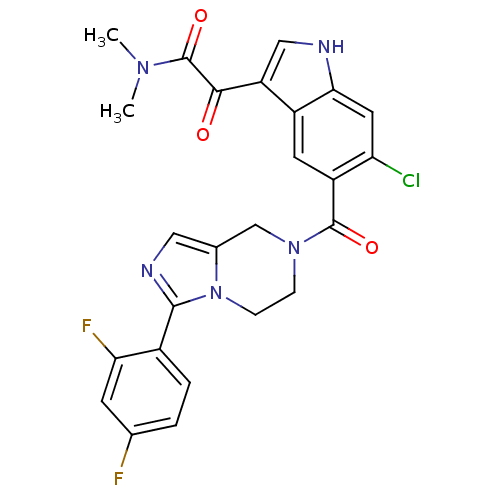
(2-(6-chloro-5-(3-(2,4-difluorophenyl)-5,6,7,8-tetr...)Show SMILES CN(C)C(=O)C(=O)c1c[nH]c2cc(Cl)c(cc12)C(=O)N1CCn2c(C1)cnc2-c1ccc(F)cc1F Show InChI InChI=1S/C25H20ClF2N5O3/c1-31(2)25(36)22(34)18-11-29-21-9-19(26)17(8-16(18)21)24(35)32-5-6-33-14(12-32)10-30-23(33)15-4-3-13(27)7-20(15)28/h3-4,7-11,29H,5-6,12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218824
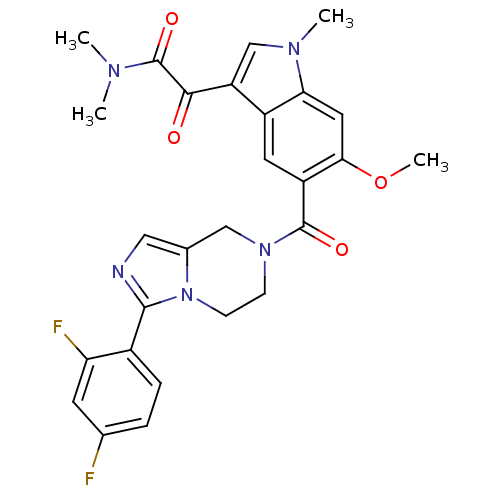
(2-(5-(3-(2,4-difluorophenyl)-5,6,7,8-tetrahydroimi...)Show SMILES COc1cc2n(C)cc(C(=O)C(=O)N(C)C)c2cc1C(=O)N1CCn2c(C1)cnc2-c1ccc(F)cc1F Show InChI InChI=1S/C27H25F2N5O4/c1-31(2)27(37)24(35)20-14-32(3)22-11-23(38-4)19(10-18(20)22)26(36)33-7-8-34-16(13-33)12-30-25(34)17-6-5-15(28)9-21(17)29/h5-6,9-12,14H,7-8,13H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218821
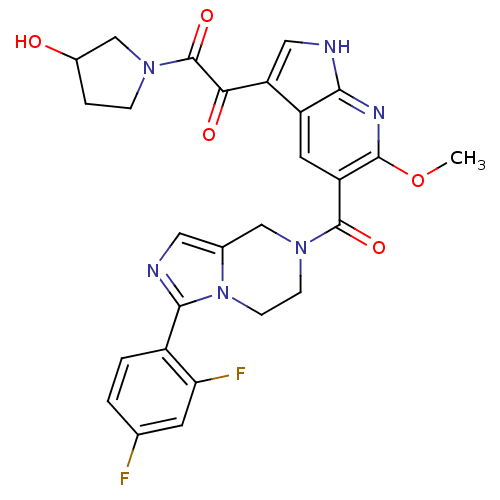
(1-(5-(3-(2,4-difluorophenyl)-5,6,7,8-tetrahydroimi...)Show SMILES COc1nc2[nH]cc(C(=O)C(=O)N3CCC(O)C3)c2cc1C(=O)N1CCn2c(C1)cnc2-c1ccc(F)cc1F Show InChI InChI=1S/C27H24F2N6O5/c1-40-25-19(9-18-20(11-30-23(18)32-25)22(37)27(39)33-5-4-16(36)13-33)26(38)34-6-7-35-15(12-34)10-31-24(35)17-3-2-14(28)8-21(17)29/h2-3,8-11,16,36H,4-7,12-13H2,1H3,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218820
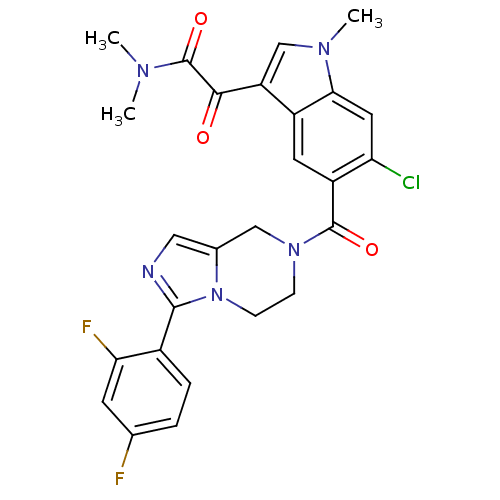
(2-(6-chloro-5-(3-(2,4-difluorophenyl)-5,6,7,8-tetr...)Show SMILES CN(C)C(=O)C(=O)c1cn(C)c2cc(Cl)c(cc12)C(=O)N1CCn2c(C1)cnc2-c1ccc(F)cc1F Show InChI InChI=1S/C26H22ClF2N5O3/c1-31(2)26(37)23(35)19-13-32(3)22-10-20(27)18(9-17(19)22)25(36)33-6-7-34-15(12-33)11-30-24(34)16-5-4-14(28)8-21(16)29/h4-5,8-11,13H,6-7,12H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218829
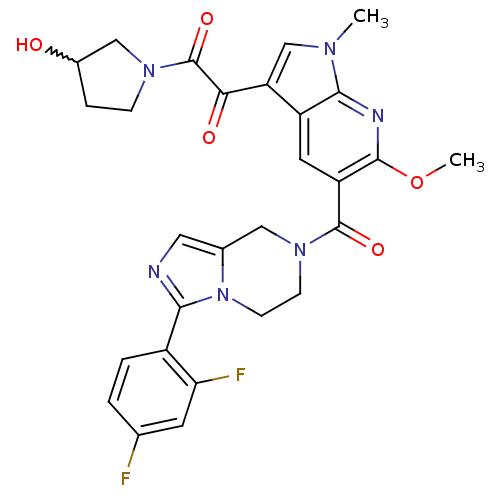
(1-(5-(3-(2,4-difluorophenyl)-5,6,7,8-tetrahydroimi...)Show SMILES COc1nc2n(C)cc(C(=O)C(=O)N3CCC(O)C3)c2cc1C(=O)N1CCn2c(C1)cnc2-c1ccc(F)cc1F |w:16.16| Show InChI InChI=1S/C28H26F2N6O5/c1-33-14-21(23(38)28(40)34-6-5-17(37)13-34)19-10-20(26(41-2)32-25(19)33)27(39)35-7-8-36-16(12-35)11-31-24(36)18-4-3-15(29)9-22(18)30/h3-4,9-11,14,17,37H,5-8,12-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50391846

(CHEMBL2147041 | US9133160, 31)Show InChI InChI=1S/C15H12F3N3/c1-10-5-6-19-7-12(10)11-3-2-4-13-14(11)20-9-21(13)8-15(16,17)18/h2-7,9H,8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50391846

(CHEMBL2147041 | US9133160, 31)Show InChI InChI=1S/C15H12F3N3/c1-10-5-6-19-7-12(10)11-3-2-4-13-14(11)20-9-21(13)8-15(16,17)18/h2-7,9H,8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP17A1 in human adrenal microsomes using [3H]-pregnenolone as substrate incubated for 90 mins by scintillation proximity assay in pres... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50156282

(CHEMBL3780847)Show SMILES [H][C@@]12CC=C3C[C@@H](O)CC[C@]3(C)C1CC[C@@]1(C)C2CC=C1c1cccnc1 |r,c:22,t:3| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP17A1 in human adrenal microsomes using [3H]-pregnenolone as substrate incubated for 90 mins by scintillation proximity assay in pres... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/beta-2
(Homo sapiens (Human)) | BDBM50154496
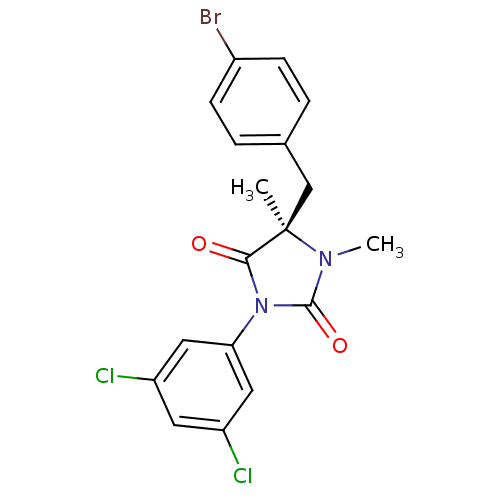
((R)-5-(4-Bromo-benzyl)-3-(3,5-dichloro-phenyl)-1,5...)Show SMILES CN1C(=O)N(C(=O)[C@@]1(C)Cc1ccc(Br)cc1)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C18H15BrCl2N2O2/c1-18(10-11-3-5-12(19)6-4-11)16(24)23(17(25)22(18)2)15-8-13(20)7-14(21)9-15/h3-9H,10H2,1-2H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Cerep
Curated by ChEMBL
| Assay Description
Inhibitory concentration against leukocyte function-associated antigen-1 in HSB-2 human lymphoblast and H1HeLa cell adhesion assay |
Bioorg Med Chem Lett 15: 1161-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.007
BindingDB Entry DOI: 10.7270/Q2NP23W2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218839
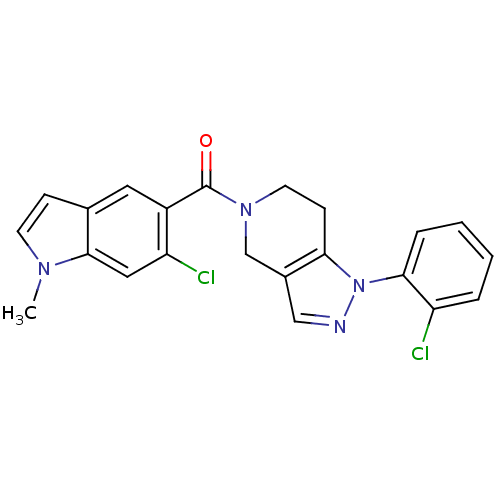
((6-chloro-1-methyl-1H-indol-5-yl)(1-(2-chloropheny...)Show SMILES Cn1ccc2cc(C(=O)N3CCc4c(C3)cnn4-c3ccccc3Cl)c(Cl)cc12 Show InChI InChI=1S/C22H18Cl2N4O/c1-26-8-6-14-10-16(18(24)11-21(14)26)22(29)27-9-7-19-15(13-27)12-25-28(19)20-5-3-2-4-17(20)23/h2-6,8,10-12H,7,9,13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218818
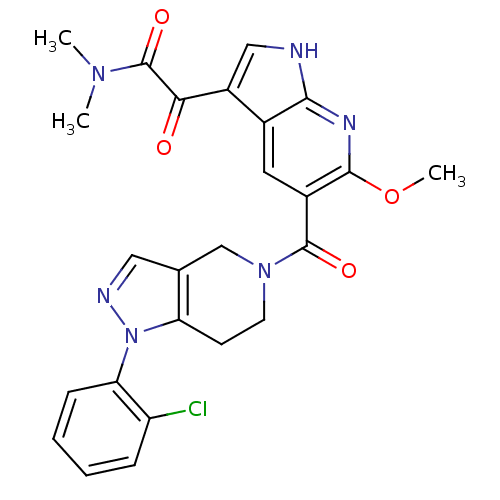
(2-(5-(1-(2-chlorophenyl)-4,5,6,7-tetrahydro-1H-pyr...)Show SMILES COc1nc2[nH]cc(C(=O)C(=O)N(C)C)c2cc1C(=O)N1CCc2c(C1)cnn2-c1ccccc1Cl Show InChI InChI=1S/C25H23ClN6O4/c1-30(2)25(35)21(33)17-12-27-22-15(17)10-16(23(29-22)36-3)24(34)31-9-8-19-14(13-31)11-28-32(19)20-7-5-4-6-18(20)26/h4-7,10-12H,8-9,13H2,1-3H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218826
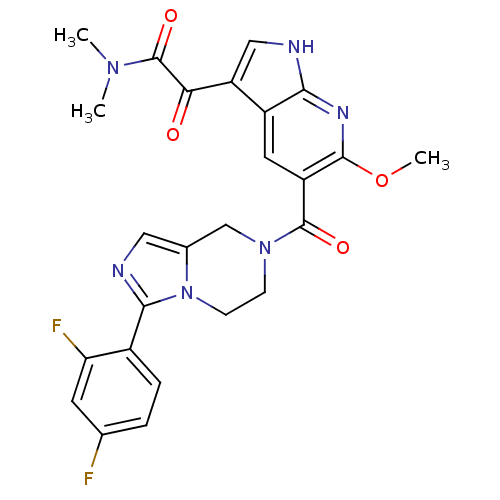
(2-(5-(3-(2,4-difluorophenyl)-5,6,7,8-tetrahydroimi...)Show SMILES COc1nc2[nH]cc(C(=O)C(=O)N(C)C)c2cc1C(=O)N1CCn2c(C1)cnc2-c1ccc(F)cc1F Show InChI InChI=1S/C25H22F2N6O4/c1-31(2)25(36)20(34)18-11-28-21-16(18)9-17(23(30-21)37-3)24(35)32-6-7-33-14(12-32)10-29-22(33)15-5-4-13(26)8-19(15)27/h4-5,8-11H,6-7,12H2,1-3H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218835
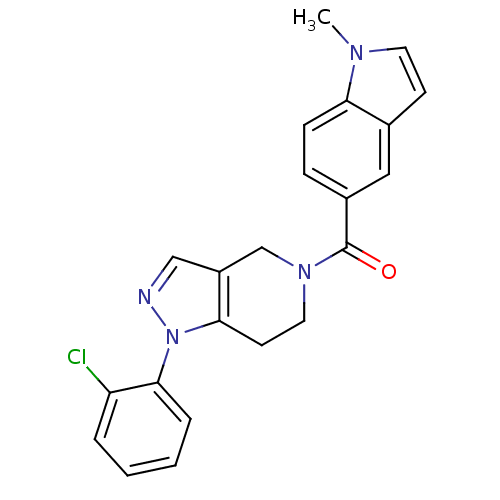
((1-(2-chlorophenyl)-6,7-dihydro-1H-pyrazolo[4,3-c]...)Show SMILES Cn1ccc2cc(ccc12)C(=O)N1CCc2c(C1)cnn2-c1ccccc1Cl Show InChI InChI=1S/C22H19ClN4O/c1-25-10-8-15-12-16(6-7-19(15)25)22(28)26-11-9-20-17(14-26)13-24-27(20)21-5-3-2-4-18(21)23/h2-8,10,12-13H,9,11,14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218828
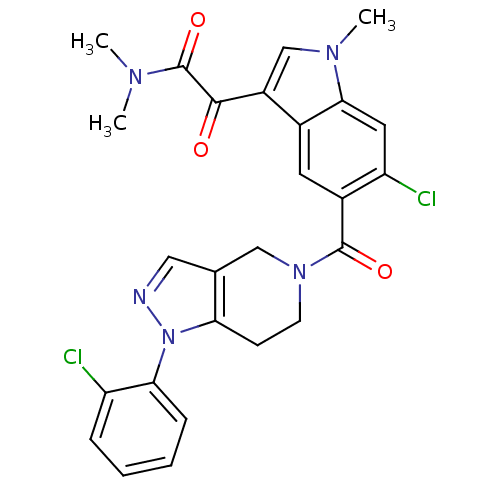
(2-(6-chloro-5-(1-(2-chlorophenyl)-4,5,6,7-tetrahyd...)Show SMILES CN(C)C(=O)C(=O)c1cn(C)c2cc(Cl)c(cc12)C(=O)N1CCc2c(C1)cnn2-c1ccccc1Cl Show InChI InChI=1S/C26H23Cl2N5O3/c1-30(2)26(36)24(34)18-14-31(3)23-11-20(28)17(10-16(18)23)25(35)32-9-8-21-15(13-32)12-29-33(21)22-7-5-4-6-19(22)27/h4-7,10-12,14H,8-9,13H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218823
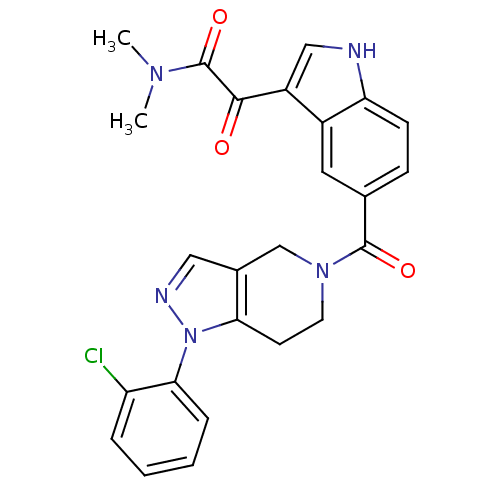
(2-(5-(1-(2-chlorophenyl)-4,5,6,7-tetrahydro-1H-pyr...)Show SMILES CN(C)C(=O)C(=O)c1c[nH]c2ccc(cc12)C(=O)N1CCc2c(C1)cnn2-c1ccccc1Cl Show InChI InChI=1S/C25H22ClN5O3/c1-29(2)25(34)23(32)18-13-27-20-8-7-15(11-17(18)20)24(33)30-10-9-21-16(14-30)12-28-31(21)22-6-4-3-5-19(22)26/h3-8,11-13,27H,9-10,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50391840
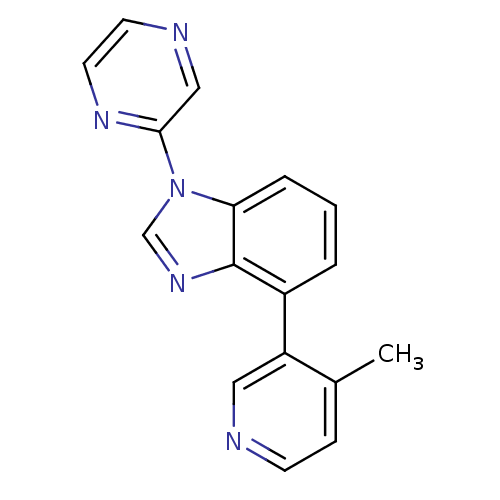
(CHEMBL2147035 | US9133160, 4)Show InChI InChI=1S/C17H13N5/c1-12-5-6-18-9-14(12)13-3-2-4-15-17(13)21-11-22(15)16-10-19-7-8-20-16/h2-11H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity... |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218840
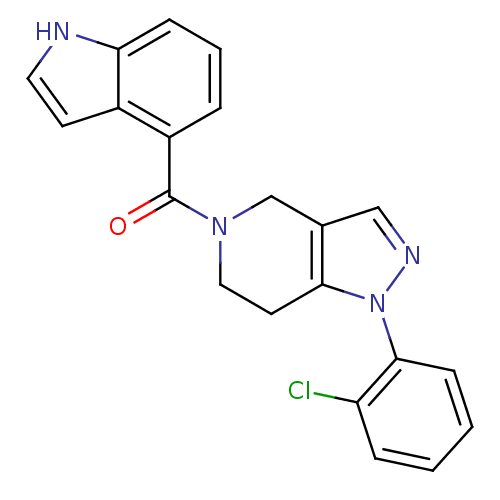
((1-(2-chlorophenyl)-6,7-dihydro-1H-pyrazolo[4,3-c]...)Show SMILES Clc1ccccc1-n1ncc2CN(CCc12)C(=O)c1cccc2[nH]ccc12 Show InChI InChI=1S/C21H17ClN4O/c22-17-5-1-2-7-20(17)26-19-9-11-25(13-14(19)12-24-26)21(27)16-4-3-6-18-15(16)8-10-23-18/h1-8,10,12,23H,9,11,13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218817
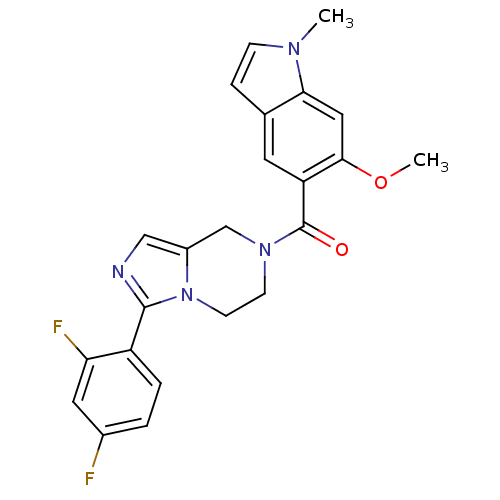
((3-(2,4-difluorophenyl)-5,6-dihydroimidazo[1,5-a]p...)Show SMILES COc1cc2n(C)ccc2cc1C(=O)N1CCn2c(C1)cnc2-c1ccc(F)cc1F Show InChI InChI=1S/C23H20F2N4O2/c1-27-6-5-14-9-18(21(31-2)11-20(14)27)23(30)28-7-8-29-16(13-28)12-26-22(29)17-4-3-15(24)10-19(17)25/h3-6,9-12H,7-8,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218833
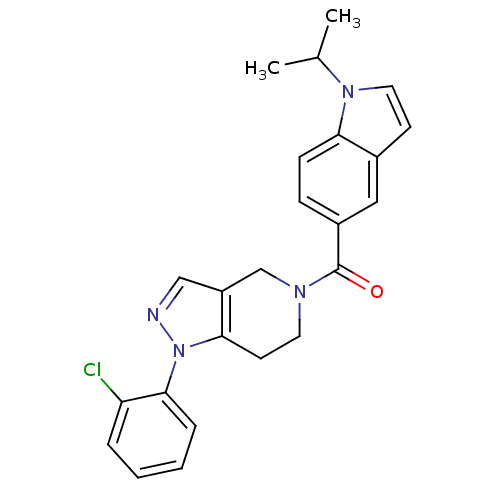
((1-(2-chlorophenyl)-6,7-dihydro-1H-pyrazolo[4,3-c]...)Show SMILES CC(C)n1ccc2cc(ccc12)C(=O)N1CCc2c(C1)cnn2-c1ccccc1Cl Show InChI InChI=1S/C24H23ClN4O/c1-16(2)28-12-9-17-13-18(7-8-21(17)28)24(30)27-11-10-22-19(15-27)14-26-29(22)23-6-4-3-5-20(23)25/h3-9,12-14,16H,10-11,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218838
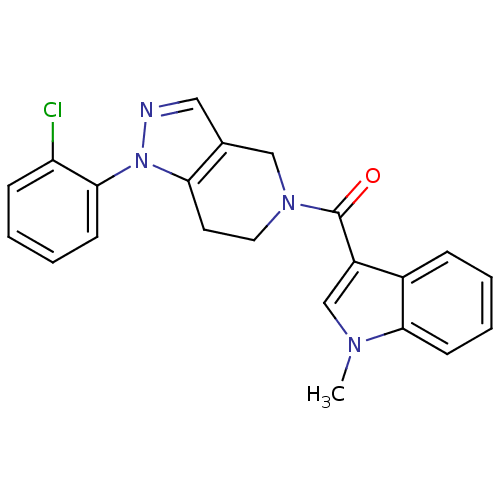
((1-(2-chlorophenyl)-6,7-dihydro-1H-pyrazolo[4,3-c]...)Show SMILES Cn1cc(C(=O)N2CCc3c(C2)cnn3-c2ccccc2Cl)c2ccccc12 Show InChI InChI=1S/C22H19ClN4O/c1-25-14-17(16-6-2-4-8-20(16)25)22(28)26-11-10-19-15(13-26)12-24-27(19)21-9-5-3-7-18(21)23/h2-9,12,14H,10-11,13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218827

((1-(2-chlorophenyl)-6,7-dihydro-1H-pyrazolo[4,3-c]...)Show SMILES Clc1ccccc1-n1ncc2CN(CCc12)C(=O)c1ccc2cc[nH]c2c1 Show InChI InChI=1S/C21H17ClN4O/c22-17-3-1-2-4-20(17)26-19-8-10-25(13-16(19)12-24-26)21(27)15-6-5-14-7-9-23-18(14)11-15/h1-7,9,11-12,23H,8,10,13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50253633
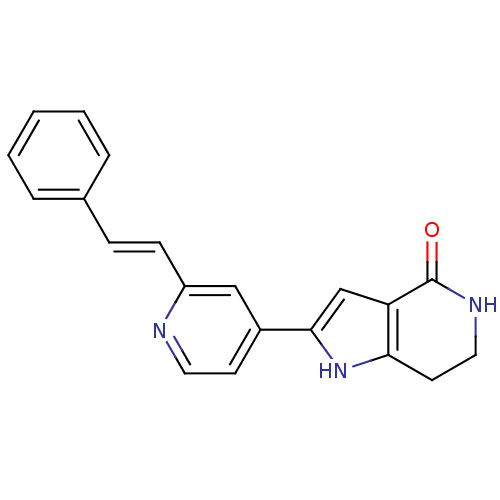
(2-(2-styrylpyridin-4-yl)-6,7-dihydro-1H-pyrrolo[3,...)Show SMILES O=C1NCCc2[nH]c(cc12)-c1ccnc(\C=C\c2ccccc2)c1 Show InChI InChI=1S/C20H17N3O/c24-20-17-13-19(23-18(17)9-11-22-20)15-8-10-21-16(12-15)7-6-14-4-2-1-3-5-14/h1-8,10,12-13,23H,9,11H2,(H,22,24)/b7-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 expressed in Escherichia coli BL21(DE3) after 60 mins |
J Med Chem 51: 6225-9 (2008)
Article DOI: 10.1021/jm800747w
BindingDB Entry DOI: 10.7270/Q2JM29GJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218825
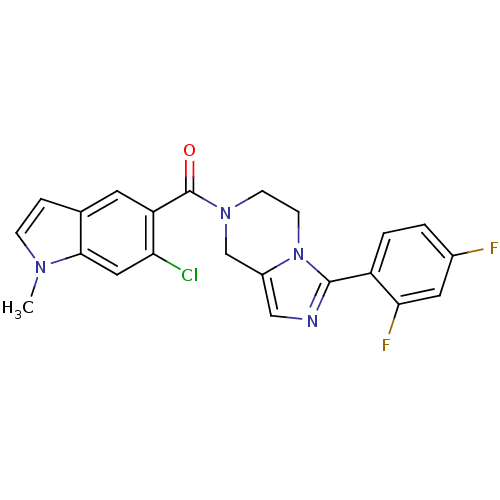
((6-chloro-1-methyl-1H-indol-5-yl)(3-(2,4-difluorop...)Show SMILES Cn1ccc2cc(C(=O)N3CCn4c(C3)cnc4-c3ccc(F)cc3F)c(Cl)cc12 Show InChI InChI=1S/C22H17ClF2N4O/c1-27-5-4-13-8-17(18(23)10-20(13)27)22(30)28-6-7-29-15(12-28)11-26-21(29)16-3-2-14(24)9-19(16)25/h2-5,8-11H,6-7,12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218822
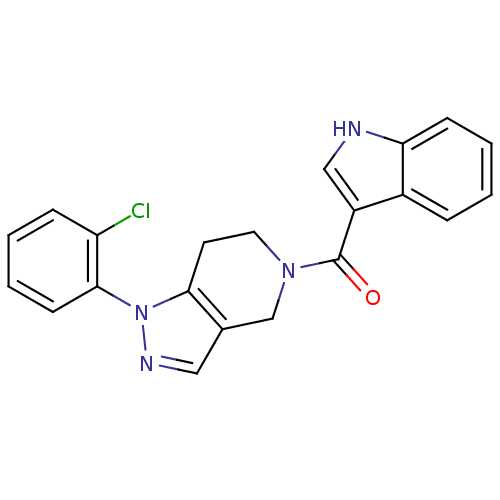
((1-(2-chlorophenyl)-6,7-dihydro-1H-pyrazolo[4,3-c]...)Show SMILES Clc1ccccc1-n1ncc2CN(CCc12)C(=O)c1c[nH]c2ccccc12 Show InChI InChI=1S/C21H17ClN4O/c22-17-6-2-4-8-20(17)26-19-9-10-25(13-14(19)11-24-26)21(27)16-12-23-18-7-3-1-5-15(16)18/h1-8,11-12,23H,9-10,13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218836
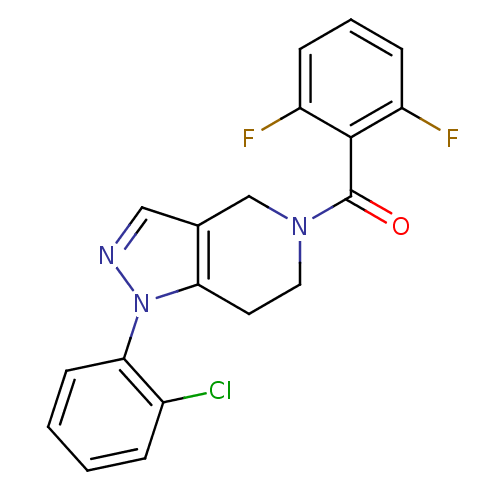
((1-(2-chlorophenyl)-6,7-dihydro-1H-pyrazolo[4,3-c]...)Show SMILES Fc1cccc(F)c1C(=O)N1CCc2c(C1)cnn2-c1ccccc1Cl Show InChI InChI=1S/C19H14ClF2N3O/c20-13-4-1-2-7-17(13)25-16-8-9-24(11-12(16)10-23-25)19(26)18-14(21)5-3-6-15(18)22/h1-7,10H,8-9,11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50156236

(CHEMBL3780048)Show InChI InChI=1S/C16H12N4S/c1-11-5-6-17-7-13(11)12-3-2-4-15-14(12)8-19-20(15)16-9-21-10-18-16/h2-10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50156237

(CHEMBL3781487)Show InChI InChI=1S/C16H12N4S/c1-11-5-6-17-9-13(11)12-3-2-4-14-15(12)19-10-20(14)16-18-7-8-21-16/h2-10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50156235

(CHEMBL3780266)Show InChI InChI=1S/C16H12N4S/c1-11-5-6-17-9-13(11)12-3-2-4-15-14(12)10-19-20(15)16-18-7-8-21-16/h2-10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/beta-2
(Homo sapiens (Human)) | BDBM50160927

((6S,7aS)-6-(4''-Bromo-biphenyl-3-ylmethoxy)-2-(3,5...)Show SMILES Oc1c2C[C@@H](Cn2c(=O)n1-c1cc(Cl)cc(Cl)c1)OCc1cccc(c1)-c1ccc(Br)cc1 Show InChI InChI=1S/C25H19BrCl2N2O3/c26-18-6-4-16(5-7-18)17-3-1-2-15(8-17)14-33-22-12-23-24(31)30(25(32)29(23)13-22)21-10-19(27)9-20(28)11-21/h1-11,22,31H,12-14H2/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Cerep
Curated by ChEMBL
| Assay Description
Inhibitory concentration against leukocyte function-associated antigen-1 in HSB-2 human lymphoblast and H1HeLa cell adhesion assay |
Bioorg Med Chem Lett 15: 1161-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.007
BindingDB Entry DOI: 10.7270/Q2NP23W2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50391839

(CHEMBL2147034 | US9133160, 2)Show InChI InChI=1S/C18H14N4/c1-13-8-10-19-11-15(13)14-5-4-6-16-18(14)21-12-22(16)17-7-2-3-9-20-17/h2-12H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
ACS Med Chem Lett 7: 40-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00310
BindingDB Entry DOI: 10.7270/Q2TH8PK5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218841
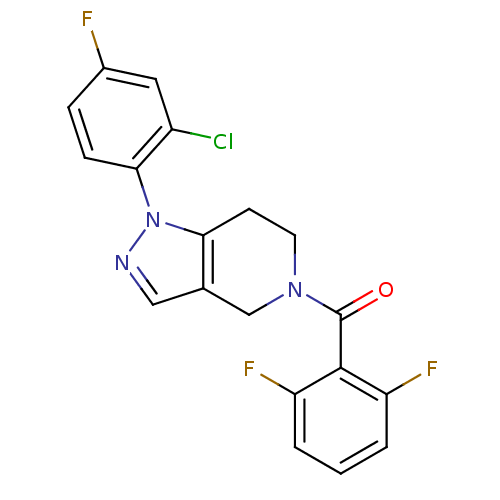
((1-(2-chloro-4-fluorophenyl)-6,7-dihydro-1H-pyrazo...)Show SMILES Fc1ccc(c(Cl)c1)-n1ncc2CN(CCc12)C(=O)c1c(F)cccc1F Show InChI InChI=1S/C19H13ClF3N3O/c20-13-8-12(21)4-5-17(13)26-16-6-7-25(10-11(16)9-24-26)19(27)18-14(22)2-1-3-15(18)23/h1-5,8-9H,6-7,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
P-selectin
(Homo sapiens (Human)) | BDBM50061124
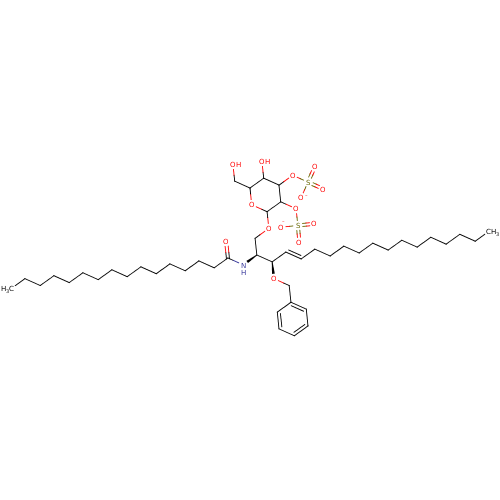
((2S,3R,4E)-3-(Benzoyloxy)-2-(hexadecanoylamino)-1-...)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](COC1OC(CO)C(O)C(OS([O-])(=O)=O)C1OS([O-])(=O)=O)[C@H](OCc1ccccc1)\C=C\CCCCCCCCCCCCC Show InChI InChI=1S/C47H83NO14S2/c1-3-5-7-9-11-13-15-17-19-21-23-25-30-34-41(58-37-39-32-28-27-29-33-39)40(48-43(50)35-31-26-24-22-20-18-16-14-12-10-8-6-4-2)38-59-47-46(62-64(55,56)57)45(61-63(52,53)54)44(51)42(36-49)60-47/h27-30,32-34,40-42,44-47,49,51H,3-26,31,35-38H2,1-2H3,(H,48,50)(H,52,53,54)(H,55,56,57)/p-2/b34-30+/t40-,41+,42?,44?,45?,46?,47?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Selectin P in a cell-free binding assay |
J Med Chem 40: 3234-47 (1997)
Article DOI: 10.1021/jm9606960
BindingDB Entry DOI: 10.7270/Q21Z454Z |
More data for this
Ligand-Target Pair | |
P-selectin
(Homo sapiens (Human)) | BDBM50369315
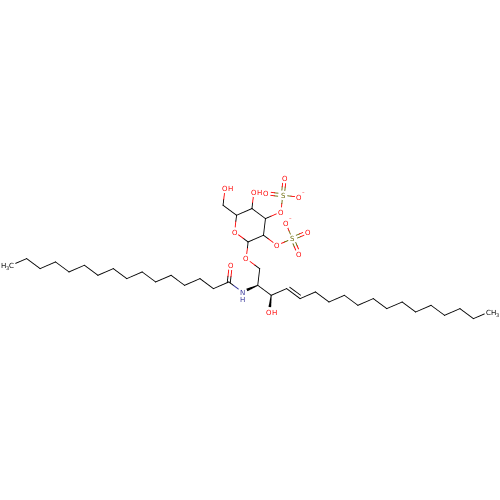
(CHEMBL1627019)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](COC1OC(CO)C(O)C(OS([O-])(=O)=O)C1OS([O-])(=O)=O)[C@H](O)\C=C\CCCCCCCCCCCCC Show InChI InChI=1S/C40H77NO14S2/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-34(43)33(41-36(44)30-28-26-24-22-20-18-16-14-12-10-8-6-4-2)32-52-40-39(55-57(49,50)51)38(54-56(46,47)48)37(45)35(31-42)53-40/h27,29,33-35,37-40,42-43,45H,3-26,28,30-32H2,1-2H3,(H,41,44)(H,46,47,48)(H,49,50,51)/p-2/b29-27+/t33-,34+,35?,37?,38?,39?,40?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Selectin P in a cell-free binding assay |
J Med Chem 40: 3234-47 (1997)
Article DOI: 10.1021/jm9606960
BindingDB Entry DOI: 10.7270/Q21Z454Z |
More data for this
Ligand-Target Pair | |
P-selectin
(Homo sapiens (Human)) | BDBM50061130

((2S,3R,4E)-3-(Benzoyloxy)-2-(hexadecanoylamino)-1-...)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](COC1OC2COC(OC2C(OS([O-])(=O)=O)C1OS([O-])(=O)=O)c1ccccc1)[C@H](OCc1ccccc1)\C=C\CCCCCCCCCCCCC Show InChI InChI=1S/C54H87NO14S2/c1-3-5-7-9-11-13-15-17-19-21-23-25-33-39-47(63-41-44-35-29-27-30-36-44)46(55-49(56)40-34-26-24-22-20-18-16-14-12-10-8-6-4-2)42-64-54-52(69-71(60,61)62)51(68-70(57,58)59)50-48(66-54)43-65-53(67-50)45-37-31-28-32-38-45/h27-33,35-39,46-48,50-54H,3-26,34,40-43H2,1-2H3,(H,55,56)(H,57,58,59)(H,60,61,62)/p-2/b39-33+/t46-,47+,48?,50?,51?,52?,53?,54?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Selectin P in a cell-free binding assay |
J Med Chem 40: 3234-47 (1997)
Article DOI: 10.1021/jm9606960
BindingDB Entry DOI: 10.7270/Q21Z454Z |
More data for this
Ligand-Target Pair | |
P-selectin
(Homo sapiens (Human)) | BDBM50061136

((2S,3R,4E)-3-(Benzoyloxy)-2-(hexadecanoylamino)-1-...)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](COC1OC(COS([O-])(=O)=O)C(OS([O-])(=O)=O)C(OCc2ccccc2)C1OCc1ccccc1)[C@H](OCc1ccccc1)\C=C\CCCCCCCCCCCCC Show InChI InChI=1S/C61H95NO14S2/c1-3-5-7-9-11-13-15-17-19-21-23-25-36-44-55(70-46-51-38-30-27-31-39-51)54(62-57(63)45-37-26-24-22-20-18-16-14-12-10-8-6-4-2)49-73-61-60(72-48-53-42-34-29-35-43-53)59(71-47-52-40-32-28-33-41-52)58(76-78(67,68)69)56(75-61)50-74-77(64,65)66/h27-36,38-44,54-56,58-61H,3-26,37,45-50H2,1-2H3,(H,62,63)(H,64,65,66)(H,67,68,69)/p-2/b44-36+/t54-,55+,56?,58?,59?,60?,61?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Selectin P in a cell-free binding assay |
J Med Chem 40: 3234-47 (1997)
Article DOI: 10.1021/jm9606960
BindingDB Entry DOI: 10.7270/Q21Z454Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data