

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen receptor (Homo sapiens (Human)) | BDBM29321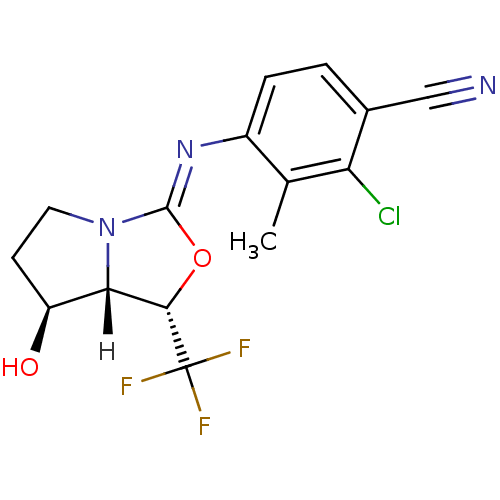 (oxazolidin-2-imine, 6d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | -54.8 | n/a | n/a | 19 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29319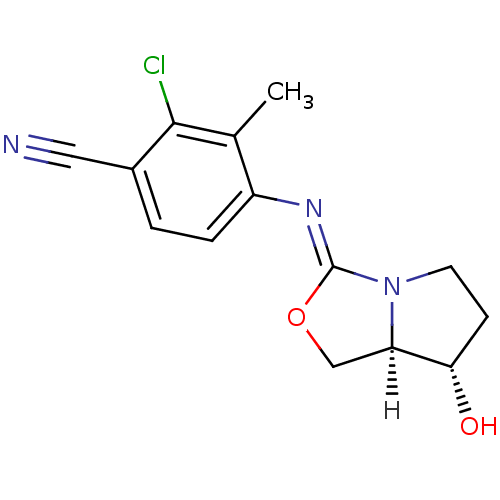 (oxazolidin-2-imine, 6b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | -53.8 | n/a | n/a | 14 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29320 (BMS-665139 | oxazolidin-2-imine, 6c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.300 | -53.8 | n/a | n/a | 0.200 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29323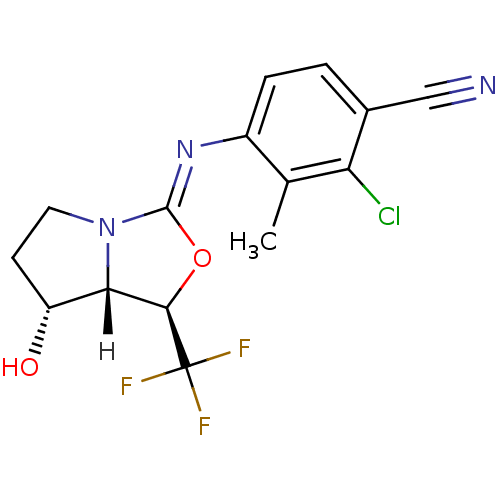 (oxazolidin-2-imine, 6f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | -53.8 | n/a | n/a | 1.40 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18188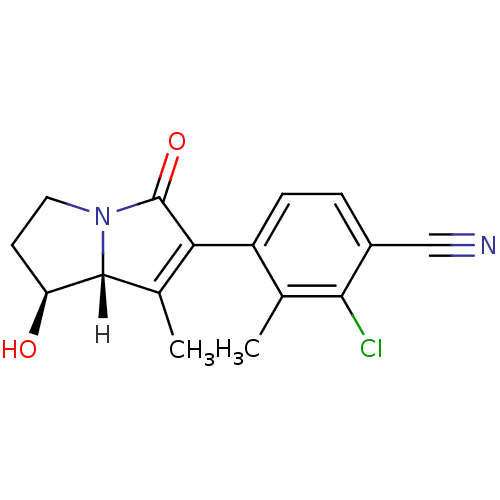 (4-[(1S,7aR)-1-hydroxy-7-methyl-5-oxo-2,3,5,7a-tetr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 0.5 | -52.6 | n/a | n/a | 2 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 50: 3015-3025 (2007) Article DOI: 10.1021/jm070312d BindingDB Entry DOI: 10.7270/Q24F1P1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29324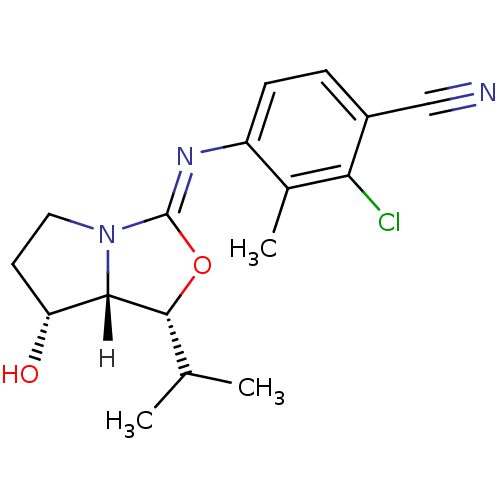 (oxazolidin-2-imine, 6g) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | -51.7 | n/a | n/a | 3.70 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18183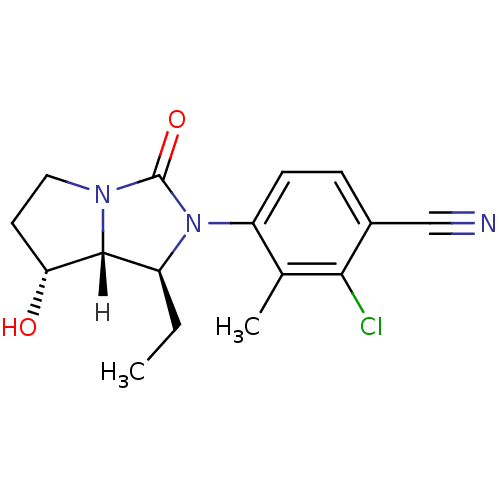 (4-[(1S,7R,7aR)-1-ethyl-7-hydroxy-3-oxo-hexahydro-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | -51.7 | n/a | n/a | 2.60 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 50: 3015-3025 (2007) Article DOI: 10.1021/jm070312d BindingDB Entry DOI: 10.7270/Q24F1P1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29318 (oxazolidin-2-imine, 6a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | -51.4 | n/a | n/a | 4.80 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18177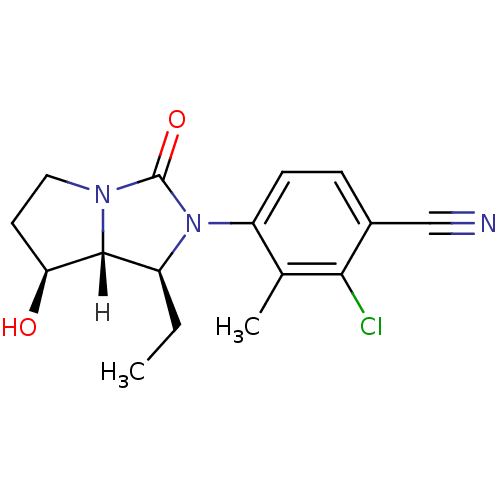 (4-[(1S,7S,7aR)-1-ethyl-7-hydroxy-3-oxo-hexahydro-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | -51.1 | n/a | n/a | 1.80 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 50: 3015-3025 (2007) Article DOI: 10.1021/jm070312d BindingDB Entry DOI: 10.7270/Q24F1P1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18178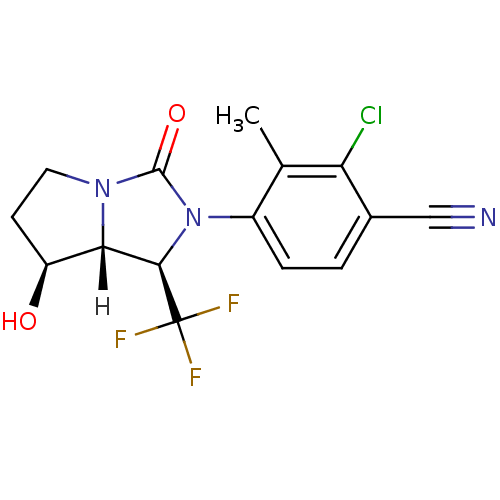 (4-[(1R,7S,7aR)-7-hydroxy-3-oxo-1-(trifluoromethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | -51.1 | n/a | n/a | 2.5 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 50: 3015-3025 (2007) Article DOI: 10.1021/jm070312d BindingDB Entry DOI: 10.7270/Q24F1P1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18184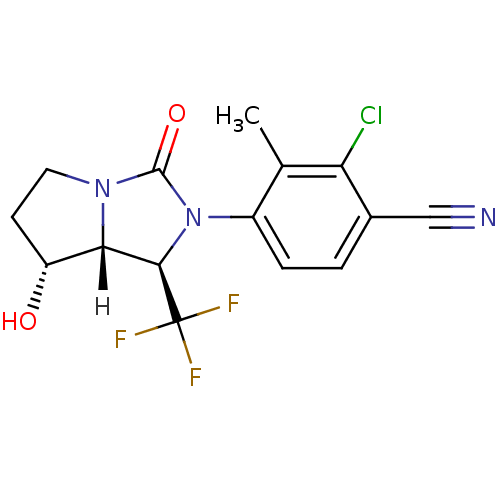 (4-[(1R,7R,7aR)-7-hydroxy-3-oxo-1-(trifluoromethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -50.9 | n/a | n/a | 2.90 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 50: 3015-3025 (2007) Article DOI: 10.1021/jm070312d BindingDB Entry DOI: 10.7270/Q24F1P1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18173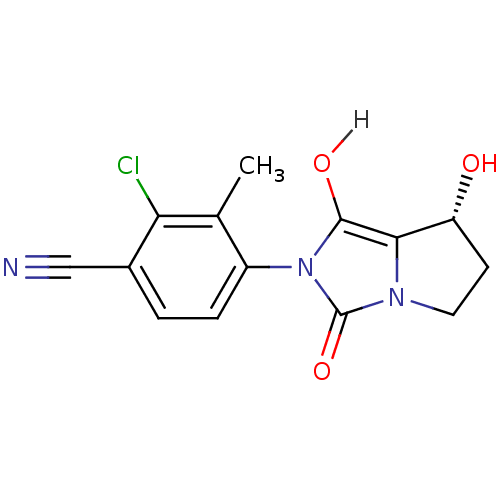 (4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.40 | -50.0 | n/a | n/a | 0.700 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 50: 3015-3025 (2007) Article DOI: 10.1021/jm070312d BindingDB Entry DOI: 10.7270/Q24F1P1F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18176 (4-[(1S,7S,7aR)-7-hydroxy-1-methyl-3-oxo-hexahydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | -49.9 | n/a | n/a | 5.20 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 50: 3015-3025 (2007) Article DOI: 10.1021/jm070312d BindingDB Entry DOI: 10.7270/Q24F1P1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18180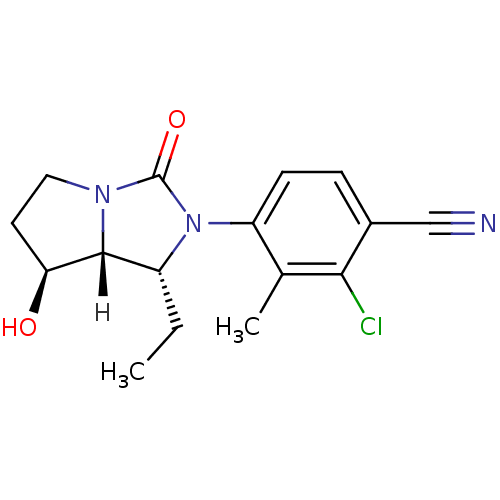 (4-[(1R,7S,7aR)-1-ethyl-7-hydroxy-3-oxo-hexahydro-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | -49.7 | n/a | n/a | 7.10 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 50: 3015-3025 (2007) Article DOI: 10.1021/jm070312d BindingDB Entry DOI: 10.7270/Q24F1P1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18176 (4-[(1S,7S,7aR)-7-hydroxy-1-methyl-3-oxo-hexahydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | -49.7 | n/a | n/a | 5.40 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29326 (guanidine derivative, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | -49.3 | n/a | n/a | 44 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18181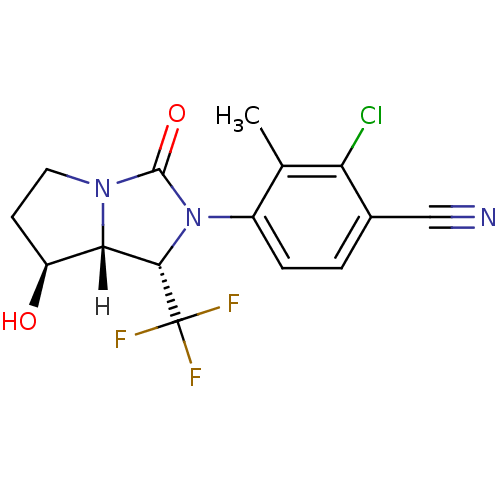 (4-[(1S,7S,7aR)-7-hydroxy-3-oxo-1-(trifluoromethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | -49.3 | n/a | n/a | 48 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 50: 3015-3025 (2007) Article DOI: 10.1021/jm070312d BindingDB Entry DOI: 10.7270/Q24F1P1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18174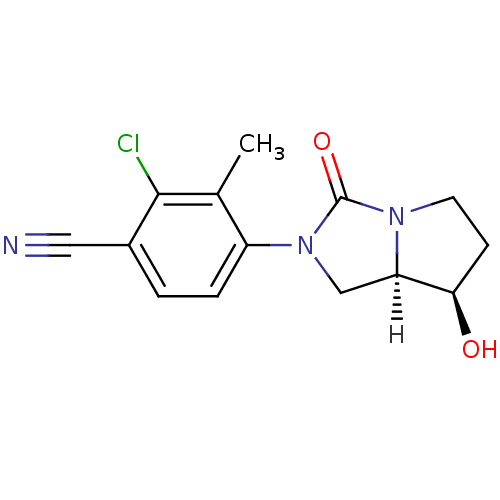 (4-[(7R,7aR)-7-hydroxy-3-oxo-hexahydro-1H-pyrrolo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | -49.2 | n/a | n/a | 15 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 50: 3015-3025 (2007) Article DOI: 10.1021/jm070312d BindingDB Entry DOI: 10.7270/Q24F1P1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18175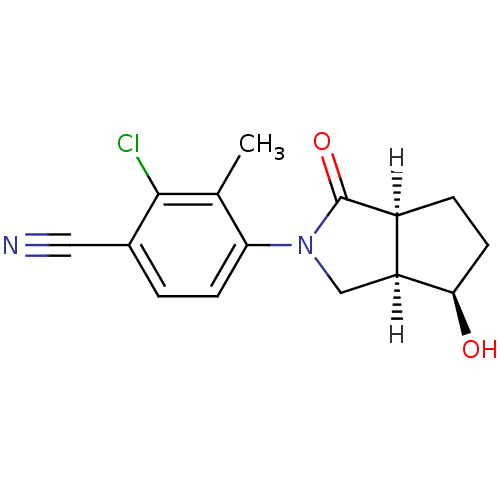 (4-[(3aR,4R,6aS)-4-hydroxy-1-oxo-octahydrocyclopent...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 2.20 | -48.9 | n/a | n/a | 20 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 50: 3015-3025 (2007) Article DOI: 10.1021/jm070312d BindingDB Entry DOI: 10.7270/Q24F1P1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18186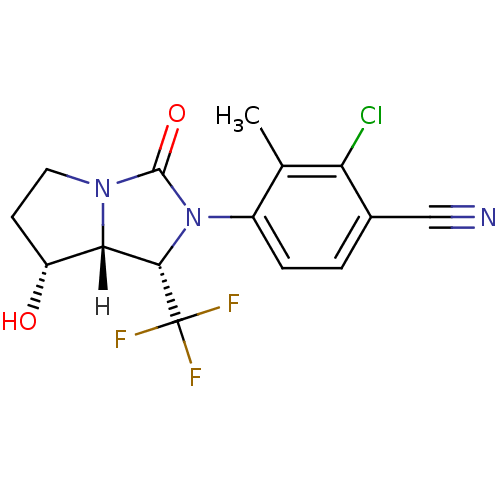 (4-[(1S,7R,7aR)-7-hydroxy-3-oxo-1-(trifluoromethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | -48.9 | n/a | n/a | 2.10 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 50: 3015-3025 (2007) Article DOI: 10.1021/jm070312d BindingDB Entry DOI: 10.7270/Q24F1P1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18185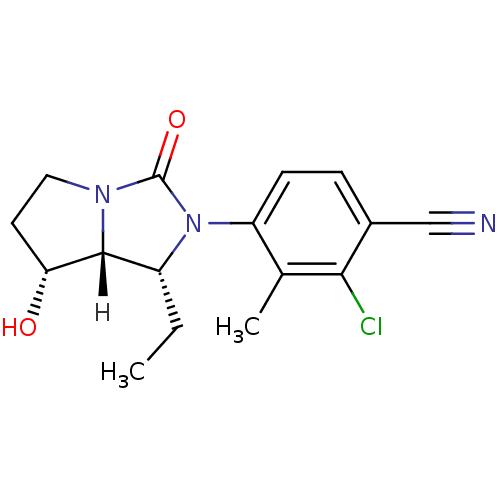 (4-[(1R,7R,7aR)-1-ethyl-7-hydroxy-3-oxo-hexahydro-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | -48.8 | n/a | n/a | 7.40 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 50: 3015-3025 (2007) Article DOI: 10.1021/jm070312d BindingDB Entry DOI: 10.7270/Q24F1P1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29322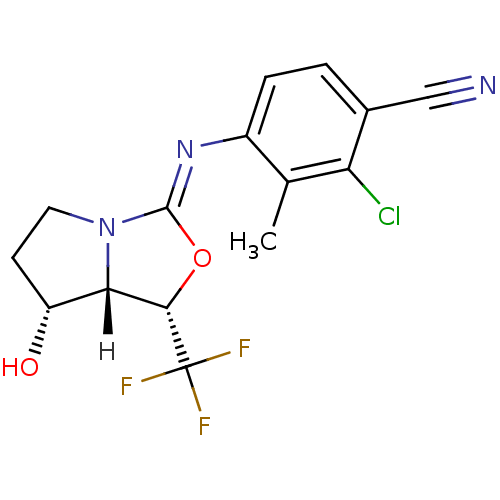 (oxazolidin-2-imine, 6e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | -48.8 | n/a | n/a | 1.10 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18182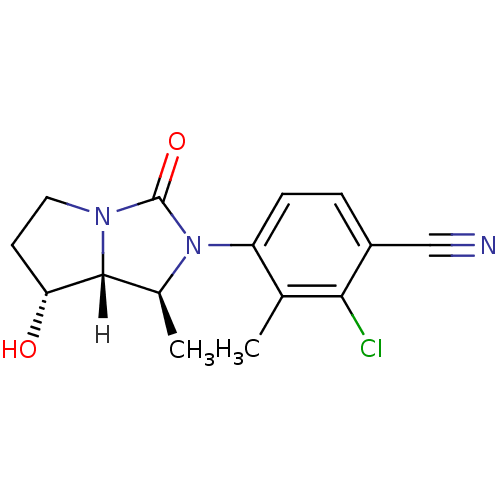 (4-[(1S,7R,7aR)-7-hydroxy-1-methyl-3-oxo-hexahydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | -48.2 | n/a | n/a | 13 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 50: 3015-3025 (2007) Article DOI: 10.1021/jm070312d BindingDB Entry DOI: 10.7270/Q24F1P1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18171 (4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3.20 | -48.0 | n/a | n/a | 2.30 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 50: 3015-3025 (2007) Article DOI: 10.1021/jm070312d BindingDB Entry DOI: 10.7270/Q24F1P1F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18179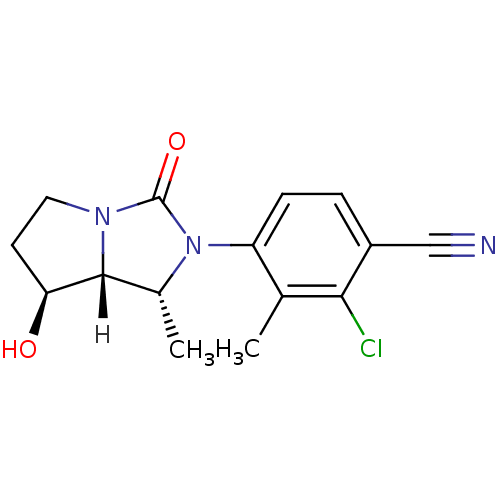 (4-[(1R,7S,7aR)-7-hydroxy-1-methyl-3-oxo-hexahydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | -47.5 | n/a | n/a | 87 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 50: 3015-3025 (2007) Article DOI: 10.1021/jm070312d BindingDB Entry DOI: 10.7270/Q24F1P1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18174 (4-[(7R,7aR)-7-hydroxy-3-oxo-hexahydro-1H-pyrrolo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | -46.5 | n/a | n/a | 6.40 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29325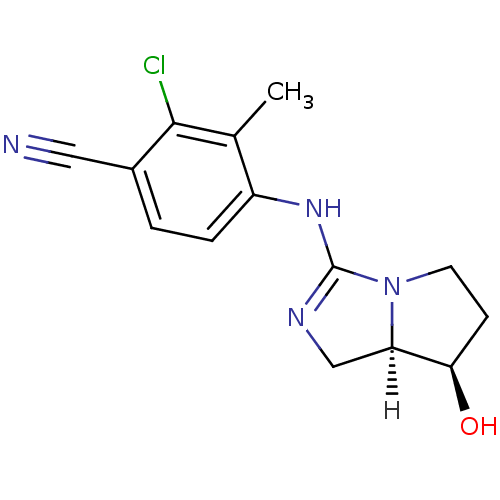 (guanidine derivative, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | -45.2 | n/a | n/a | 1.80E+3 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18173 (4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 14 | -44.4 | n/a | n/a | 7.80 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29328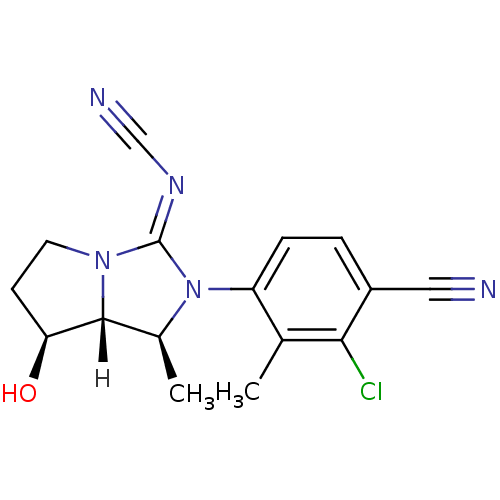 (cyanoguanidine, 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 17 | -43.9 | n/a | n/a | 1.11E+3 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29327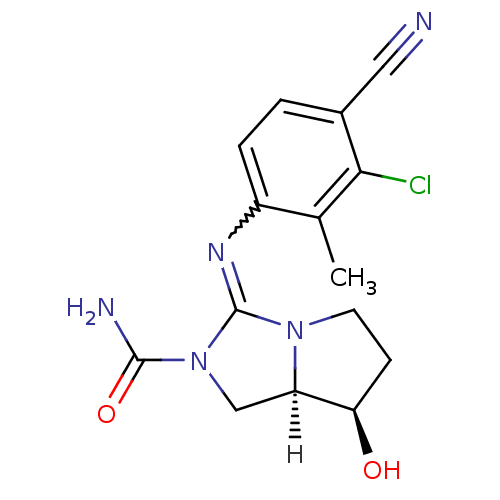 (guanidine derivative, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18187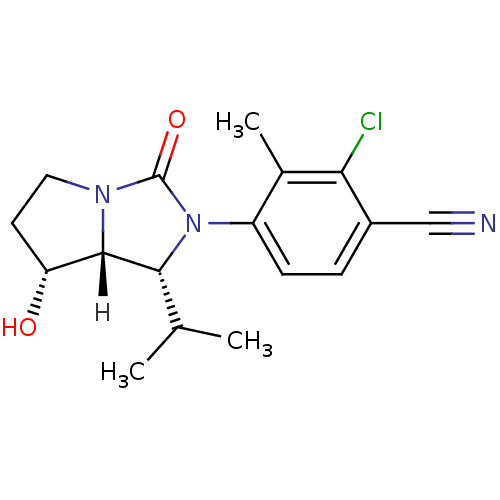 (4-[(1R,7R,7aR)-7-hydroxy-3-oxo-1-(propan-2-yl)-hex...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 23 | -43.2 | n/a | n/a | 108 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 50: 3015-3025 (2007) Article DOI: 10.1021/jm070312d BindingDB Entry DOI: 10.7270/Q24F1P1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50184807 ((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against VEGFR2 | J Med Chem 49: 2143-6 (2006) Article DOI: 10.1021/jm051106d BindingDB Entry DOI: 10.7270/Q2WW7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50184807 ((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against VEGFR2 | J Med Chem 49: 2143-6 (2006) Article DOI: 10.1021/jm051106d BindingDB Entry DOI: 10.7270/Q2WW7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Mus musculus) | BDBM50184807 ((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Flk1 | J Med Chem 49: 2143-6 (2006) Article DOI: 10.1021/jm051106d BindingDB Entry DOI: 10.7270/Q2WW7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM50184807 ((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against VEGFR1 | J Med Chem 49: 2143-6 (2006) Article DOI: 10.1021/jm051106d BindingDB Entry DOI: 10.7270/Q2WW7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50168389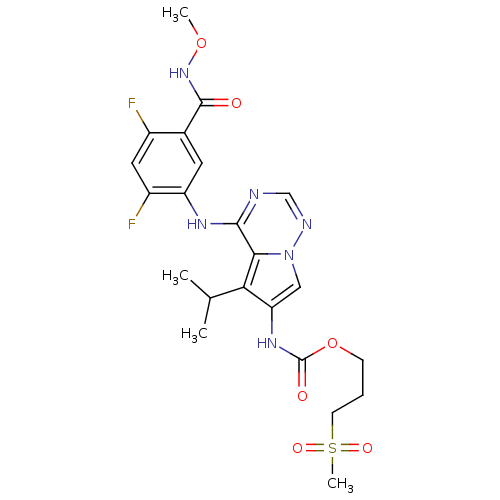 (CHEMBL195218 | [4-(2,4-Difluoro-5-methoxycarbamoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human vascular endothelial growth factor receptor 2 (VEGFR-2) | J Med Chem 48: 3991-4008 (2005) Article DOI: 10.1021/jm0501275 BindingDB Entry DOI: 10.7270/Q2KP81QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105327 (JNJ-26481585 | Quisinostat) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113332 BindingDB Entry DOI: 10.7270/Q2F76HH8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50105327 (JNJ-26481585 | Quisinostat) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113332 BindingDB Entry DOI: 10.7270/Q2F76HH8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50105327 (JNJ-26481585 | Quisinostat) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113332 BindingDB Entry DOI: 10.7270/Q2F76HH8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM50105327 (JNJ-26481585 | Quisinostat) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113332 BindingDB Entry DOI: 10.7270/Q2F76HH8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50105327 (JNJ-26481585 | Quisinostat) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113332 BindingDB Entry DOI: 10.7270/Q2F76HH8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1/2/3/4 (Homo sapiens (Human)) | BDBM50168394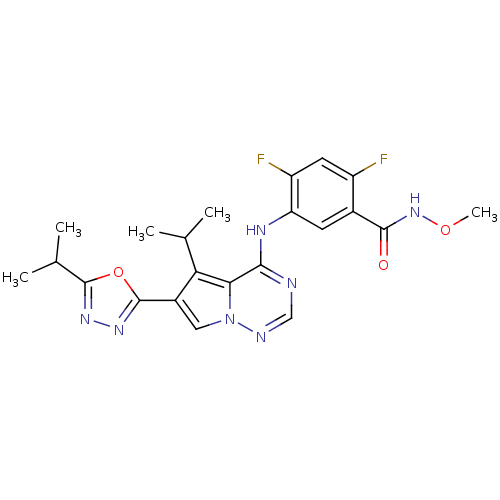 (2,4-Difluoro-5-[5-isopropyl-6-(5-isopropyl-[1,3,4]...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of FGF-stimulated human umbilical vein endothelial cell proliferation | J Med Chem 48: 3991-4008 (2005) Article DOI: 10.1021/jm0501275 BindingDB Entry DOI: 10.7270/Q2KP81QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1/2/3 (Homo sapiens (Human)) | BDBM50168397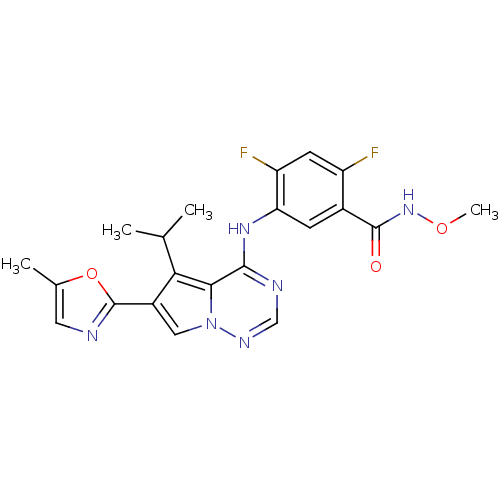 (2,4-Difluoro-5-[5-isopropyl-6-(5-methyl-oxazol-2-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of VEGF-stimulated human umbilical vein endothelial cell proliferation | J Med Chem 48: 3991-4008 (2005) Article DOI: 10.1021/jm0501275 BindingDB Entry DOI: 10.7270/Q2KP81QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50188961 (CHEMBL3622533) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113332 BindingDB Entry DOI: 10.7270/Q2F76HH8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50188961 (CHEMBL3622533) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113332 BindingDB Entry DOI: 10.7270/Q2F76HH8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1/2/3 (Homo sapiens (Human)) | BDBM50168410 (5-[6-(5-Ethyl-[1,3,4]oxadiazol-2-yl)-5-isopropyl-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of VEGF-stimulated human umbilical vein endothelial cell proliferation | J Med Chem 48: 3991-4008 (2005) Article DOI: 10.1021/jm0501275 BindingDB Entry DOI: 10.7270/Q2KP81QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1/2/3 (Homo sapiens (Human)) | BDBM50168398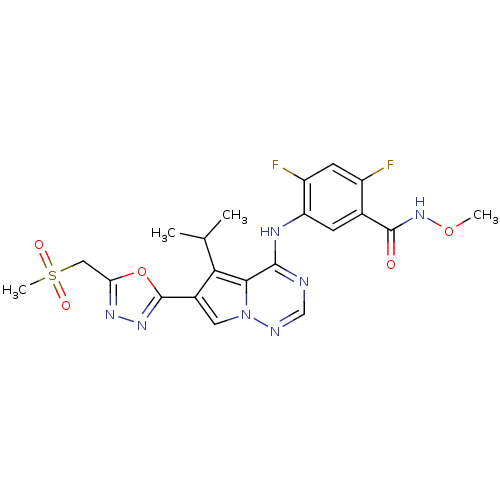 (2,4-Difluoro-5-[5-isopropyl-6-(5-methanesulfonylme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of VEGF-stimulated human umbilical vein endothelial cell proliferation | J Med Chem 48: 3991-4008 (2005) Article DOI: 10.1021/jm0501275 BindingDB Entry DOI: 10.7270/Q2KP81QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1/2/3 (Homo sapiens (Human)) | BDBM50168390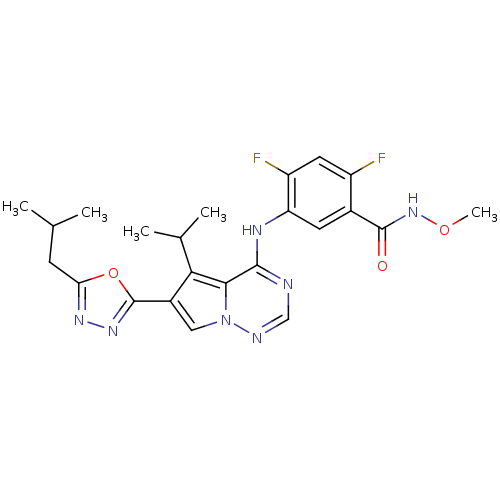 (2,4-Difluoro-5-[6-(5-isobutyl-[1,3,4]oxadiazol-2-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of VEGF-stimulated human umbilical vein endothelial cell proliferation | J Med Chem 48: 3991-4008 (2005) Article DOI: 10.1021/jm0501275 BindingDB Entry DOI: 10.7270/Q2KP81QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max (Soybean)) | BDBM192705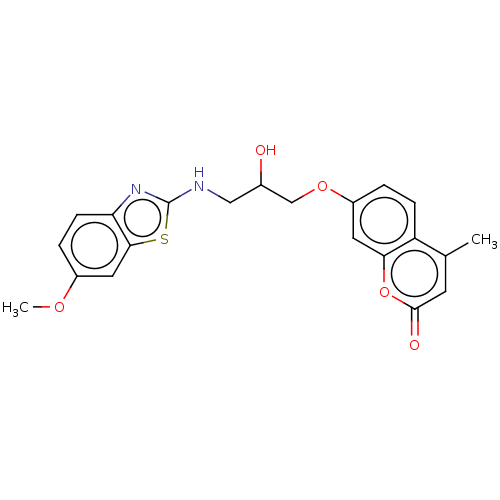 (7-(2-hydroxy-3-(6-methoxybenzo[d]thiazol-2-ylamino...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.12 | n/a | n/a | n/a | n/a | 9.0 | 25 |
Nirma University | Assay Description It is determined by kinetic mode of spectro-photometric determination method,which was performed by recording the rate of change in absorbance at 234... | Bioorg Chem 67: 130-8 (2016) Article DOI: 10.1016/j.bioorg.2016.06.004 BindingDB Entry DOI: 10.7270/Q2M907GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max (Soybean)) | BDBM192703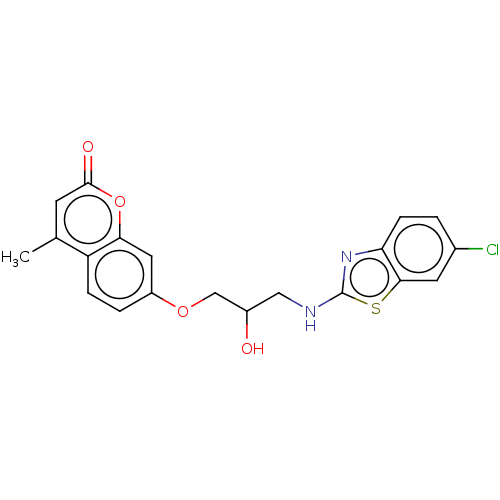 (7-(3-(6-chlorobenzo[d]thiazol-2-ylamino)-2-hydroxy...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.79 | n/a | n/a | n/a | n/a | 9.0 | 25 |
Nirma University | Assay Description It is determined by kinetic mode of spectro-photometric determination method,which was performed by recording the rate of change in absorbance at 234... | Bioorg Chem 67: 130-8 (2016) Article DOI: 10.1016/j.bioorg.2016.06.004 BindingDB Entry DOI: 10.7270/Q2M907GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 350 total ) | Next | Last >> |