

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Lactobacillus casei) | BDBM50022238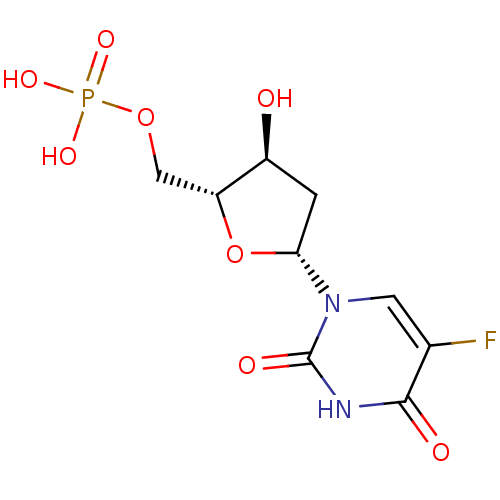 ((R)-5-Fluoro-1-((4S,5R)-4-hydroxy-5-methylphosphat...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010241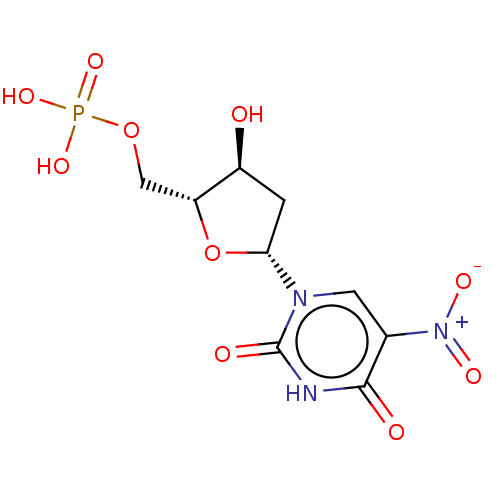 (CHEMBL1234672) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010236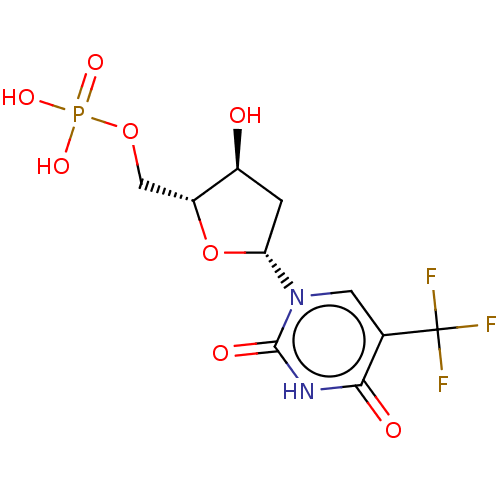 (CHEMBL3144200) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50000038 (CHEMBL3228321) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010239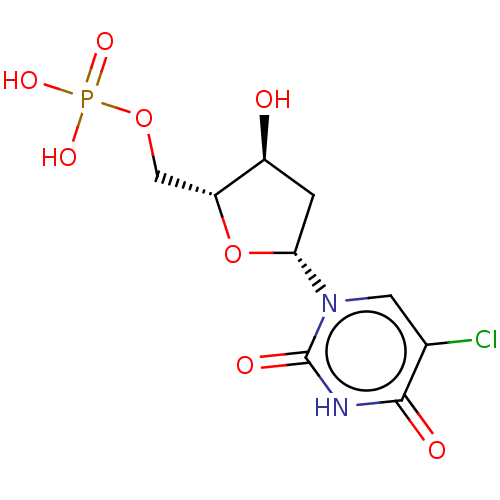 (CHEMBL1236538) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010240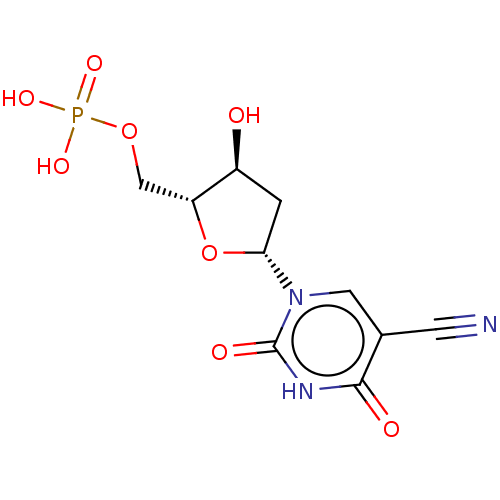 (CHEMBL3246102) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010238 (CHEMBL1160593) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50010242 (CHEMBL1160594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of thymidylate synthase purified from methotrexate-resistant Lactobacillus casei using 2'-deoxy[5-3H]uridine 5'-phosphate by r... | J Med Chem 22: 1137-9 (1979) BindingDB Entry DOI: 10.7270/Q2XK8H3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085553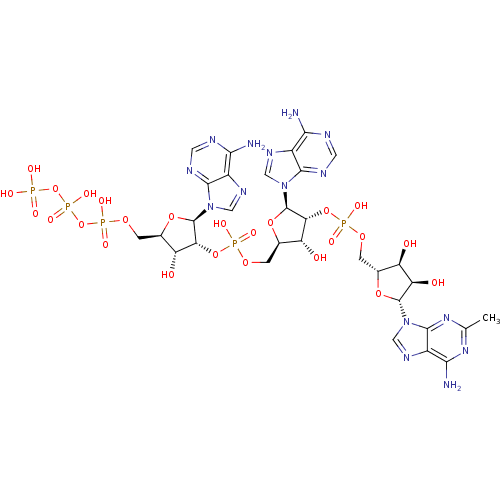 (CHEMBL214603 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Compound was tested for its binding ability, by displacement of p(A2'p)3A3'[32p]p5'Cp from recombinant human ribonuclease L | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Mus musculus) | BDBM50421415 (CHEMBL414948) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity to prevent binding of added ppp5'A2'p5'A2'pA2'p5'A3'[32P]p5' (c3 label) to RNase L in mouse L cells | J Med Chem 28: 1376-80 (1985) BindingDB Entry DOI: 10.7270/Q26W9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50421415 (CHEMBL414948) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity to prevent binding of added ppp5'A2'p5'A2'pA2'p5'A3'[32P]p5' (c3 label) to RNase L in human Daudi lymphoblastoid cells | J Med Chem 28: 1376-80 (1985) BindingDB Entry DOI: 10.7270/Q26W9BNH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 2-5A-dependent ribonuclease (Mus musculus) | BDBM50025002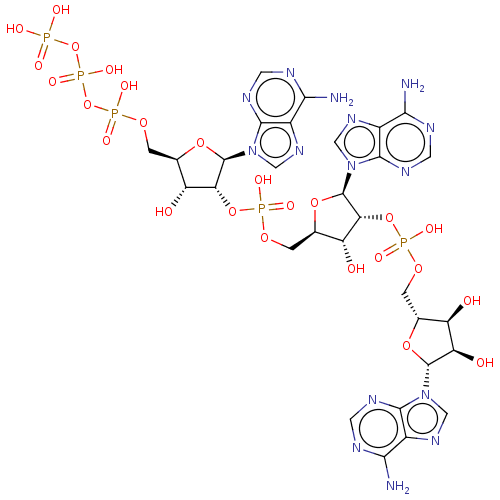 (CHEMBL404038 | ppp'A2'p5'A2'p5'A | ppp5'A2'p5'A2'p...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for activation of RNase L by measuring concentration required for 50% inhibition of protein synthesis in mouse L cell extracts | J Med Chem 29: 1015-22 (1986) BindingDB Entry DOI: 10.7270/Q2SX6C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085558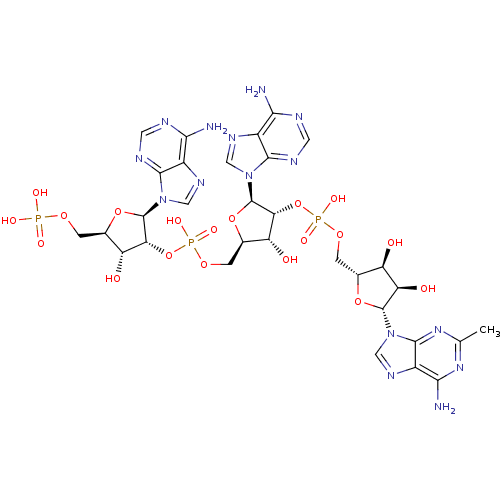 (CHEMBL402676 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Activation of purified recombinant human Ribonuclease L by the compound was measured as degradation of poly (U) 3'[32P]p5'C3'p | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085554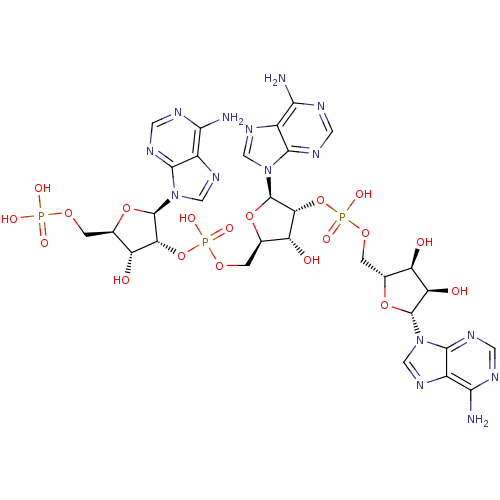 (5'-O-MONOPHOSPHORYLADENYLYL(2'->5')ADENYLYL(2'->5'...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Activation of purified recombinant human Ribonuclease L by the compound was measured as degradation of [32P]-pC11U2C7 | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50421415 (CHEMBL414948) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive antd Kidney Diseases Curated by ChEMBL | Assay Description Displacement of the probe bound to Ribonuclease L by compound in radiobinding assay was evaluated | Bioorg Med Chem Lett 10: 1357-60 (2000) BindingDB Entry DOI: 10.7270/Q2TT4Q67 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50421415 (CHEMBL414948) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity to prevent binding of added ppp5'A2'p5'A2'pA2'p5'A3'[32P]p5' (c3 label) to RNase L in rabbit reticulocytes | J Med Chem 28: 1376-80 (1985) BindingDB Entry DOI: 10.7270/Q26W9BNH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085556 (CHEMBL405496 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Compound was tested for its binding ability, by displacement of p(A2'p)3A3'[32p]p5'Cp from recombinant human ribonuclease L | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Mus musculus) | BDBM50025005 (CHEMBL3143469 | pp5'(br8A)2'p5'(br8A)2'p5'(br8A)) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for activation of RNase L by measuring concentration required for 50% inhibition of protein synthesis in mouse L cell extracts | J Med Chem 29: 1015-22 (1986) BindingDB Entry DOI: 10.7270/Q2SX6C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085555 (CHEMBL384725 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Activation of purified recombinant human Ribonuclease L by the compound was measured as degradation of poly (U) 3'[32P]p5'C3'p | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085559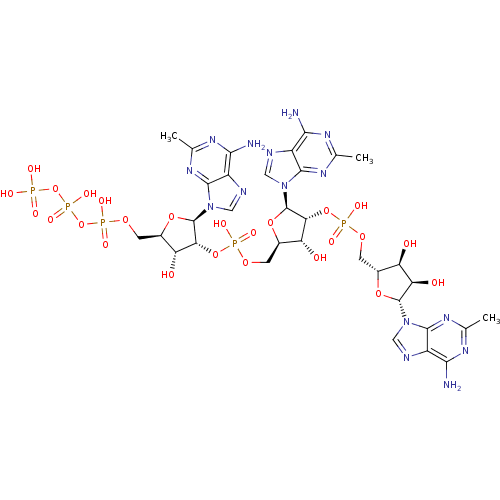 (CHEMBL410213 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Compound was tested for its binding ability, by displacement of p(A2'p)3A3'[32p]p5'Cp from recombinant human ribonuclease L | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Mus musculus) | BDBM50025001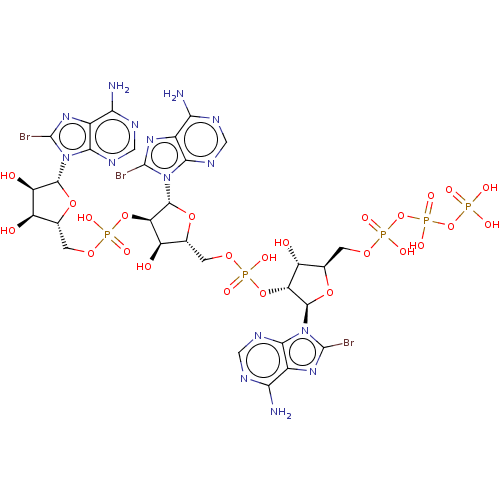 (CHEMBL3143411 | pp5'(br8A)2'p5'(br8A)2'p5'(br8A)2'...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for activation of RNase L by measuring concentration required for 50% inhibition of protein synthesis in mouse L cell extracts | J Med Chem 29: 1015-22 (1986) BindingDB Entry DOI: 10.7270/Q2SX6C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50021104 ((2'-5'(pAris)3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity to prevent binding of added ppp5'A2'p5'A2'pA2'p5'A3'[32P]p5' (c3 label) to RNase L in human Daudi lymphoblastoid cells | J Med Chem 28: 1376-80 (1985) BindingDB Entry DOI: 10.7270/Q26W9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Mus musculus) | BDBM50025000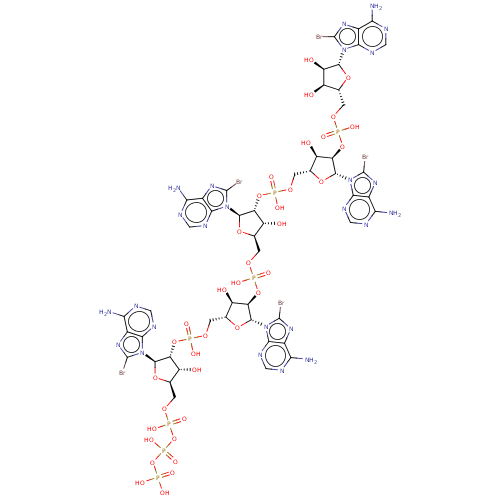 (CHEMBL3143409 | ppp5'(br8A)2'p5'(br8A)2'p5'(br8A)) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for activation of RNase L by measuring concentration required for 50% inhibition of protein synthesis in mouse L cell extracts | J Med Chem 29: 1015-22 (1986) BindingDB Entry DOI: 10.7270/Q2SX6C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Mus musculus) | BDBM50021104 ((2'-5'(pAris)3) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity to prevent binding of added ppp5'A2'p5'A2'pA2'p5'A3'[32P]p5' (c3 label) to RNase L in mouse L cells | J Med Chem 28: 1376-80 (1985) BindingDB Entry DOI: 10.7270/Q26W9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Mus musculus) | BDBM50024997 (CHEMBL3143462 | p5'(br8A)2'p5'(br8A)2'p5'(br8A)) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for activation of RNase L by measuring concentration required for 50% inhibition of protein synthesis in mouse L cell extracts | J Med Chem 29: 1015-22 (1986) BindingDB Entry DOI: 10.7270/Q2SX6C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Mus musculus) | BDBM50025003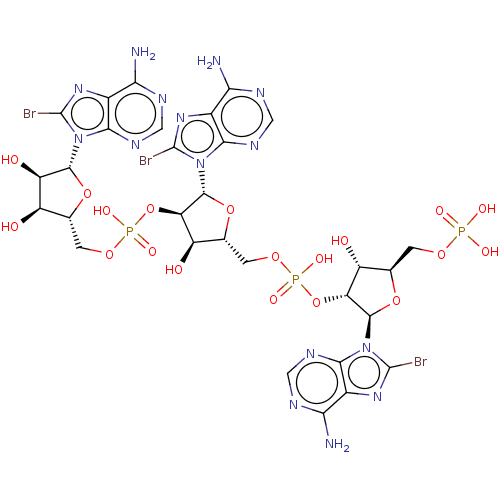 ((br8A)2'p5'(br8A)2'p5'(br8A) | CHEMBL3143413) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description compound was tested for activation of RNase L by measuring concentration required for 50% inhibition of protein synthesis in mouse L cell extracts | J Med Chem 29: 1015-22 (1986) BindingDB Entry DOI: 10.7270/Q2SX6C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085557 (CHEMBL407239 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Compound was tested for its binding ability, by displacement of p(A2'p)3A3'[32p]p5'Cp from recombinant human ribonuclease L | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50021104 ((2'-5'(pAris)3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity to prevent binding of added ppp5'A2'p5'A2'pA2'p5'A3'[32P]p5' (c3 label) to RNase L in rabbit reticulocytes | J Med Chem 28: 1376-80 (1985) BindingDB Entry DOI: 10.7270/Q26W9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Mus musculus) | BDBM50025006 ((br8A)2'p5'(br8A)2'p5'(br8A)2'p5'(br8A) | CHEMBL31...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description compound was tested for activation of RNase L by measuring concentration required for 50% inhibition of protein synthesis in mouse L cell extracts | J Med Chem 29: 1015-22 (1986) BindingDB Entry DOI: 10.7270/Q2SX6C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Mus musculus) | BDBM50025004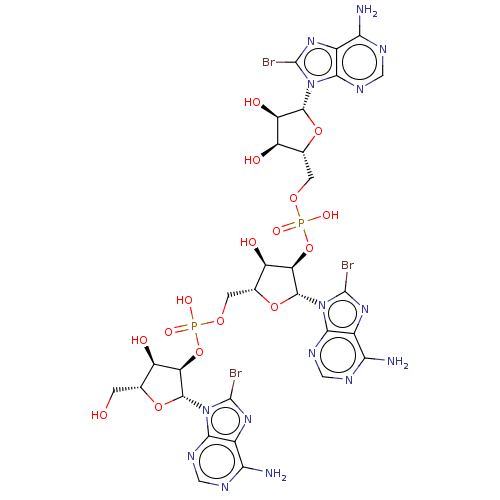 (CHEMBL3143467 | p5'(br8A)2'p5'(br8A)2'p5'(br8A)2'p...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description compound was tested for activation of RNase L by measuring concentration required for 50% inhibition of protein synthesis in mouse L cell extracts | J Med Chem 29: 1015-22 (1986) BindingDB Entry DOI: 10.7270/Q2SX6C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Mus musculus) | BDBM50024999 (CHEMBL414948 | Sulfuric acid mono-{5-(6-amino-puri...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for activation of RNase L by measuring concentration required for 50% inhibition of protein synthesis in mouse L cell extracts | J Med Chem 29: 1015-22 (1986) BindingDB Entry DOI: 10.7270/Q2SX6C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Mus musculus) | BDBM50024998 (CHEMBL3143412 | p5'(br8A)2'p5'(br8A)2'p5'(br8A)2'p...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description compound was tested for activation of RNase L by measuring concentration required for 50% inhibition of protein synthesis in mouse L cell extracts | J Med Chem 29: 1015-22 (1986) BindingDB Entry DOI: 10.7270/Q2SX6C6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085558 (CHEMBL402676 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Activation of purified recombinant human Ribonuclease L by the compound was measured as degradation of [32P]-pC11U2C7 | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085555 (CHEMBL384725 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Compound was tested for its binding ability, by displacement of p(A2'p)3A3'[32p]p5'Cp from recombinant human ribonuclease L | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085554 (5'-O-MONOPHOSPHORYLADENYLYL(2'->5')ADENYLYL(2'->5'...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB PubMed | n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Activation of purified recombinant human Ribonuclease L by the compound was measured as degradation of poly (U) 3'[32P]p5'C3'p | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085559 (CHEMBL410213 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Compound was tested for its binding ability, by displacement of p(A2'p)3A3'[32p]p5'Cp from recombinant human ribonuclease L | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085556 (CHEMBL405496 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Compound was tested for its binding ability, by displacement of p(A2'p)3A3'[32p]p5'Cp from recombinant human ribonuclease L | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085559 (CHEMBL410213 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Activation of purified recombinant human Ribonuclease L by the compound was measured as degradation of [32P]-pC11U2C7 | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085555 (CHEMBL384725 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Activation of purified recombinant human Ribonuclease L by the compound was measured as degradation of [32P]-pC11U2C7 | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085556 (CHEMBL405496 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Activation of purified recombinant human Ribonuclease L by the compound was measured as degradation of [32P]-pC11U2C7 | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085557 (CHEMBL407239 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Activation of purified recombinant human Ribonuclease L by the compound was measured as degradation of poly (U) 3'[32P]p5'C3'p | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085554 (5'-O-MONOPHOSPHORYLADENYLYL(2'->5')ADENYLYL(2'->5'...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Activation of purified recombinant human Ribonuclease L by the compound was measured as degradation of [32P]-pC11U2C7 | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085557 (CHEMBL407239 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Activation of purified recombinant human Ribonuclease L by the compound was measured as degradation of [32P]-pC11U2C7 | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50421415 (CHEMBL414948) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive antd Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity of compound to human recombinant Ribonuclease L was evaluated | Bioorg Med Chem Lett 10: 1357-60 (2000) BindingDB Entry DOI: 10.7270/Q2TT4Q67 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085553 (CHEMBL214603 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Activation of purified recombinant human Ribonuclease L by the compound was measured as degradation of poly (U) 3'[32P]p5'C3'p | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085558 (CHEMBL402676 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Activation of purified recombinant human Ribonuclease L by the compound was measured as degradation of poly (U) 3'[32P]p5'C3'p | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-5A-dependent ribonuclease (Homo sapiens (Human)) | BDBM50085553 (CHEMBL214603 | Oligoadenylate analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Activation of purified recombinant human Ribonuclease L by the compound was measured as degradation of poly (U) 3'[32P]p5'C3'p | Bioorg Med Chem Lett 10: 329-31 (2000) BindingDB Entry DOI: 10.7270/Q2TM79B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||