

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075813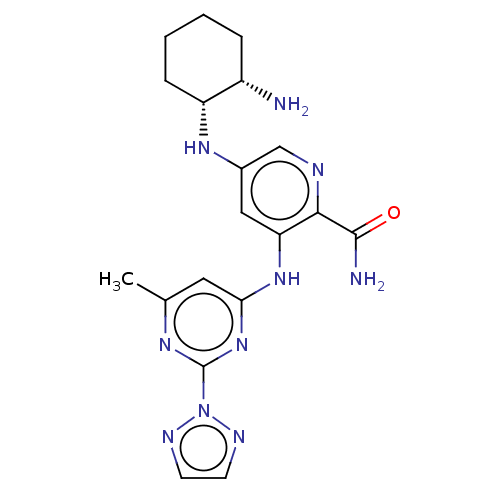 (CHEMBL3415598) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075735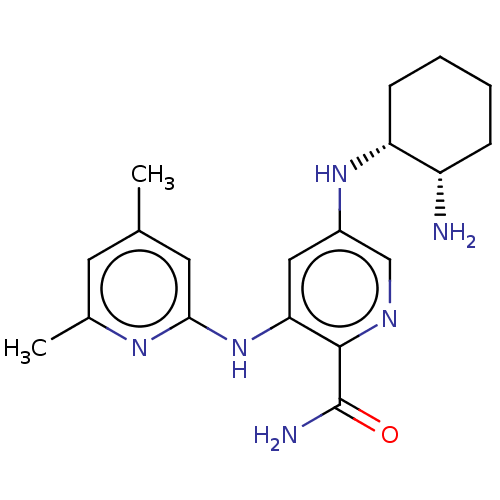 (CHEMBL3415583) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075744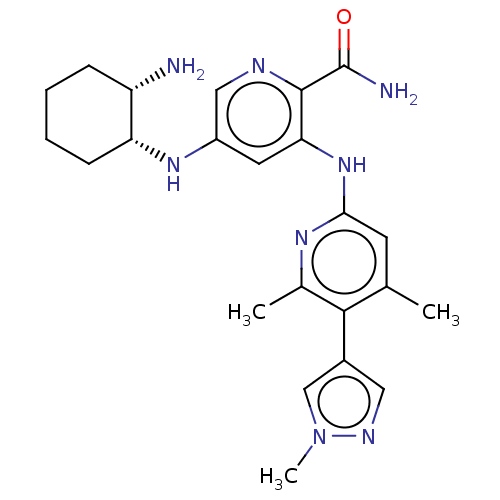 (CHEMBL3415594) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075740 (CHEMBL3415589) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075922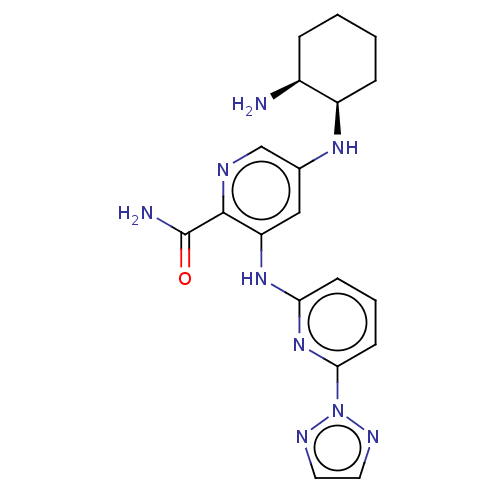 (CHEMBL3415606) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075747 (CHEMBL3415597) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50104550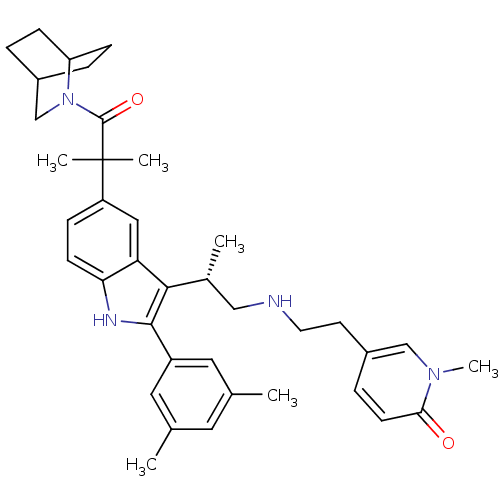 (5-(2-{(S)-2-[5-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition towards human pituitary gonadotropin-releasing hormone receptor using [125I]-buserelin. | Bioorg Med Chem Lett 11: 2597-602 (2001) BindingDB Entry DOI: 10.7270/Q2NV9HKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110617 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-[3-[(S)-2-(2-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110618 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-(2-(3,5-dimethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110623 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-[3-{(S)-2-[2-(1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110626 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-[3-{(S)-2-[2-(1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110618 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-(2-(3,5-dimethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50104554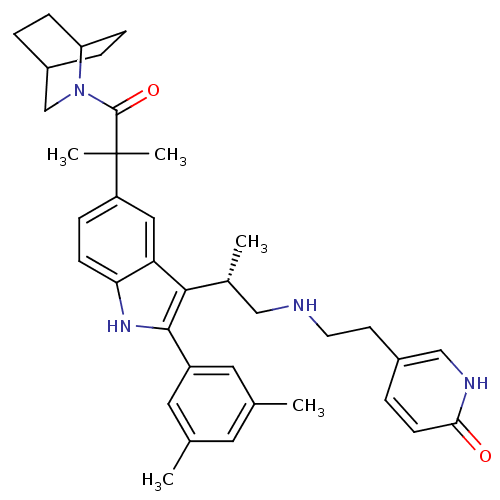 (5-(2-{(S)-2-[5-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro functional antagonism via inhibition of GnRH-stimulated phosphatidylinositol (PI) hydrolysis in cloned Chinese hamster ovary (CHO) cells sta... | Bioorg Med Chem Lett 11: 2597-602 (2001) BindingDB Entry DOI: 10.7270/Q2NV9HKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50120666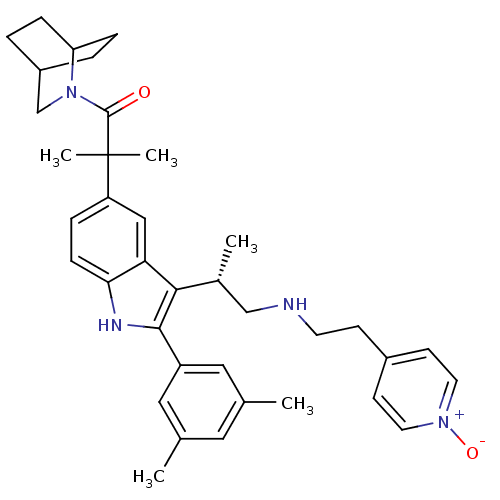 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-(2-(3,5-dimethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for the inhibition of I-125 labeled buserelin binding to the human Gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 3329-32 (2002) BindingDB Entry DOI: 10.7270/Q2668CHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50120658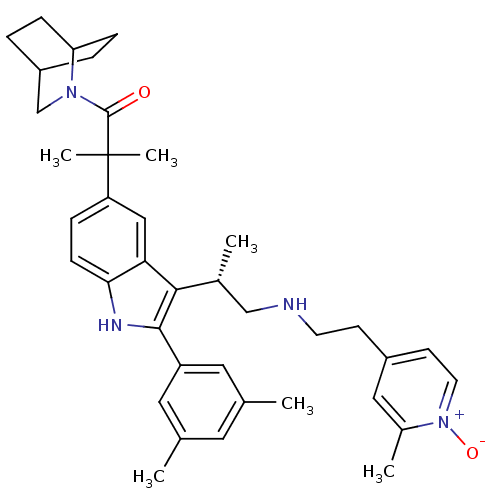 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-(2-(3,5-dimethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for the inhibition of I-125 labeled buserelin binding to the human Gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 3329-32 (2002) BindingDB Entry DOI: 10.7270/Q2668CHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50120662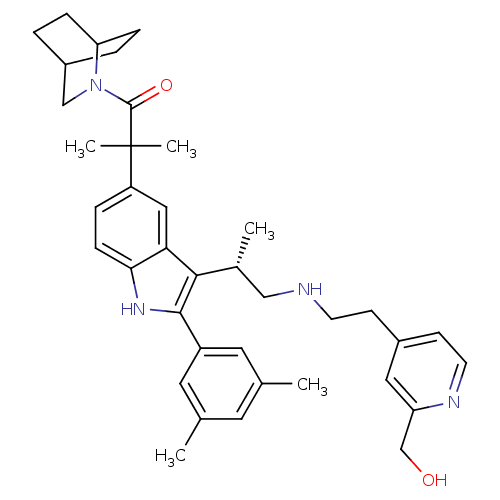 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-(2-(3,5-dimethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for the inhibition of phosphatidyl inositol hydrolysis in chinese hamster ovary cells expressing human GnRH receptor (Gonadotr... | Bioorg Med Chem Lett 12: 3329-32 (2002) BindingDB Entry DOI: 10.7270/Q2668CHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50120667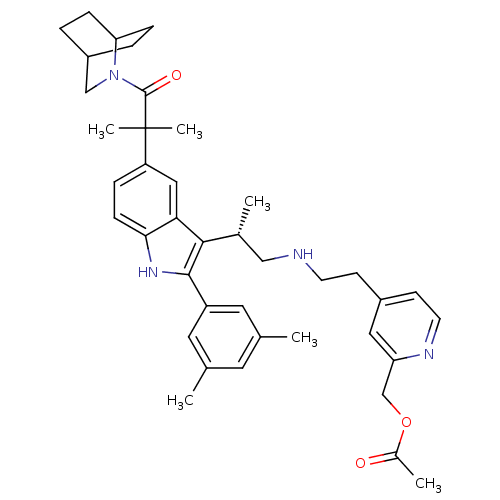 (Acetic acid 4-(2-{(S)-2-[5-[2-(2-aza-bicyclo[2.2.2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for the inhibition of phosphatidyl inositol hydrolysis in chinese hamster ovary cells expressing human GnRH receptor (Gonadotr... | Bioorg Med Chem Lett 12: 3329-32 (2002) BindingDB Entry DOI: 10.7270/Q2668CHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50120665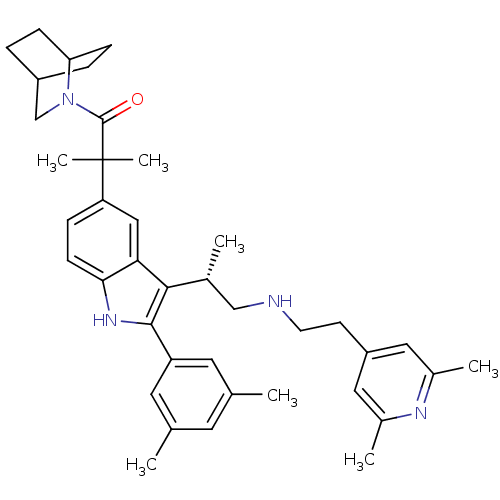 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-(2-(3,5-dimethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for the inhibition of I-125 labeled buserelin binding to the human Gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 3329-32 (2002) BindingDB Entry DOI: 10.7270/Q2668CHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50104549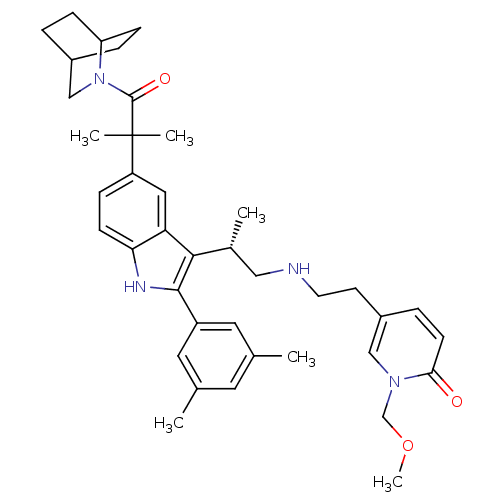 (5-(2-{(S)-2-[5-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition towards human pituitary gonadotropin-releasing hormone receptor using [125I]-buserelin. | Bioorg Med Chem Lett 11: 2597-602 (2001) BindingDB Entry DOI: 10.7270/Q2NV9HKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110597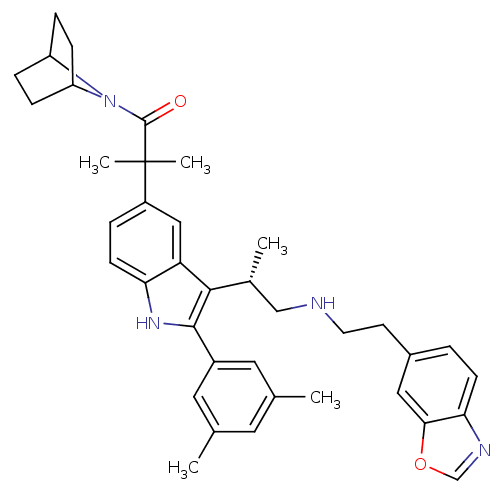 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-[3-[(S)-2-(2-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110627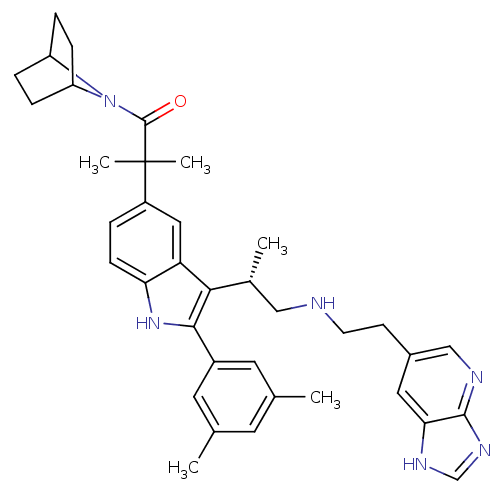 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-(2-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110599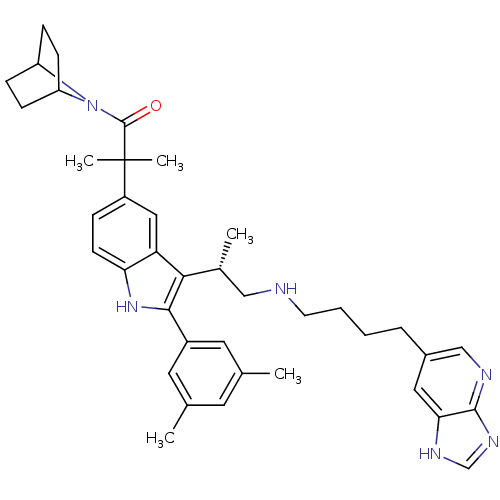 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-(2-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110600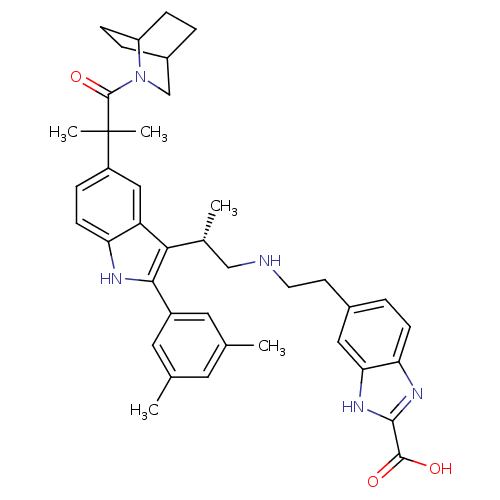 (5-(2-{(S)-2-[5-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gonadotropin-releasing hormone receptor-stimulated [3H]-inositol phosphate hydrolysis | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110627 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-(2-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gonadotropin-releasing hormone receptor-stimulated [3H]-inositol phosphate hydrolysis | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50104551 (5-(2-{(S)-2-[5-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition towards human pituitary gonadotropin-releasing hormone receptor using [125I]-buserelin. | Bioorg Med Chem Lett 11: 2597-602 (2001) BindingDB Entry DOI: 10.7270/Q2NV9HKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50104564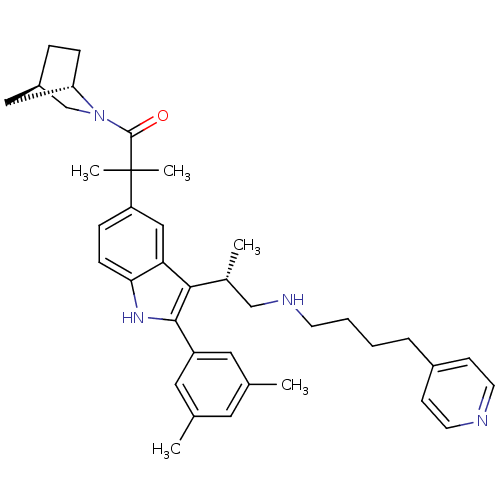 (1-(1S,4R)-2-Aza-bicyclo[2.2.1]hept-2-yl-2-{2-(3,5-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition towards human pituitary gonadotropin-releasing hormone receptor using [125I]-buserelin. | Bioorg Med Chem Lett 11: 2597-602 (2001) BindingDB Entry DOI: 10.7270/Q2NV9HKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110619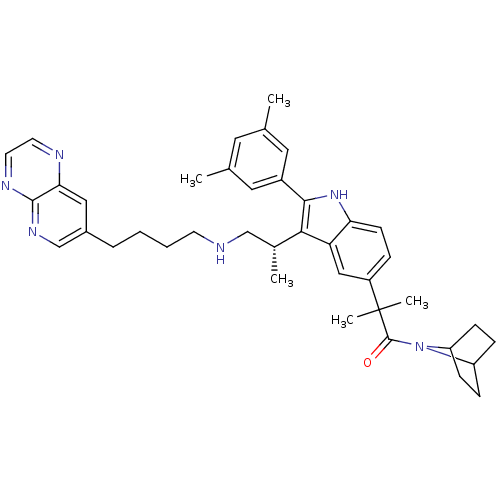 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-{2-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gonadotropin-releasing hormone receptor-stimulated [3H]-inositol phosphate hydrolysis | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110611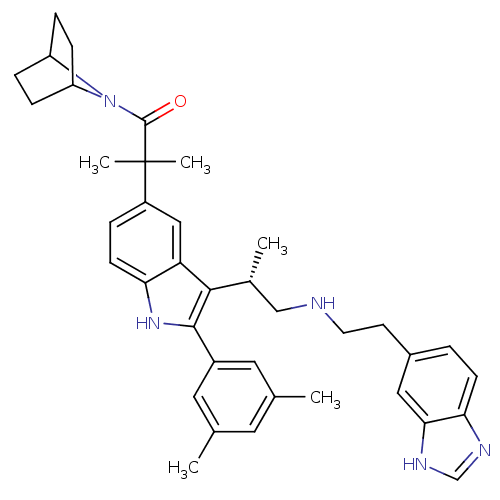 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-[3-{(S)-2-[2-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110613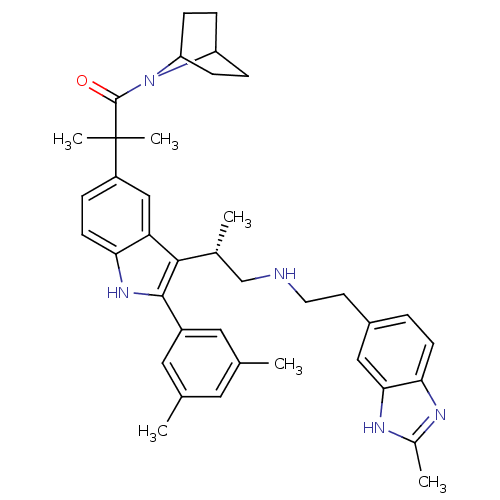 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-(2-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075819 (CHEMBL3415604) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075742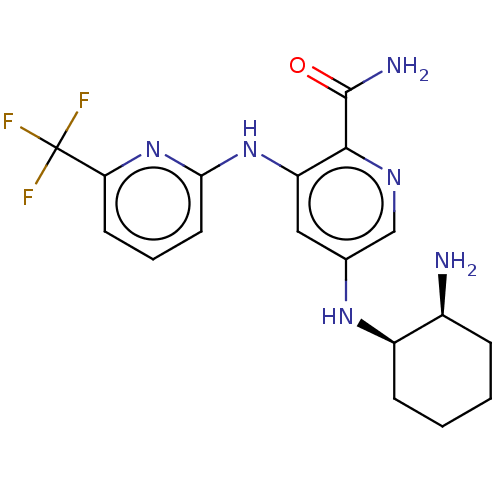 (CHEMBL3415592) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075732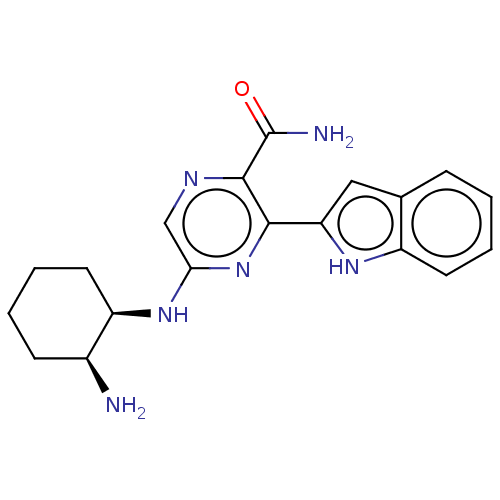 (CHEMBL3414584 | US9775839, 2.1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075738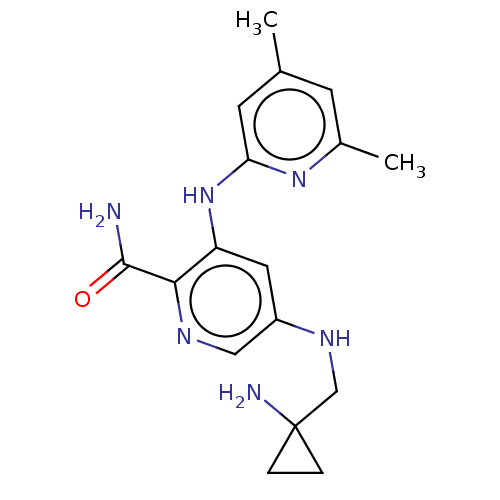 (CHEMBL3415587) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110614 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-[3-[(S)-2-(2-be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50120661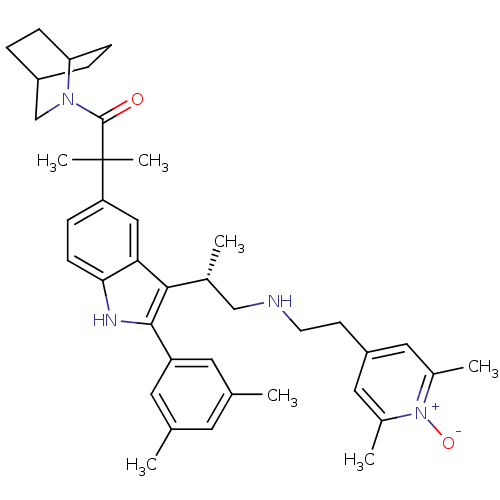 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-[3-{(S)-2-[2-(2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for the inhibition of I-125 labeled buserelin binding to the human Gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 3329-32 (2002) BindingDB Entry DOI: 10.7270/Q2668CHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50120664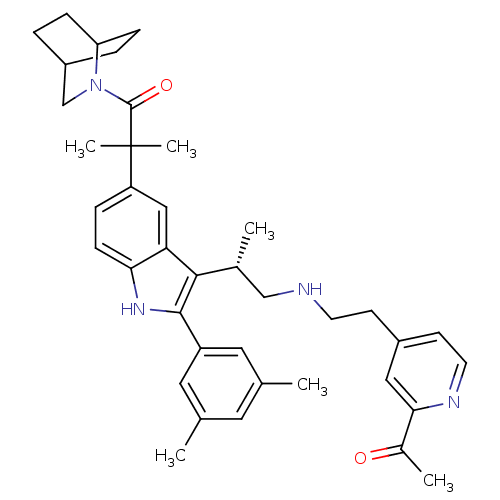 (2-[3-{(S)-2-[2-(2-Acetyl-pyridin-4-yl)-ethylamino]...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for the inhibition of I-125 labeled buserelin binding to the human Gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 3329-32 (2002) BindingDB Entry DOI: 10.7270/Q2668CHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50120657 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-[2-(3,5-dimethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for the inhibition of I-125 labeled buserelin binding to the human Gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 3329-32 (2002) BindingDB Entry DOI: 10.7270/Q2668CHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110596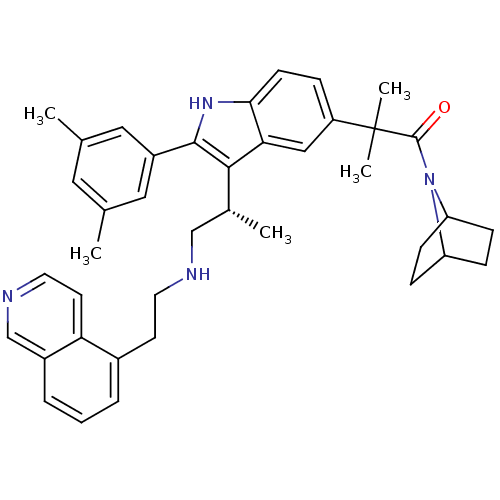 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-{2-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gonadotropin-releasing hormone receptor-stimulated [3H]-inositol phosphate hydrolysis | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075730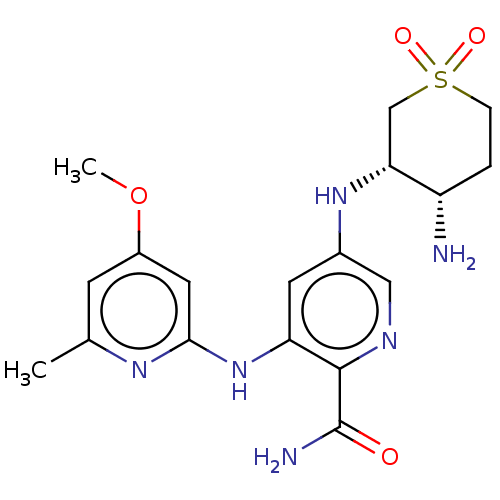 (CHEMBL3415599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110620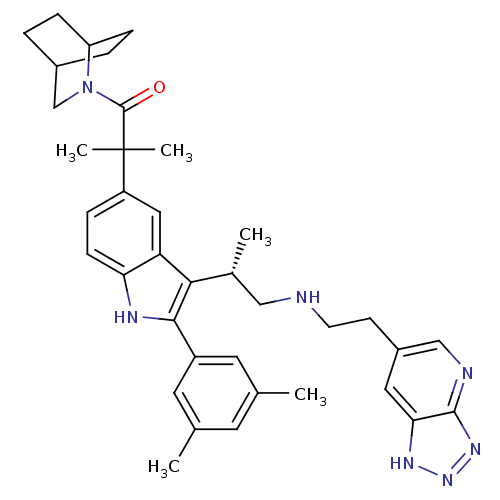 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-(2-(3,5-dimethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110593 (5-(2-{(S)-2-[5-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110594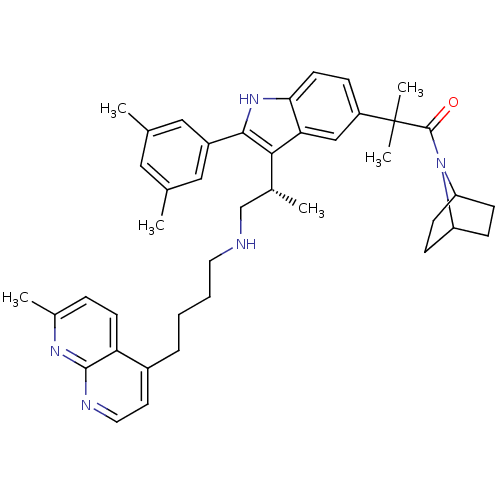 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-(2-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gonadotropin-releasing hormone receptor-stimulated [3H]-inositol phosphate hydrolysis | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110606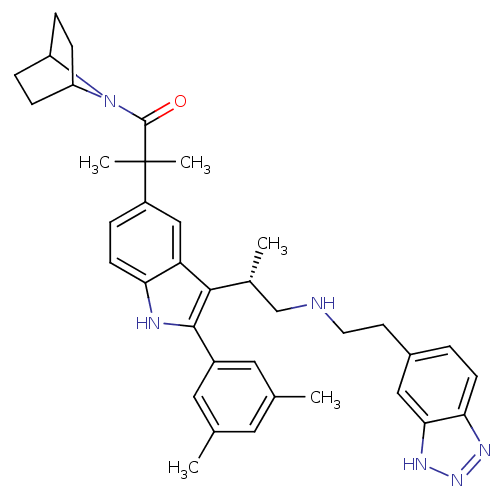 (1-(7-Aza-bicyclo[2.2.1]hept-7-yl)-2-[3-{(S)-2-[2-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gonadotropin-releasing hormone receptor-stimulated [3H]-inositol phosphate hydrolysis | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110593 (5-(2-{(S)-2-[5-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110595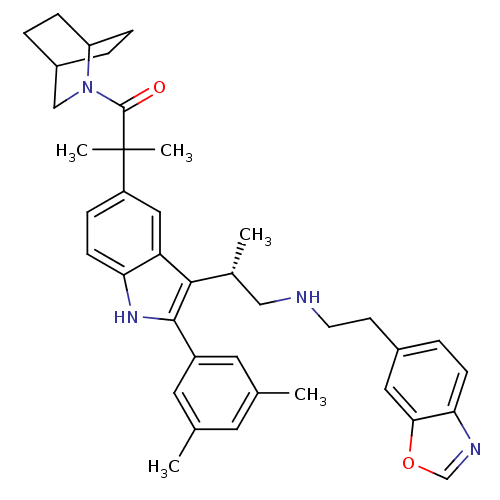 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-[3-[(S)-2-(2-be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-buserelin binding to human pituitary gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50104552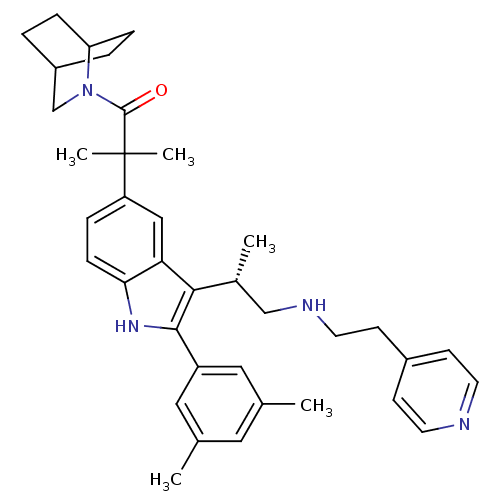 ((S)-1-(2-aza-bicyclo[2.2.2]octan-2-yl)-2-(2-(3,5-d...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition towards human pituitary gonadotropin-releasing hormone receptor using [125I]-buserelin. | Bioorg Med Chem Lett 11: 2597-602 (2001) BindingDB Entry DOI: 10.7270/Q2NV9HKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50104540 (5-(2-{(S)-2-[5-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition towards human pituitary gonadotropin-releasing hormone receptor using [125I]-buserelin. | Bioorg Med Chem Lett 11: 2597-602 (2001) BindingDB Entry DOI: 10.7270/Q2NV9HKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50104542 (1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-{2-(3,5-dimethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro functional antagonism via inhibition of GnRH-stimulated phosphatidylinositol (PI) hydrolysis in cloned Chinese hamster ovary (CHO) cells sta... | Bioorg Med Chem Lett 11: 2597-602 (2001) BindingDB Entry DOI: 10.7270/Q2NV9HKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50104546 (2-{2-(3,5-Dimethyl-phenyl)-3-[(S)-1-methyl-2-(4-py...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition towards human pituitary gonadotropin-releasing hormone receptor using [125I]-buserelin. | Bioorg Med Chem Lett 11: 2597-602 (2001) BindingDB Entry DOI: 10.7270/Q2NV9HKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50104558 (1-(3,3-Dimethyl-azetidin-1-yl)-2-{2-(3,5-dimethyl-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition towards human pituitary gonadotropin-releasing hormone receptor using [125I]-buserelin. | Bioorg Med Chem Lett 11: 2597-602 (2001) BindingDB Entry DOI: 10.7270/Q2NV9HKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 432 total ) | Next | Last >> |