

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202986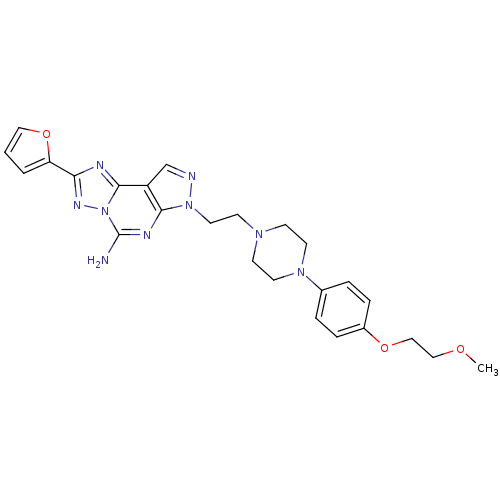 (2-furan-2-yl-7-(2-{4-[4-(2-methoxy-ethoxy)-phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336715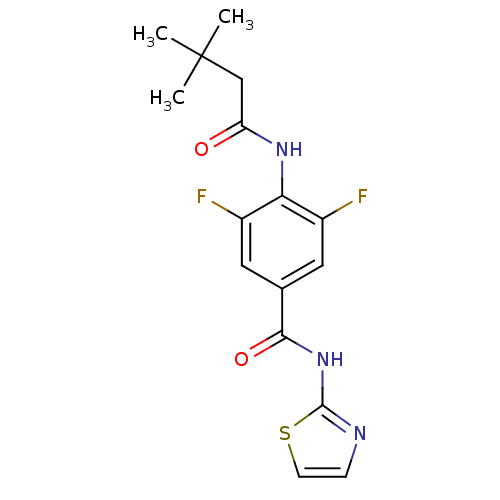 (4-(3,3-Dimethyl-butyrylamino)-3,5-difluoro-N-thiaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336712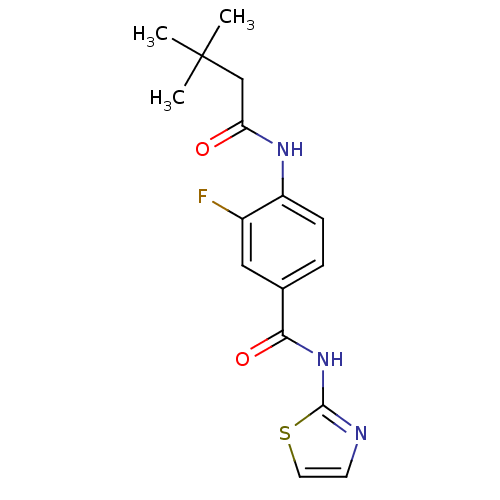 (4-(3,3-Dimethyl-butyrylamino)-3-fluoro-N-thiazol-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336719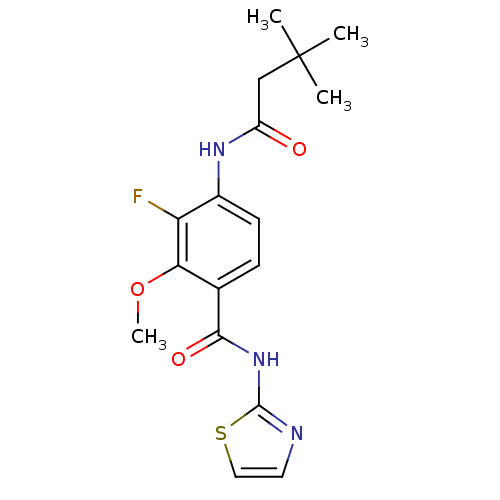 (4-(3,3-Dimethyl-butyrylamino)-5-fluoro-2-methoxy-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336717 (4-(3,3-Dimethyl-butyrylamino)-3-methoxy-N-thiazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336714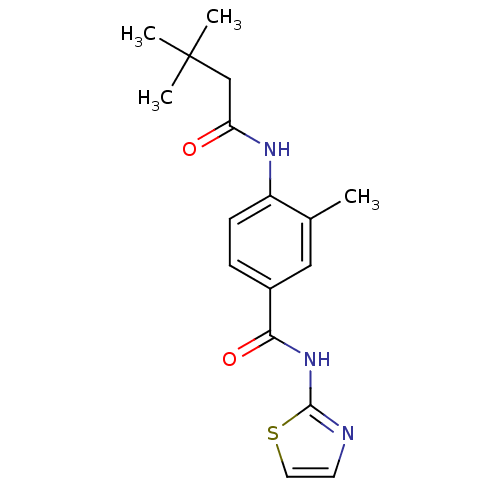 (4-(3,3-Dimethyl-butyrylamino)-3-methyl-N-thiazol-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336723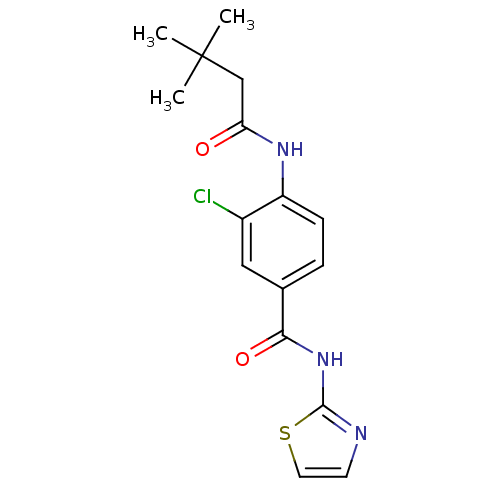 (3-Chloro-4-(3,3-dimethyl-butyrylamino)-N-thiazol-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336720 (CHEMBL1671923 | N-(5-Chloro-thiazol-2-yl)-4-(3,3-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336713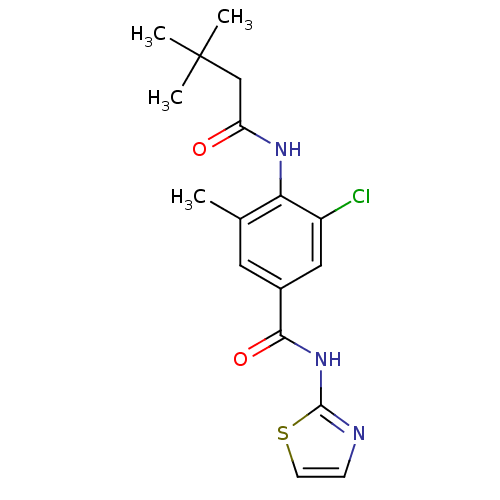 (3-Chloro-4-(3,3-dimethyl-butyrylamino)-5-methyl-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336729 (4-(3,3-Dimethyl-butyrylamino)-N-thiazol-2-yl-benza...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336709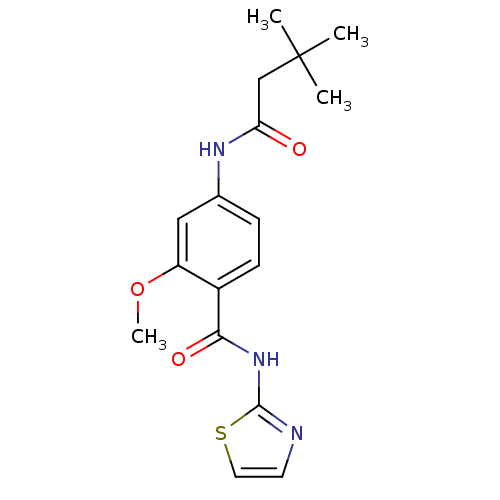 (4-(3,3-Dimethyl-butyrylamino)-2-methoxy-N-thiazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50176050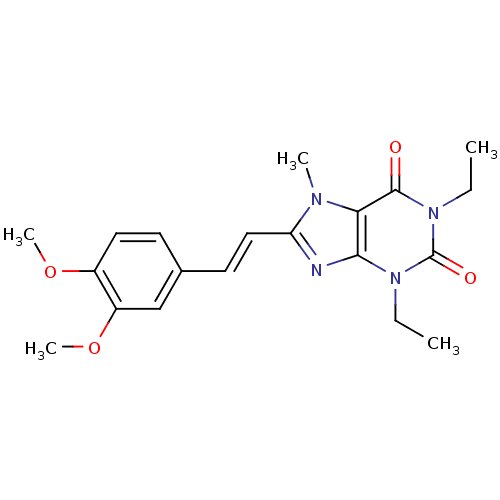 (8-(3,4-dimethoxystyryl)-1,3-diethyl-7-methyl-1H-pu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336728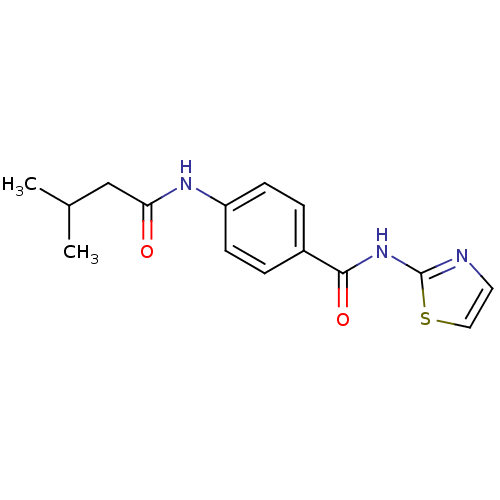 (4-(3-Methyl-butyrylamino)-N-thiazol-2-yl-benzamide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336727 (CHEMBL1671928 | N-(2,2-Dimethyl-propyl)-N'-thiazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336710 (4-(3,3-Dimethyl-butyrylamino)-N-(5-methyl-thiazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336726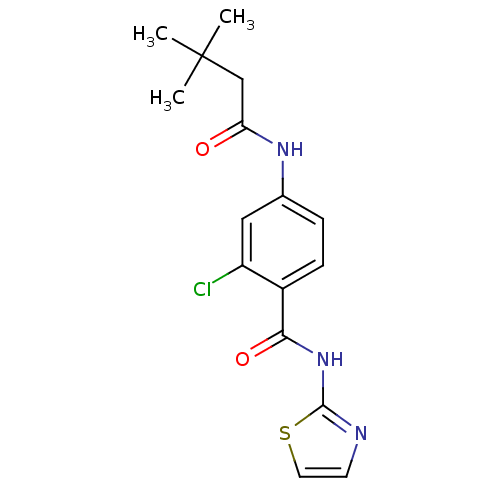 (2-Chloro-4-(3,3-dimethyl-butyrylamino)-N-thiazol-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336725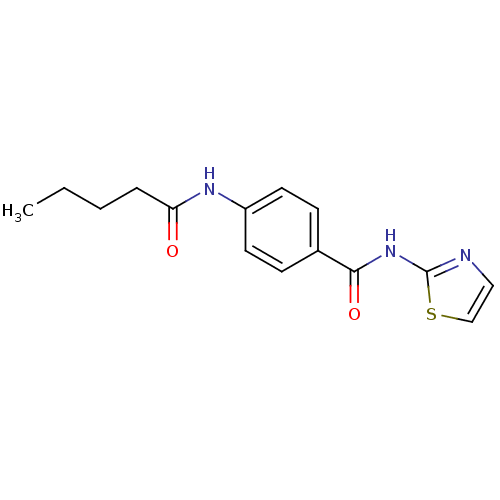 (4-pentanamido-N-(thiazol-2-yl)benzamide | CHEMBL16...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336724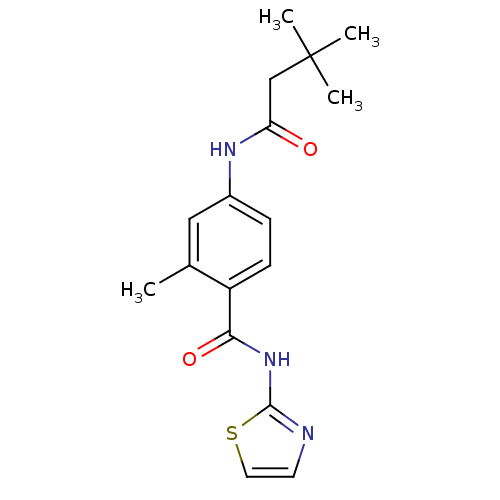 (4-(3,3-Dimethyl-butyrylamino)-2-methyl-N-thiazol-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50336723 (3-Chloro-4-(3,3-dimethyl-butyrylamino)-N-thiazol-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-DPCPX from human adenosine A1 receptor expressed in CHO cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336722 (2-methyl-N-(4-(thiazol-2-ylcarbamoyl)phenyl)benzam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336721 (4-(3,3-Dimethyl-butyrylamino)-N-(4,5-dimethyl-thia...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50336719 (4-(3,3-Dimethyl-butyrylamino)-5-fluoro-2-methoxy-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-DPCPX from human adenosine A1 receptor expressed in CHO cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50336720 (CHEMBL1671923 | N-(5-Chloro-thiazol-2-yl)-4-(3,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-DPCPX from human adenosine A1 receptor expressed in CHO cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50336715 (4-(3,3-Dimethyl-butyrylamino)-3,5-difluoro-N-thiaz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-MRS1754 from human recombinant adenosine A2B receptor expressed in HEK293 cells after 60 mins | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336718 (4-Isobutyrylamino-N-thiazol-2-yl-benzamide | CHEMB...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50336717 (4-(3,3-Dimethyl-butyrylamino)-3-methoxy-N-thiazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-DPCPX from human adenosine A1 receptor expressed in CHO cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336716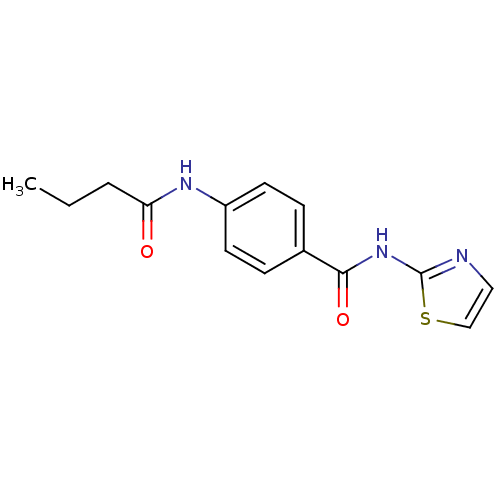 (4-Butyrylamino-N-thiazol-2-yl-benzamide | CHEMBL16...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50336715 (4-(3,3-Dimethyl-butyrylamino)-3,5-difluoro-N-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-DPCPX from human adenosine A1 receptor expressed in CHO cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50336714 (4-(3,3-Dimethyl-butyrylamino)-3-methyl-N-thiazol-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-DPCPX from human adenosine A1 receptor expressed in CHO cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50336713 (3-Chloro-4-(3,3-dimethyl-butyrylamino)-5-methyl-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-DPCPX from human adenosine A1 receptor expressed in CHO cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50336712 (4-(3,3-Dimethyl-butyrylamino)-3-fluoro-N-thiazol-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-DPCPX from human adenosine A1 receptor expressed in CHO cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50336711 (4-Propionylamino-N-thiazol-2-yl-benzamide | CHEMBL...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in high five cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50336710 (4-(3,3-Dimethyl-butyrylamino)-N-(5-methyl-thiazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-DPCPX from human adenosine A1 receptor expressed in CHO cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50336709 (4-(3,3-Dimethyl-butyrylamino)-2-methoxy-N-thiazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-DPCPX from human adenosine A1 receptor expressed in CHO cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50176050 (8-(3,4-dimethoxystyryl)-1,3-diethyl-7-methyl-1H-pu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-DPCPX from human adenosine A1 receptor expressed in CHO cells after 30 mins by scintillation counting | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50336715 (4-(3,3-Dimethyl-butyrylamino)-3,5-difluoro-N-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-AB-MECA from human recombinant adenosine A3 receptor expressed in CHO-K1 cells after 60 mins | J Med Chem 54: 751-64 (2012) Article DOI: 10.1021/jm1008659 BindingDB Entry DOI: 10.7270/Q23X87N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 beta (Homo sapiens (Human)) | BDBM50110881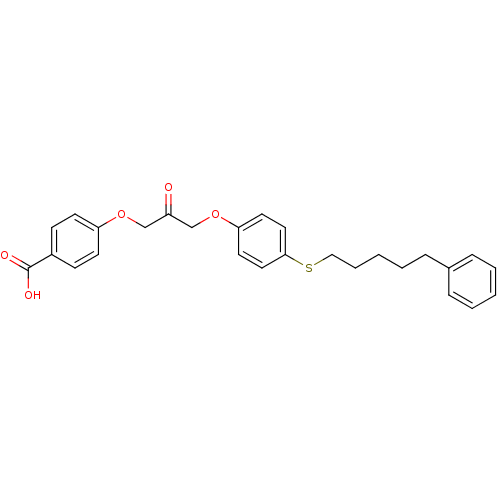 (4-(2-oxo-3-(4-(5-phenylpentylthio)phenoxy)propoxy)...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Inhibitory activity against cytosolic Phospholipase A2 (PLA2) by bilayer assay | J Med Chem 45: 1348-62 (2002) BindingDB Entry DOI: 10.7270/Q2F76BVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 beta (Homo sapiens (Human)) | BDBM50110846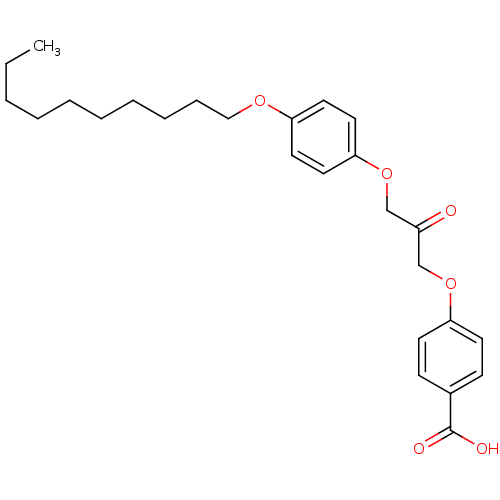 (4-(3-(4-(decyloxy)phenoxy)-2-oxopropoxy)benzoic ac...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Inhibitory activity against cytosolic Phospholipase A2 (PLA2) by bilayer assay | J Med Chem 45: 1348-62 (2002) BindingDB Entry DOI: 10.7270/Q2F76BVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 beta (Homo sapiens (Human)) | BDBM50110859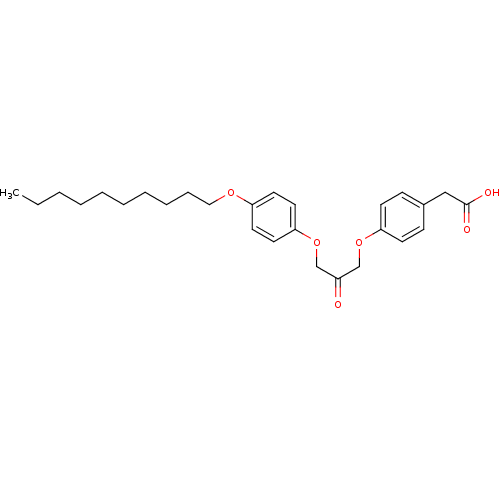 (CHEMBL26200 | {4-[3-(4-Decyloxy-phenoxy)-2-oxo-pro...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Inhibitory activity against cytosolic Phospholipase A2 (PLA2) by soluble assay | J Med Chem 45: 1348-62 (2002) BindingDB Entry DOI: 10.7270/Q2F76BVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 beta (Homo sapiens (Human)) | BDBM50110854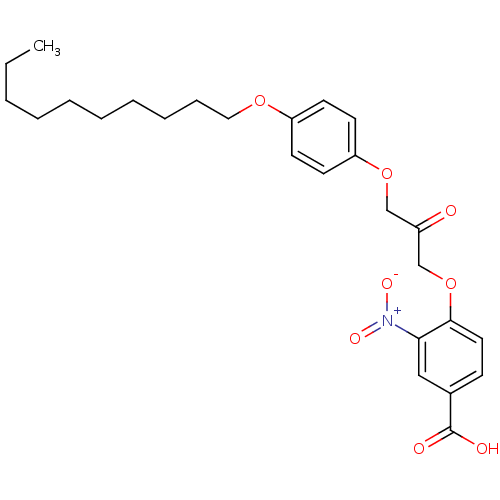 (4-[3-(4-Decyloxy-phenoxy)-2-oxo-propoxy]-3-nitro-b...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Inhibitory activity against cytosolic Phospholipase A2 (PLA2) by soluble assay | J Med Chem 45: 1348-62 (2002) BindingDB Entry DOI: 10.7270/Q2F76BVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 beta (Homo sapiens (Human)) | BDBM50110873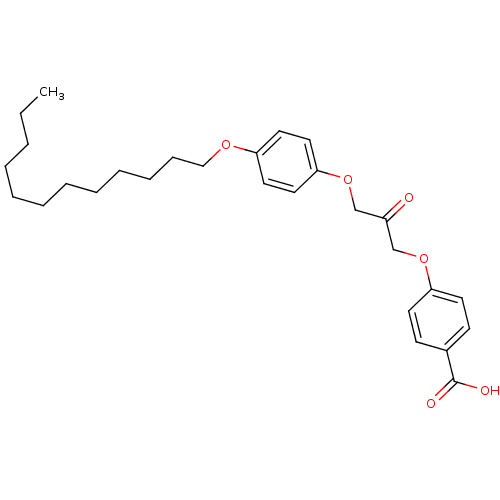 (4-[3-(4-Dodecyloxy-phenoxy)-2-oxo-propoxy]-benzoic...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Inhibitory activity against cytosolic Phospholipase A2 (PLA2) by soluble assay | J Med Chem 45: 1348-62 (2002) BindingDB Entry DOI: 10.7270/Q2F76BVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 beta (Homo sapiens (Human)) | BDBM50110875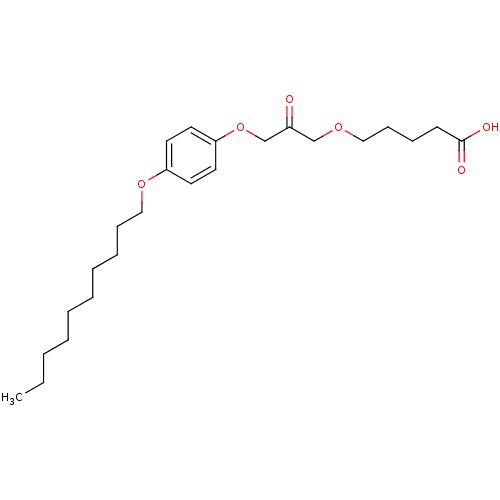 (5-[3-(4-Decyloxy-phenoxy)-2-oxo-propoxy]-pentanoic...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Inhibitory activity against cytosolic Phospholipase A2 (PLA2) by soluble assay | J Med Chem 45: 1348-62 (2002) BindingDB Entry DOI: 10.7270/Q2F76BVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 beta (Homo sapiens (Human)) | BDBM50110846 (4-(3-(4-(decyloxy)phenoxy)-2-oxopropoxy)benzoic ac...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Inhibitory activity against cytosolic Phospholipase A2 (PLA2) by soluble assay | J Med Chem 45: 1348-62 (2002) BindingDB Entry DOI: 10.7270/Q2F76BVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 beta (Homo sapiens (Human)) | BDBM50110865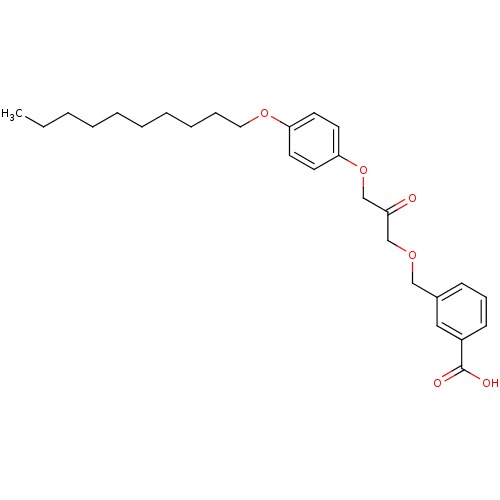 (3-[3-(4-Decyloxy-phenoxy)-2-oxo-propoxymethyl]-ben...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Inhibitory activity against cytosolic Phospholipase A2 (PLA2) by soluble assay | J Med Chem 45: 1348-62 (2002) BindingDB Entry DOI: 10.7270/Q2F76BVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 beta (Homo sapiens (Human)) | BDBM50110872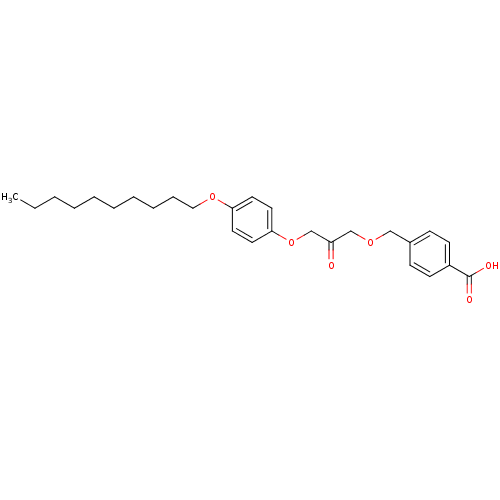 (4-[3-(4-Decyloxy-phenoxy)-2-oxo-propoxymethyl]-ben...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Inhibitory activity against cytosolic Phospholipase A2 (PLA2) by soluble assay | J Med Chem 45: 1348-62 (2002) BindingDB Entry DOI: 10.7270/Q2F76BVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 beta (Homo sapiens (Human)) | BDBM50110884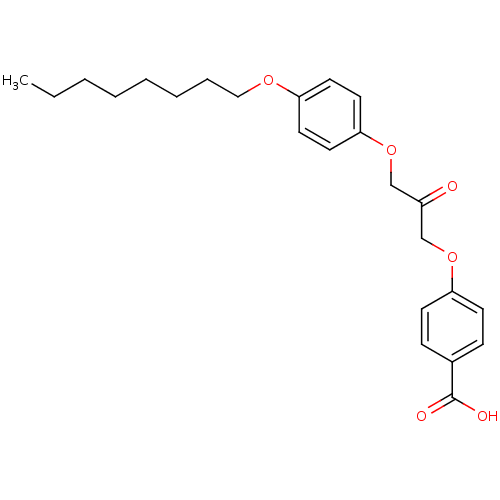 (4-[3-(4-Octyloxy-phenoxy)-2-oxo-propoxy]-benzoic a...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Inhibitory activity against cytosolic Phospholipase A2 (PLA2) by soluble assay | J Med Chem 45: 1348-62 (2002) BindingDB Entry DOI: 10.7270/Q2F76BVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 beta (Homo sapiens (Human)) | BDBM50110879 (4-{2-Oxo-3-[4-(5-phenyl-pentyloxy)-phenoxy]-propox...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Inhibitory activity against cytosolic Phospholipase A2 (PLA2) by soluble assay | J Med Chem 45: 1348-62 (2002) BindingDB Entry DOI: 10.7270/Q2F76BVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 beta (Homo sapiens (Human)) | BDBM50110888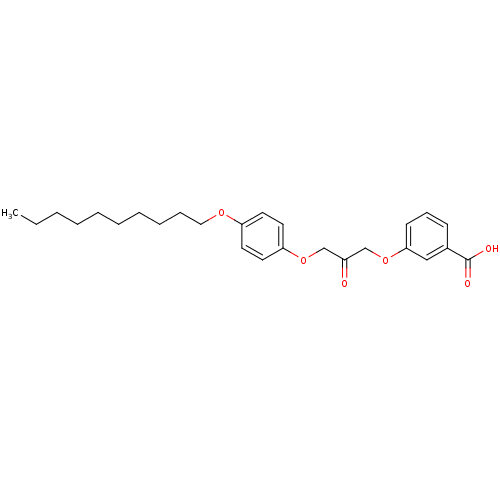 (3-[3-(4-Decyloxy-phenoxy)-2-oxo-propoxy]-benzoic a...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Inhibitory activity against cytosolic Phospholipase A2 (PLA2) by soluble assay | J Med Chem 45: 1348-62 (2002) BindingDB Entry DOI: 10.7270/Q2F76BVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 beta (Homo sapiens (Human)) | BDBM50110860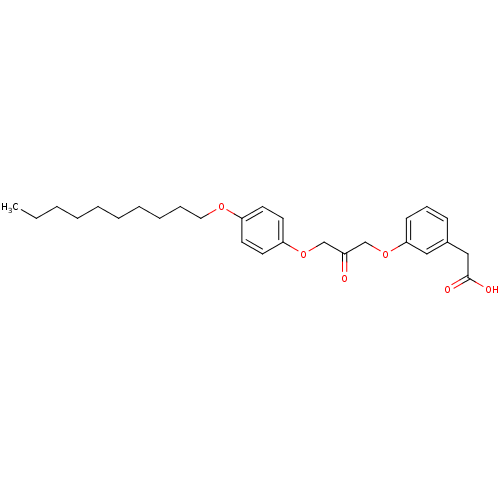 (CHEMBL29247 | {3-[3-(4-Decyloxy-phenoxy)-2-oxo-pro...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Inhibitory activity against cytosolic Phospholipase A2 (PLA2) by soluble assay | J Med Chem 45: 1348-62 (2002) BindingDB Entry DOI: 10.7270/Q2F76BVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 beta (Homo sapiens (Human)) | BDBM50110853 (CHEMBL29246 | {3-[3-(4-Decyloxy-phenoxy)-2-oxo-pro...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Inhibitory activity against cytosolic Phospholipase A2 (PLA2) by bilayer assay | J Med Chem 45: 1348-62 (2002) BindingDB Entry DOI: 10.7270/Q2F76BVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 141 total ) | Next | Last >> |