 Found 2832 hits with Last Name = 'toma' and Initial = 's'
Found 2832 hits with Last Name = 'toma' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50361606
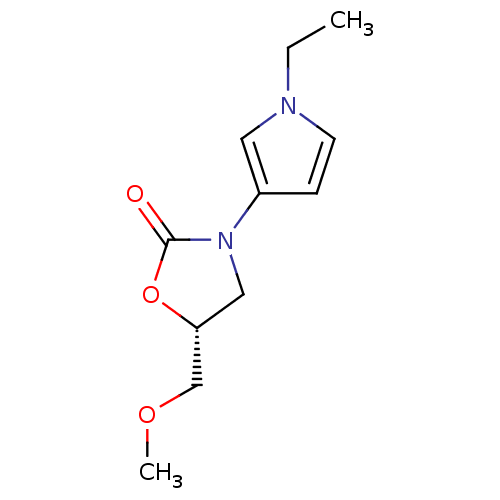
(CHEMBL1939851)Show InChI InChI=1S/C11H16N2O3/c1-3-12-5-4-9(6-12)13-7-10(8-15-2)16-11(13)14/h4-6,10H,3,7-8H2,1-2H3/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOA using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
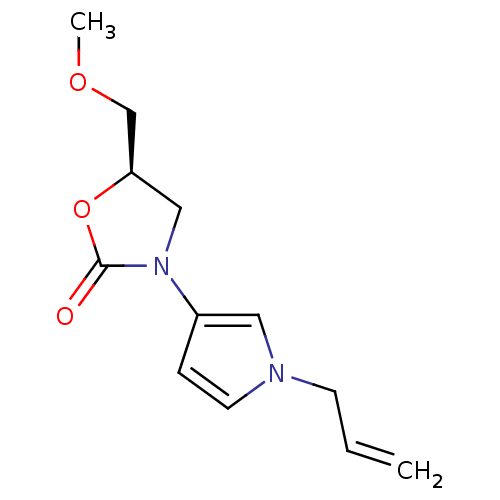
(Bos taurus) | BDBM50361609
(CHEMBL1939853)Show InChI InChI=1S/C10H13N5O2/c1-2-14-4-3-8(6-14)15-7-9(5-12-13-11)17-10(15)16/h3-4,6,9H,2,5,7H2,1H3/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOA using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50361607
(CHEMBL1938411)Show InChI InChI=1S/C12H16N2O3/c1-3-5-13-6-4-10(7-13)14-8-11(9-16-2)17-12(14)15/h3-4,6-7,11H,1,5,8-9H2,2H3/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOA using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50361617
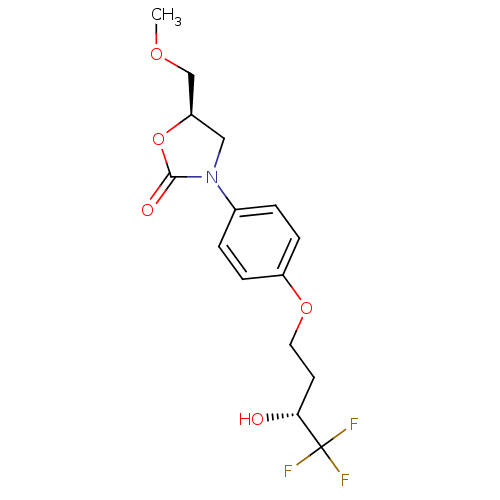
(BEFLOXATONE)Show SMILES COC[C@H]1CN(C(=O)O1)c1ccc(OCC[C@@H](O)C(F)(F)F)cc1 Show InChI InChI=1S/C15H18F3NO5/c1-22-9-12-8-19(14(21)24-12)10-2-4-11(5-3-10)23-7-6-13(20)15(16,17)18/h2-5,12-13,20H,6-9H2,1H3/t12-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOA using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50361603
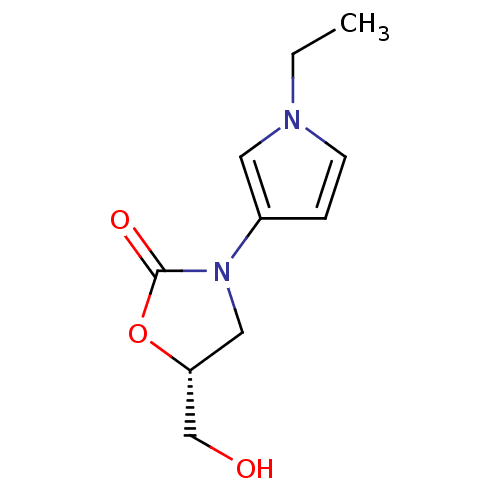
(CHEMBL1939848)Show InChI InChI=1S/C10H14N2O3/c1-2-11-4-3-8(5-11)12-6-9(7-13)15-10(12)14/h3-5,9,13H,2,6-7H2,1H3/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOA using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
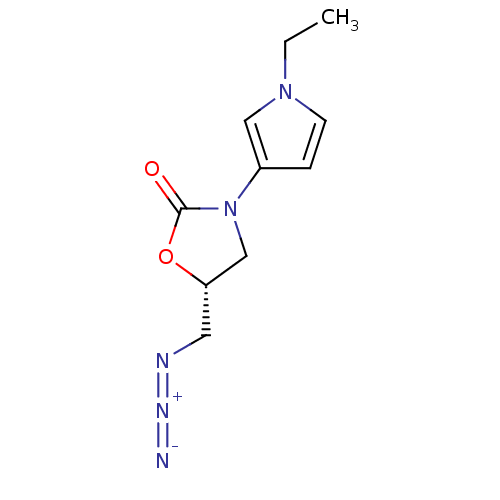
(Bos taurus) | BDBM50361610

(CHEMBL1939854)Show SMILES C=CCn1ccc(c1)N1C[C@H](CN=[N+]=[N-])OC1=O |r| Show InChI InChI=1S/C11H13N5O2/c1-2-4-15-5-3-9(7-15)16-8-10(6-13-14-12)18-11(16)17/h2-3,5,7,10H,1,4,6,8H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOA using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50237140

(CHEMBL4068763)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1n[nH]c2cc(Nc3cccc(F)c3)ccc12 Show InChI InChI=1S/C28H32FN7O2/c1-6-27(37)31-23-16-24(26(38-5)17-25(23)36(4)13-12-35(2)3)32-28-21-11-10-20(15-22(21)33-34-28)30-19-9-7-8-18(29)14-19/h6-11,14-17,30H,1,12-13H2,2-5H3,(H,31,37)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Reversible inhibition of wild-type human N-terminal GST-tagged EGFR cytoplasmic domain (669 to 1210 end amino acid residues) expressed in baculovirus... |
J Med Chem 60: 2361-2372 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01626
BindingDB Entry DOI: 10.7270/Q2FB556R |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50361614
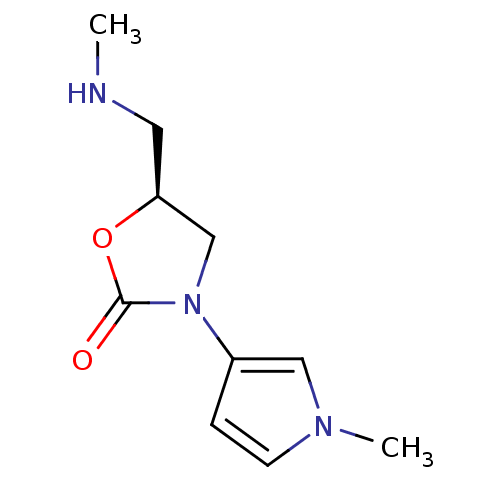
(CHEMBL1939858)Show InChI InChI=1S/C10H15N3O2/c1-11-5-9-7-13(10(14)15-9)8-3-4-12(2)6-8/h3-4,6,9,11H,5,7H2,1-2H3/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOA using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50361611
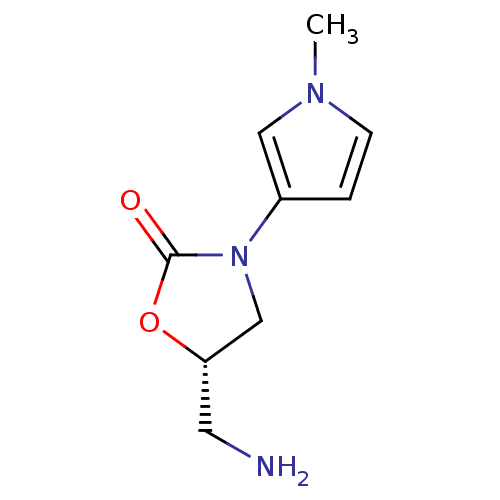
(CHEMBL1939855)Show InChI InChI=1S/C9H13N3O2/c1-11-3-2-7(5-11)12-6-8(4-10)14-9(12)13/h2-3,5,8H,4,6,10H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOA using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Reversible inhibition of wild-type human N-terminal GST-tagged EGFR cytoplasmic domain (669 to 1210 end amino acid residues) expressed in baculovirus... |
J Med Chem 60: 2361-2372 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01626
BindingDB Entry DOI: 10.7270/Q2FB556R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50353128

(CHEMBL1231795)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C30H47N5O2/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23/h20-24H,4-19H2,1-3H3,(H,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50262048
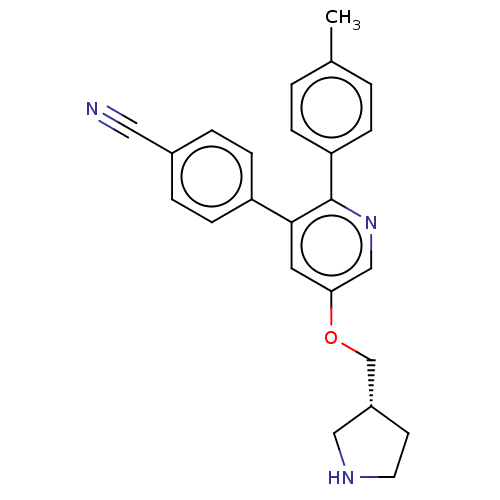
(CHEMBL3134377)Show SMILES Cc1ccc(cc1)-c1ncc(OC[C@@H]2CCNC2)cc1-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C24H23N3O/c1-17-2-6-21(7-3-17)24-23(20-8-4-18(13-25)5-9-20)12-22(15-27-24)28-16-19-10-11-26-14-19/h2-9,12,15,19,26H,10-11,14,16H2,1H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50229787

((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...)Show SMILES COc1ccc(C2=N[C@H]([C@H](N2C(=O)N2CCNC(=O)C2)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(OC(C)C)c1 |t:6| Show InChI InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37)/t27-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Binding affinity to 6xHis-taged MDM2 (unknown origin) expressed in Escherichia coli Gold (DE3) assessed as inhibition constant |
ACS Med Chem Lett 11: 1047-1053 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00620
BindingDB Entry DOI: 10.7270/Q20K2D3J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM149404
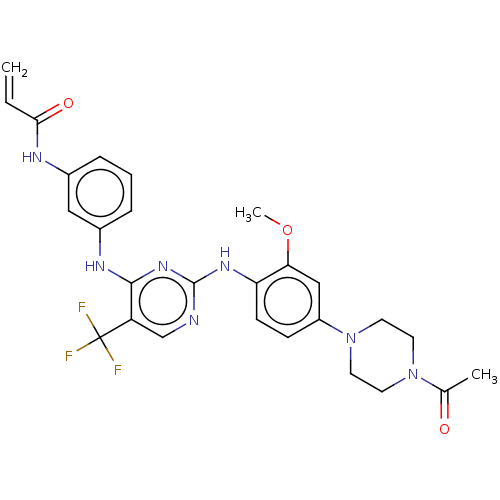
(AVL-301 | CHEMBL3545308 | CNX-419 | CO-1686 | Roci...)Show SMILES COc1cc(ccc1Nc1ncc(c(Nc2cccc(NC(=O)C=C)c2)n1)C(F)(F)F)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C27H28F3N7O3/c1-4-24(39)32-18-6-5-7-19(14-18)33-25-21(27(28,29)30)16-31-26(35-25)34-22-9-8-20(15-23(22)40-3)37-12-10-36(11-13-37)17(2)38/h4-9,14-16H,1,10-13H2,2-3H3,(H,32,39)(H2,31,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Reversible inhibition of wild-type human N-terminal GST-tagged EGFR cytoplasmic domain (669 to 1210 end amino acid residues) expressed in baculovirus... |
J Med Chem 60: 2361-2372 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01626
BindingDB Entry DOI: 10.7270/Q2FB556R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50361615

(CHEMBL1939859)Show InChI InChI=1S/C11H17N3O2/c1-3-13-5-4-9(7-13)14-8-10(6-12-2)16-11(14)15/h4-5,7,10,12H,3,6,8H2,1-2H3/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOA using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50361616
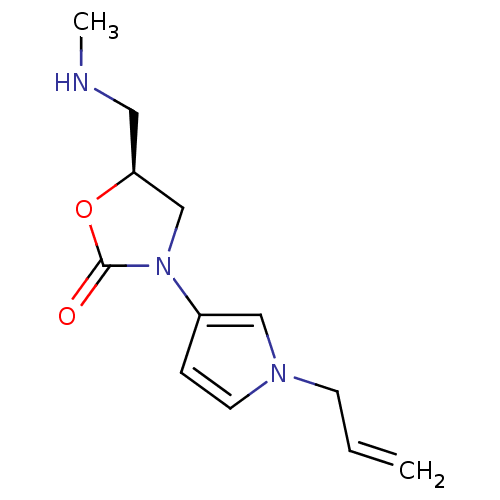
(CHEMBL1939860)Show InChI InChI=1S/C12H17N3O2/c1-3-5-14-6-4-10(8-14)15-9-11(7-13-2)17-12(15)16/h3-4,6,8,11,13H,1,5,7,9H2,2H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOA using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594106
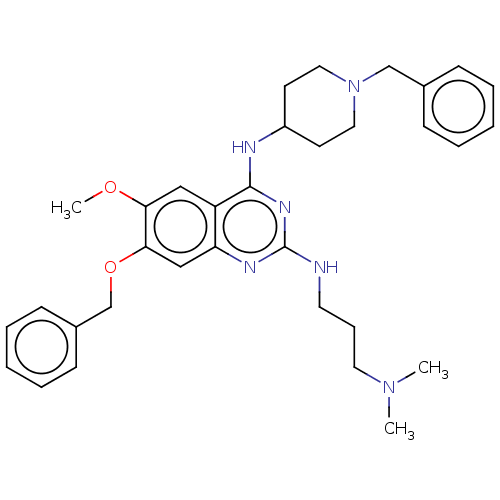
(CHEMBL5202637)Show SMILES COc1cc2c(NC3CCN(Cc4ccccc4)CC3)nc(NCCCN(C)C)nc2cc1OCc1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50361612
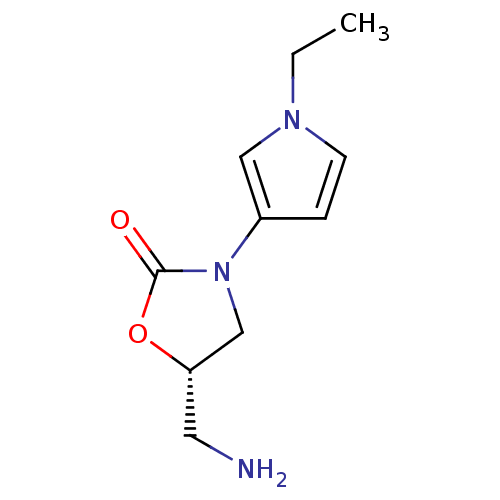
(CHEMBL1939856)Show InChI InChI=1S/C10H15N3O2/c1-2-12-4-3-8(6-12)13-7-9(5-11)15-10(13)14/h3-4,6,9H,2,5,7,11H2,1H3/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOA using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594086

(CHEMBL5170197)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(Cc4ccc(cc4)-c4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50237139

(CHEMBL4089863)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1n[nH]c2ccc(cc12)-c1ccc2ccccc2c1 Show InChI InChI=1S/C32H34N6O2/c1-6-31(39)33-27-19-28(30(40-5)20-29(27)38(4)16-15-37(2)3)34-32-25-18-24(13-14-26(25)35-36-32)23-12-11-21-9-7-8-10-22(21)17-23/h6-14,17-20H,1,15-16H2,2-5H3,(H,33,39)(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards 5-hydroxytryptamine 3 receptor was determined |
J Med Chem 60: 2361-2372 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01626
BindingDB Entry DOI: 10.7270/Q2FB556R |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594083
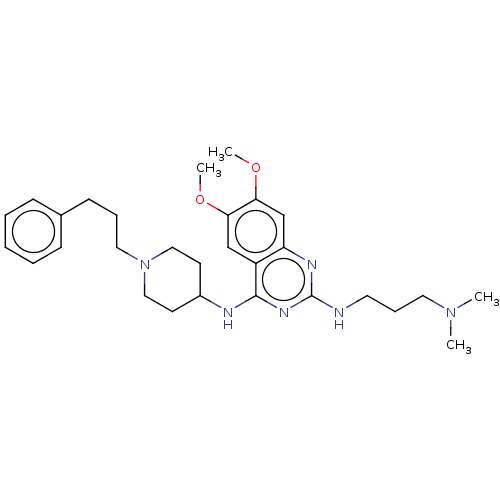
(CHEMBL5179091)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(CCCc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594098
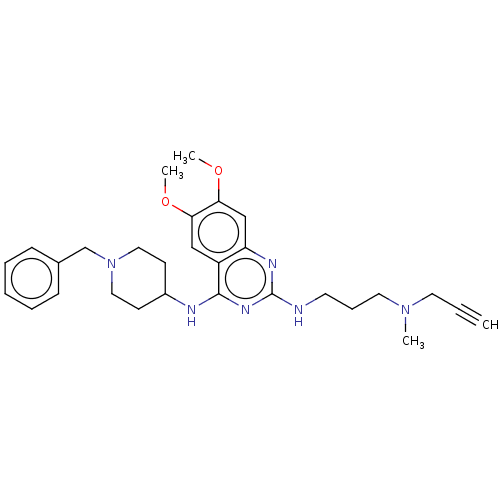
(CHEMBL5198334)Show SMILES COc1cc2nc(NCCCN(C)CC#C)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594085
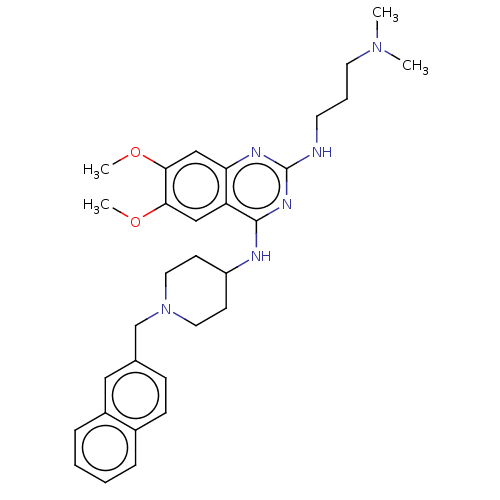
(CHEMBL5178765)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(Cc4ccc5ccccc5c4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594101
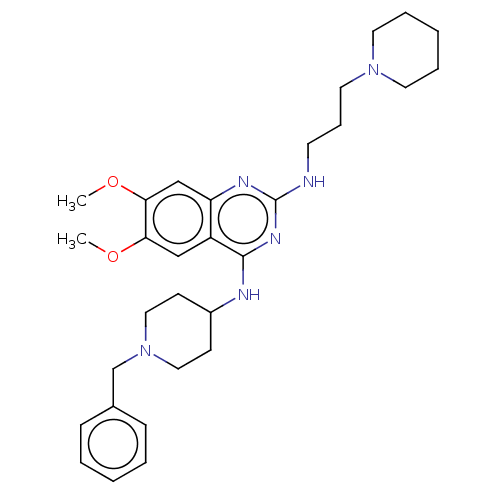
(CHEMBL5175171)Show SMILES COc1cc2nc(NCCCN3CCCCC3)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594099
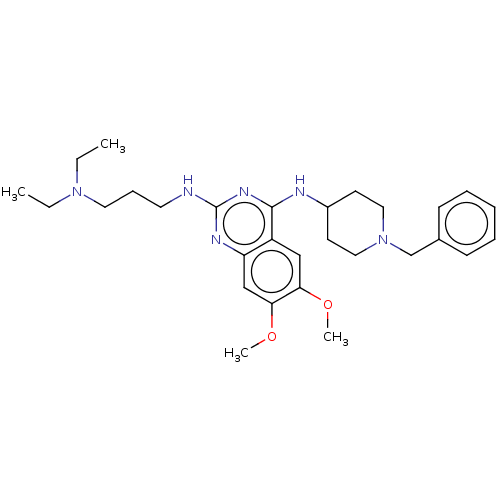
(CHEMBL5186174)Show SMILES CCN(CC)CCCNc1nc(NC2CCN(Cc3ccccc3)CC2)c2cc(OC)c(OC)cc2n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594100

(CHEMBL5181017)Show SMILES COc1cc2nc(NCCCN3CCCC3)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50361608
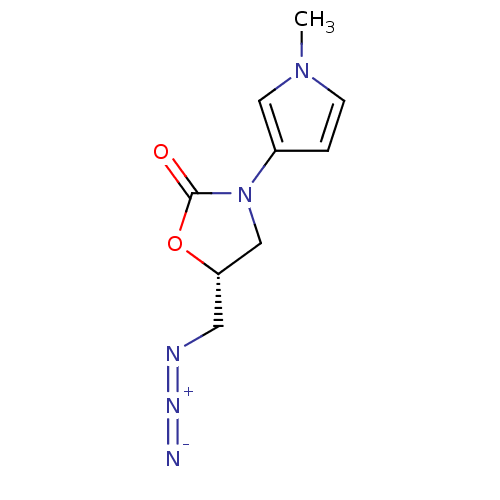
(CHEMBL1939852)Show InChI InChI=1S/C9H11N5O2/c1-13-3-2-7(5-13)14-6-8(4-11-12-10)16-9(14)15/h2-3,5,8H,4,6H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOA using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594090
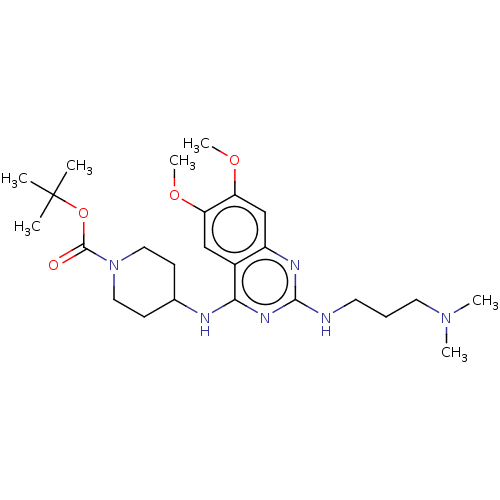
(CHEMBL5205888)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(CC3)C(=O)OC(C)(C)C)c2cc1OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594084
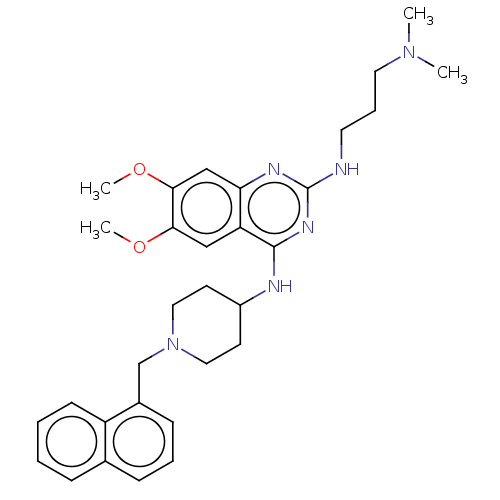
(CHEMBL5204694)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(Cc4cccc5ccccc45)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 212 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50361617

(BEFLOXATONE)Show SMILES COC[C@H]1CN(C(=O)O1)c1ccc(OCC[C@@H](O)C(F)(F)F)cc1 Show InChI InChI=1S/C15H18F3NO5/c1-22-9-12-8-19(14(21)24-12)10-2-4-11(5-3-10)23-7-6-13(20)15(16,17)18/h2-5,12-13,20H,6-9H2,1H3/t12-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOB using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50237153
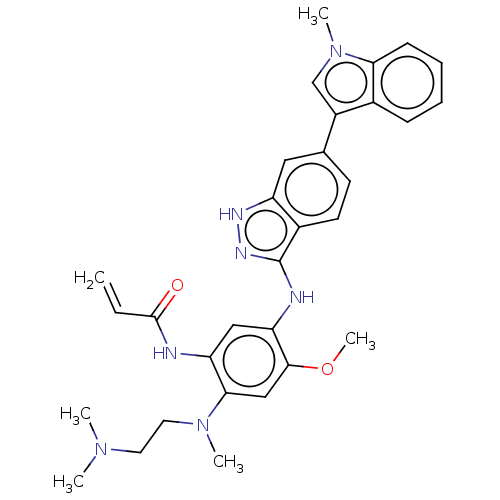
(CHEMBL4098444)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1n[nH]c2cc(ccc12)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C31H35N7O2/c1-7-30(39)32-25-17-26(29(40-6)18-28(25)37(4)15-14-36(2)3)33-31-22-13-12-20(16-24(22)34-35-31)23-19-38(5)27-11-9-8-10-21(23)27/h7-13,16-19H,1,14-15H2,2-6H3,(H,32,39)(H2,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Inhibition of cloned isozyme, human carbonic anhydrase II |
J Med Chem 60: 2361-2372 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01626
BindingDB Entry DOI: 10.7270/Q2FB556R |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50361605
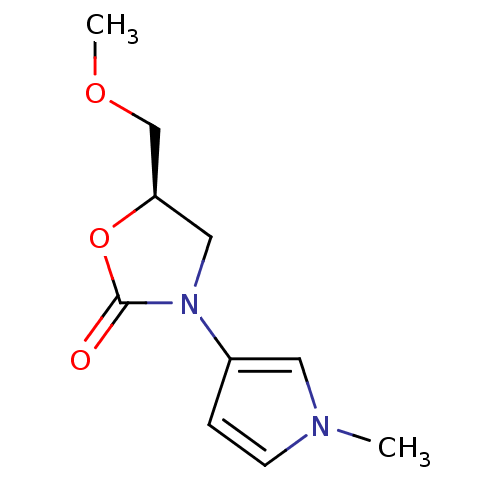
(CHEMBL1939850)Show InChI InChI=1S/C10H14N2O3/c1-11-4-3-8(5-11)12-6-9(7-14-2)15-10(12)13/h3-5,9H,6-7H2,1-2H3/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOA using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50361603

(CHEMBL1939848)Show InChI InChI=1S/C10H14N2O3/c1-2-11-4-3-8(5-11)12-6-9(7-13)15-10(12)14/h3-5,9,13H,2,6-7H2,1H3/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOB using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50361604
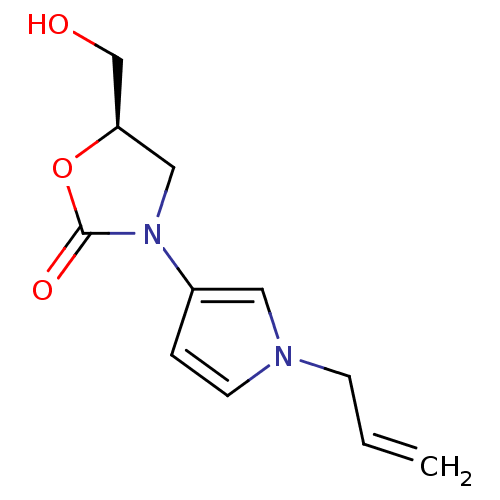
(CHEMBL1939849)Show InChI InChI=1S/C11H14N2O3/c1-2-4-12-5-3-9(6-12)13-7-10(8-14)16-11(13)15/h2-3,5-6,10,14H,1,4,7-8H2/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOA using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50110725
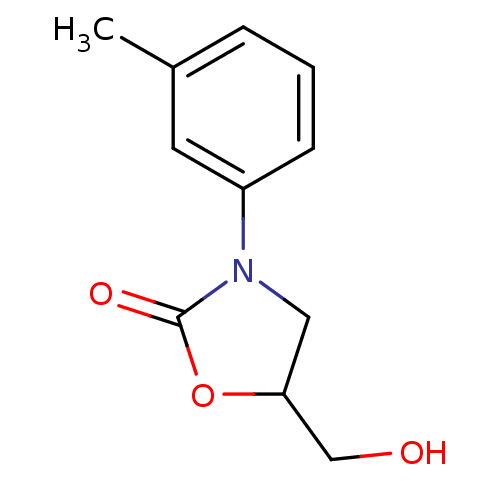
(5-(hydroxymethyl)-3-(3-methylphenyl)-oxazolidin-2-...)Show InChI InChI=1S/C11H13NO3/c1-8-3-2-4-9(5-8)12-6-10(7-13)15-11(12)14/h2-5,10,13H,6-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOA using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50507295
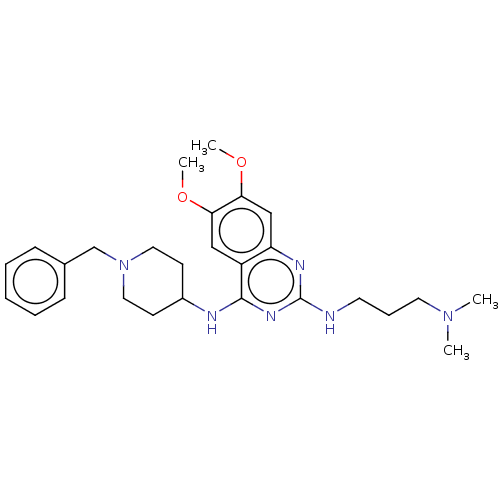
(CHEMBL1232432)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC Show InChI InChI=1S/C27H38N6O2/c1-32(2)14-8-13-28-27-30-23-18-25(35-4)24(34-3)17-22(23)26(31-27)29-21-11-15-33(16-12-21)19-20-9-6-5-7-10-20/h5-7,9-10,17-18,21H,8,11-16,19H2,1-4H3,(H2,28,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594087
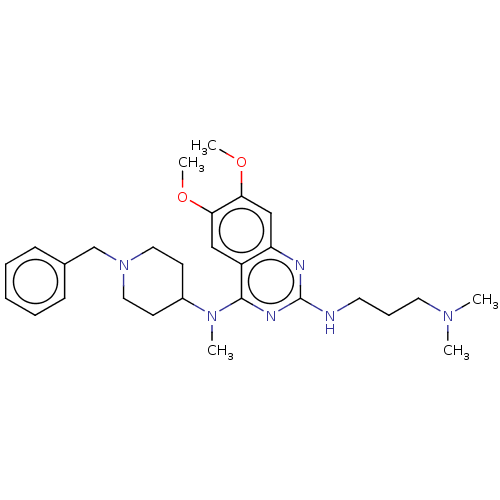
(CHEMBL5189687)Show SMILES COc1cc2nc(NCCCN(C)C)nc(N(C)C3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 569 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50507295

(CHEMBL1232432)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC Show InChI InChI=1S/C27H38N6O2/c1-32(2)14-8-13-28-27-30-23-18-25(35-4)24(34-3)17-22(23)26(31-27)29-21-11-15-33(16-12-21)19-20-9-6-5-7-10-20/h5-7,9-10,17-18,21H,8,11-16,19H2,1-4H3,(H2,28,29,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594083

(CHEMBL5179091)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(CCCc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50361613

(CHEMBL1939857)Show InChI InChI=1S/C11H15N3O2/c1-2-4-13-5-3-9(7-13)14-8-10(6-12)16-11(14)15/h2-3,5,7,10H,1,4,6,8,12H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOA using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50361602
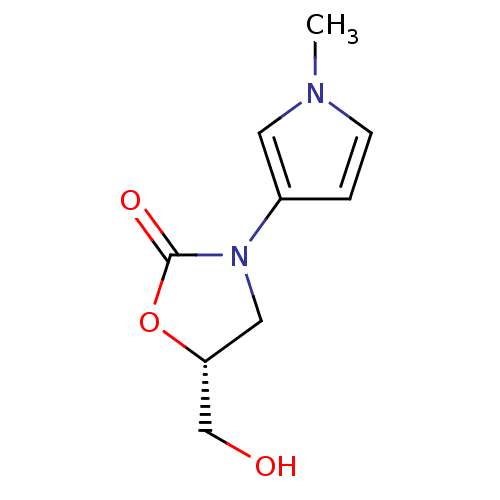
(CHEMBL1939847)Show InChI InChI=1S/C9H12N2O3/c1-10-3-2-7(4-10)11-5-8(6-12)14-9(11)13/h2-4,8,12H,5-6H2,1H3/t8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOA using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594086

(CHEMBL5170197)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(Cc4ccc(cc4)-c4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50361606

(CHEMBL1939851)Show InChI InChI=1S/C11H16N2O3/c1-3-12-5-4-9(6-12)13-7-10(8-15-2)16-11(13)14/h4-6,10H,3,7-8H2,1-2H3/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOB using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50361616

(CHEMBL1939860)Show InChI InChI=1S/C12H17N3O2/c1-3-5-14-6-4-10(8-14)15-9-11(7-13-2)17-12(15)16/h3-4,6,8,11,13H,1,5,7,9H2,2H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain MAOB using kynuramine as a substrate after 30 mins by spectrofluorometric method |
J Med Chem 54: 8228-32 (2011)
Article DOI: 10.1021/jm201011x
BindingDB Entry DOI: 10.7270/Q2GX4C1S |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594108
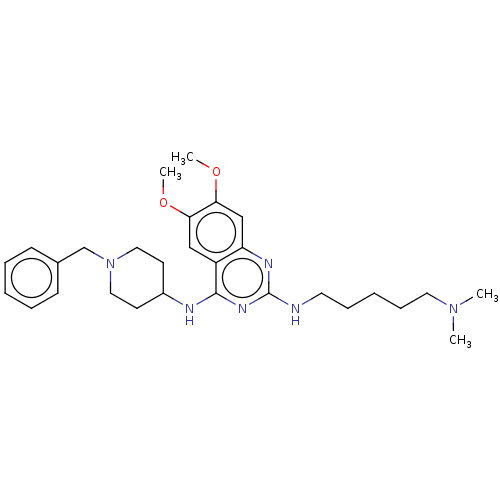
(CHEMBL5175660)Show SMILES COc1cc2nc(NCCCCCN(C)C)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594085

(CHEMBL5178765)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(Cc4ccc5ccccc5c4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594084

(CHEMBL5204694)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(Cc4cccc5ccccc45)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594082
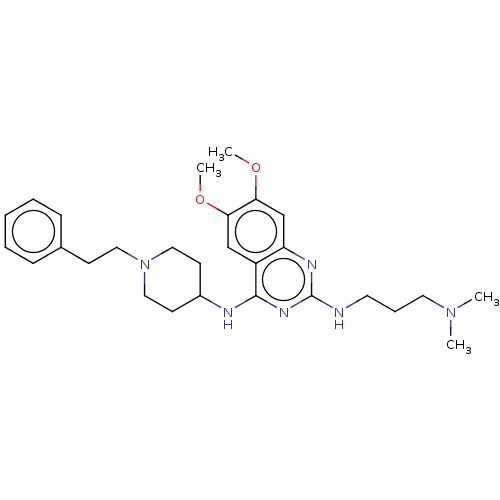
(CHEMBL5197685)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(CCc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594101

(CHEMBL5175171)Show SMILES COc1cc2nc(NCCCN3CCCCC3)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594099

(CHEMBL5186174)Show SMILES CCN(CC)CCCNc1nc(NC2CCN(Cc3ccccc3)CC2)c2cc(OC)c(OC)cc2n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data