

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50228308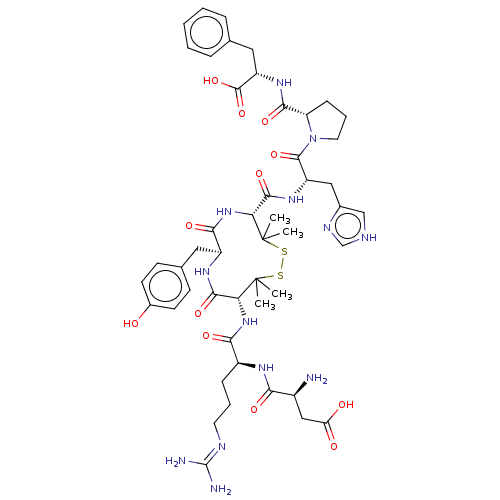 (CHEMBL413740) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albumin | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase type 1G (Homo sapiens (Human)) | BDBM50545573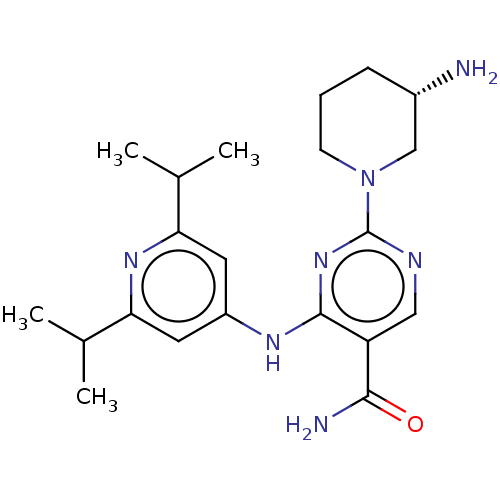 (CHEMBL4635883 | US11530193, Example 54) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of human CAMK1G using KKALRRQETVDAL as substrate in presence of [gamma-33P]-ATP by hotspot assay | J Med Chem 63: 6784-6801 (2020) Article DOI: 10.1021/acs.jmedchem.9b01803 BindingDB Entry DOI: 10.7270/Q2QF8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase type 1 (Homo sapiens (Human)) | BDBM50545573 (CHEMBL4635883 | US11530193, Example 54) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of human CAMK1A using KKALRRQETVDAL as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay | J Med Chem 63: 6784-6801 (2020) Article DOI: 10.1021/acs.jmedchem.9b01803 BindingDB Entry DOI: 10.7270/Q2QF8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50228272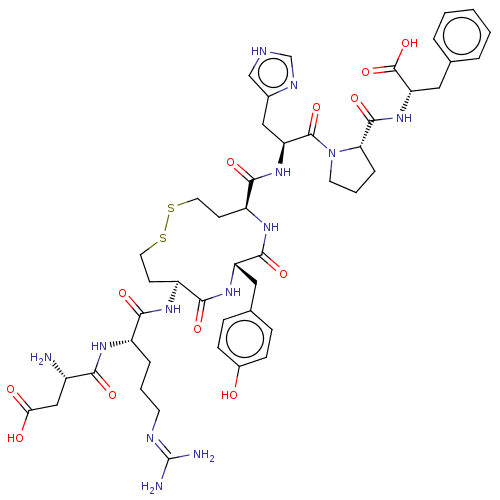 (CHEMBL411997) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albumin | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM82258 (CAS_114798-26-4 | Losartan | NSC_3961) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albumin | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50471553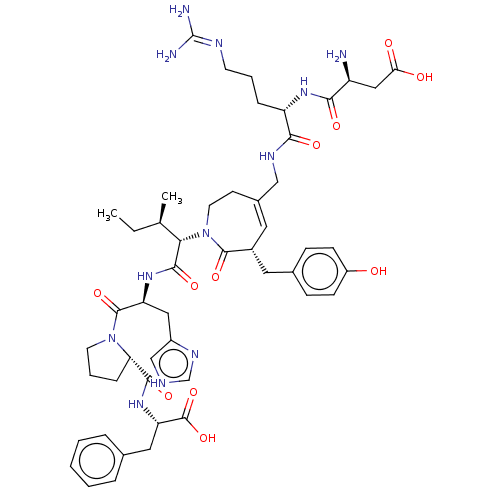 (CHEMBL1791261) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albumin | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50228195 (Angiotensin Ii | CHEBI:2719) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albumin | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase type 1 (Homo sapiens (Human)) | BDBM50545576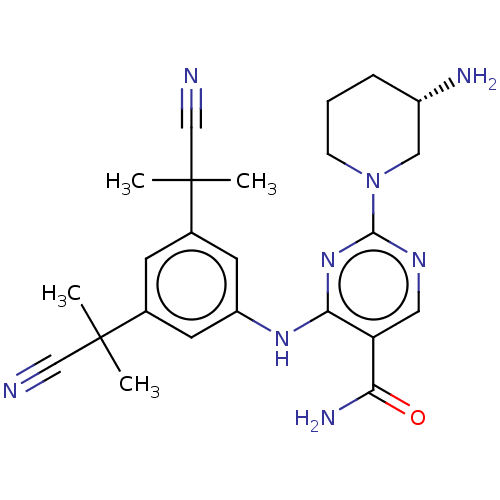 (CHEMBL4640712 | US11530193, Example 50) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of human CAMK1A using KKALRRQETVDAL as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay | J Med Chem 63: 6784-6801 (2020) Article DOI: 10.1021/acs.jmedchem.9b01803 BindingDB Entry DOI: 10.7270/Q2QF8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257167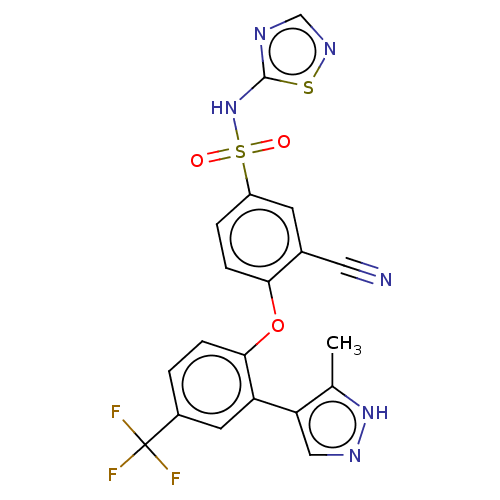 (CHEMBL2325619) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50228272 (CHEMBL411997) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against specific binding of [125 I]Ang II to rat uterine membranes (Angiotensin II receptor, type 1) | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50368147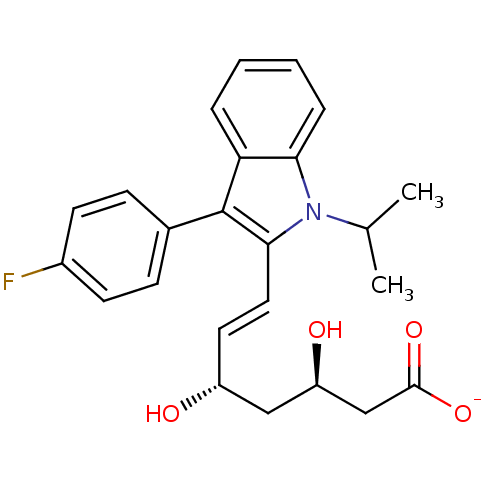 ((+)-(3R,5S)-fluvastatin | (3R,5S)-fluvastatin | (3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against partially purified rat liver HMG-CoA reductase in vitro; 0.0015-0.0040 | J Med Chem 36: 3674-85 (1994) BindingDB Entry DOI: 10.7270/Q2R78FVG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50228308 (CHEMBL413740) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against specific binding of [125 I]Ang II to rat uterine membranes (Angiotensin II receptor, type 1) | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50471554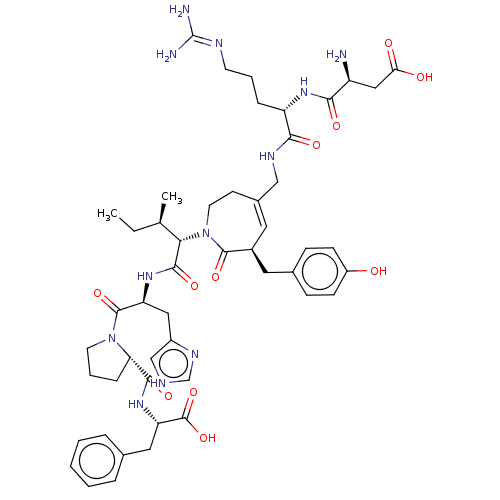 (CHEMBL1791267) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albumin | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50228196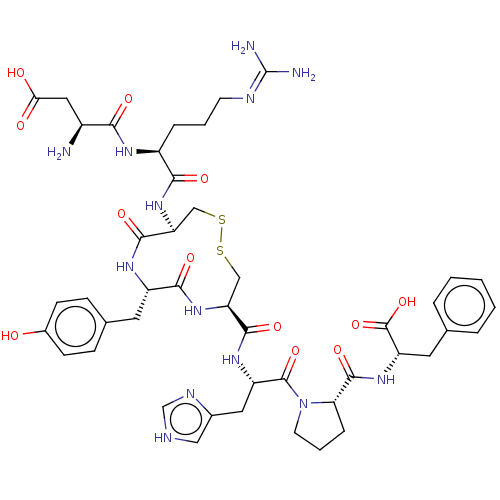 (CHEMBL405464) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albumin | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase type 1B (Homo sapiens (Human)) | BDBM50545573 (CHEMBL4635883 | US11530193, Example 54) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of human CAMK1B using KKALRRQETVDAL as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay | J Med Chem 63: 6784-6801 (2020) Article DOI: 10.1021/acs.jmedchem.9b01803 BindingDB Entry DOI: 10.7270/Q2QF8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257179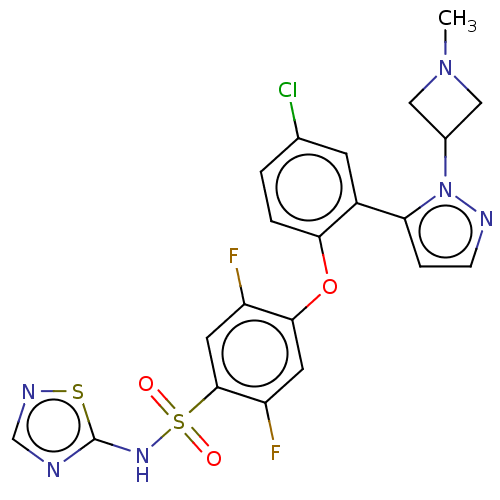 (CHEMBL2325622) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042682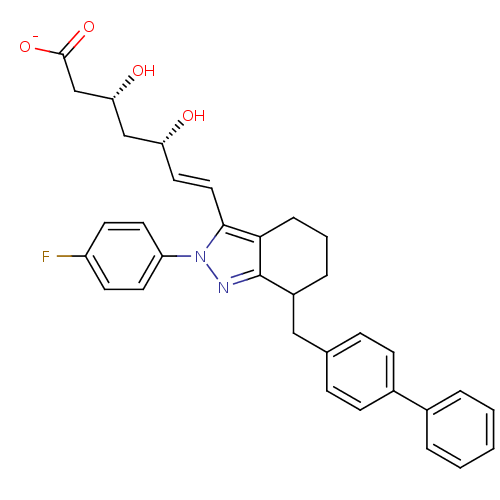 (CHEMBL122674 | Sodium; 7-[7-biphenyl-4-ylmethyl-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against partially purified rat liver HMG-CoA reductase in vitro; 0.0015-0.0049 | J Med Chem 36: 3674-85 (1994) BindingDB Entry DOI: 10.7270/Q2R78FVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50228308 (CHEMBL413740) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against specific binding of [125 I]Ang II to rat pituitary membranes (Angiotensin II receptor, type 1) in presence of 0.2% b... | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257166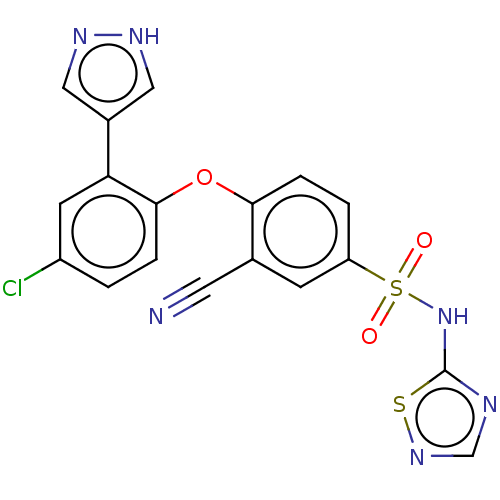 (CHEMBL2325330) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257216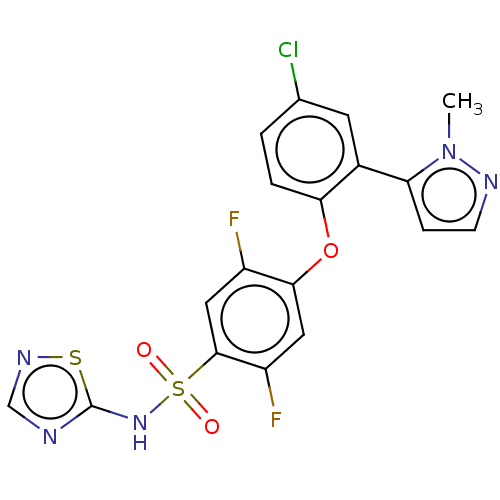 (CHEMBL2325350) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257180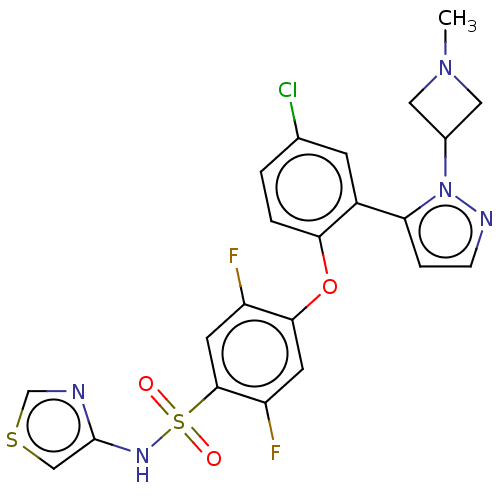 (CHEMBL2325317) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50249319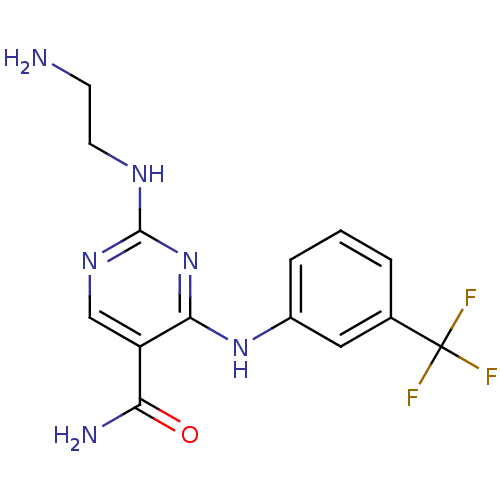 (2-(2-aminoethylamino)-4-(3-(trifluoromethyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of human SYK using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay | J Med Chem 63: 6784-6801 (2020) Article DOI: 10.1021/acs.jmedchem.9b01803 BindingDB Entry DOI: 10.7270/Q2QF8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257178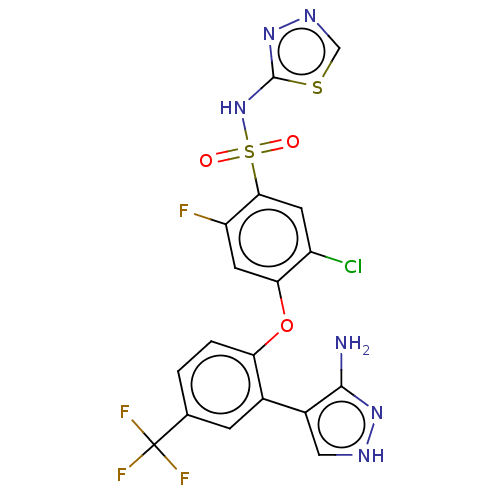 (CHEMBL2325627) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase type 1D (Homo sapiens (Human)) | BDBM50545573 (CHEMBL4635883 | US11530193, Example 54) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length His-tagged CAMK1D expressed in baculovirus expression system using autocamtide-2 as substrate preincubate... | J Med Chem 63: 6784-6801 (2020) Article DOI: 10.1021/acs.jmedchem.9b01803 BindingDB Entry DOI: 10.7270/Q2QF8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase type 1D (Homo sapiens (Human)) | BDBM50545573 (CHEMBL4635883 | US11530193, Example 54) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of human CAMK1D using KKALRRQETVDAL as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay | J Med Chem 63: 6784-6801 (2020) Article DOI: 10.1021/acs.jmedchem.9b01803 BindingDB Entry DOI: 10.7270/Q2QF8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Rattus norvegicus) | BDBM50254439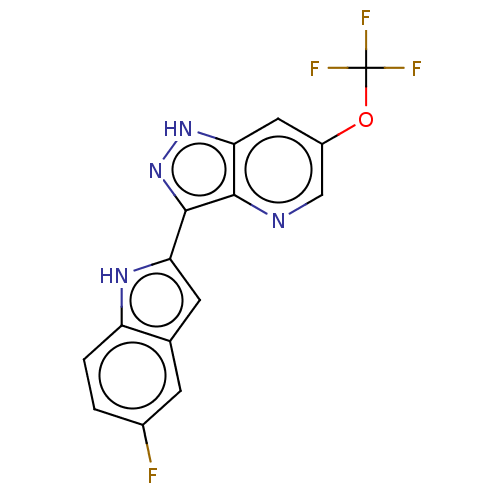 (CHEMBL4091459) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Medicinal Chemistry, Neuroscience and Pain Research Unit, Portway Building, Granta Park, Great Abington, Cambridgeshire CB21 6GS, U.K. Curated by ChEMBL | Assay Description Inhibition of full length rat TRPA1 at a holding potential of 15 mV measured after 1 min by PatchXpress electrophysiology assay | ACS Med Chem Lett 8: 666-671 (2017) Article DOI: 10.1021/acsmedchemlett.7b00140 BindingDB Entry DOI: 10.7270/Q2C24ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase type 1D (Homo sapiens (Human)) | BDBM50545576 (CHEMBL4640712 | US11530193, Example 50) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of HA-tagged CAMK1D (unknown origin) expressed in human MDA-MB-231 cells assessed as reduction in CAMK1D autophosphorylation measured afte... | J Med Chem 63: 6784-6801 (2020) Article DOI: 10.1021/acs.jmedchem.9b01803 BindingDB Entry DOI: 10.7270/Q2QF8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Rattus norvegicus) | BDBM50254442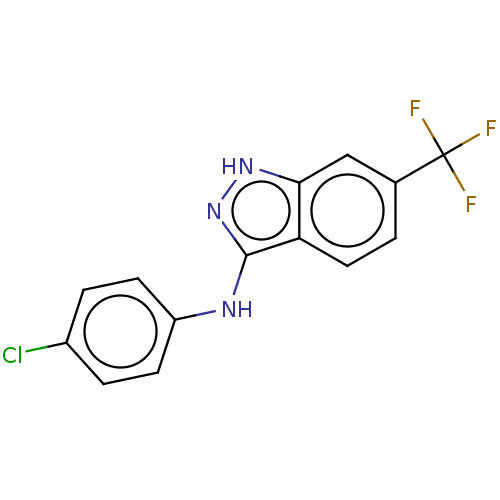 (CHEMBL4095993) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Medicinal Chemistry, Neuroscience and Pain Research Unit, Portway Building, Granta Park, Great Abington, Cambridgeshire CB21 6GS, U.K. Curated by ChEMBL | Assay Description Inhibition of full length rat TRPA1 at a holding potential of 15 mV measured after 1 min by PatchXpress electrophysiology assay | ACS Med Chem Lett 8: 666-671 (2017) Article DOI: 10.1021/acsmedchemlett.7b00140 BindingDB Entry DOI: 10.7270/Q2C24ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042665 (CHEMBL332812 | Sodium; 7-[2-(4-fluoro-phenyl)-7-na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against partially purified rat liver HMG-CoA reductase in vitro; 0.0050-0.016 | J Med Chem 36: 3674-85 (1994) BindingDB Entry DOI: 10.7270/Q2R78FVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257214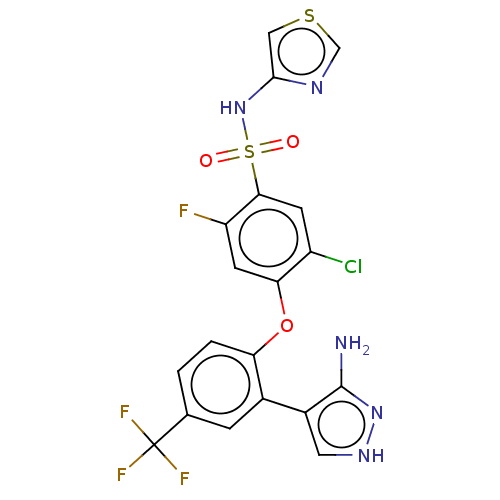 (CHEMBL2325601) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257168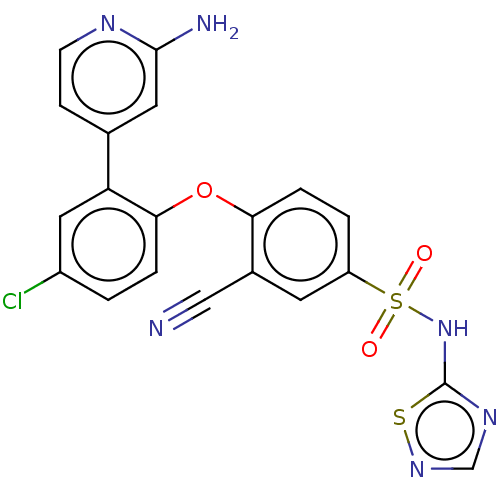 (CHEMBL2325013) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Rattus norvegicus) | BDBM50254402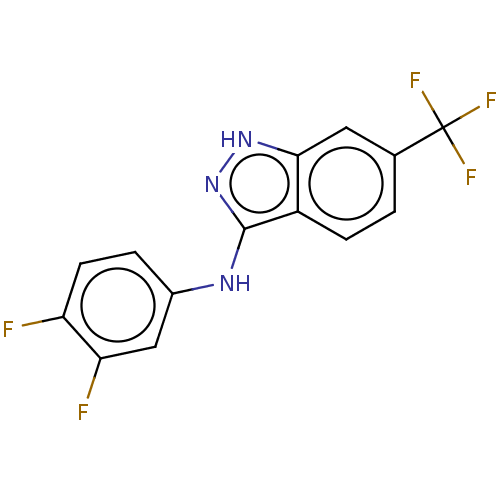 (CHEMBL4098431) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Medicinal Chemistry, Neuroscience and Pain Research Unit, Portway Building, Granta Park, Great Abington, Cambridgeshire CB21 6GS, U.K. Curated by ChEMBL | Assay Description Inhibition of full length rat TRPA1 at a holding potential of 15 mV measured after 1 min by PatchXpress electrophysiology assay | ACS Med Chem Lett 8: 666-671 (2017) Article DOI: 10.1021/acsmedchemlett.7b00140 BindingDB Entry DOI: 10.7270/Q2C24ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042675 (CHEMBL421436 | Sodium;2-{2-[2,6-dimethyl-8-(2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against partially purified rat liver HMG-CoA reductase in vitro; 0.0073-0.017 | J Med Chem 36: 3674-85 (1994) BindingDB Entry DOI: 10.7270/Q2R78FVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 by fluorescence method | J Med Chem 62: 10124-10143 (2019) Article DOI: 10.1021/acs.jmedchem.9b00952 BindingDB Entry DOI: 10.7270/Q2QF8XB9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257181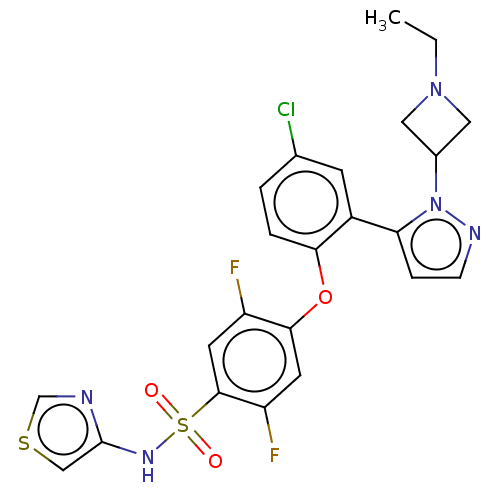 (CHEMBL2325020) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 by fluorescence method | J Med Chem 62: 10124-10143 (2019) Article DOI: 10.1021/acs.jmedchem.9b00952 BindingDB Entry DOI: 10.7270/Q2QF8XB9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Rattus norvegicus) | BDBM50254387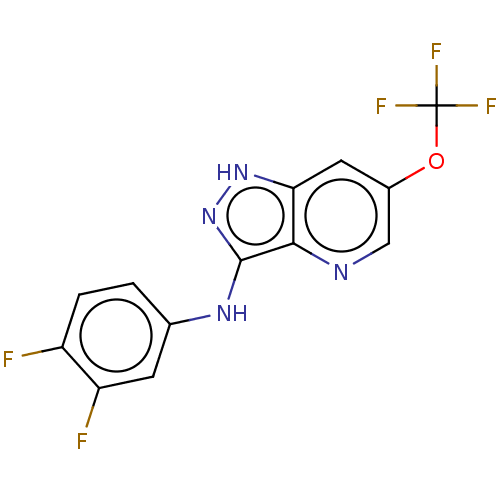 (CHEMBL4062193) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Medicinal Chemistry, Neuroscience and Pain Research Unit, Portway Building, Granta Park, Great Abington, Cambridgeshire CB21 6GS, U.K. Curated by ChEMBL | Assay Description Inhibition of rat TRPA1 at a holding potential of 15 mV measured after 1 min by whole-cell manual patch clamp electrophysiology assay | ACS Med Chem Lett 8: 666-671 (2017) Article DOI: 10.1021/acsmedchemlett.7b00140 BindingDB Entry DOI: 10.7270/Q2C24ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase type 1D (Homo sapiens (Human)) | BDBM50545573 (CHEMBL4635883 | US11530193, Example 54) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of HA-tagged CAMK1D (unknown origin) expressed in human MDA-MB-231 cells assessed as reduction in CAMK1D autophosphorylation measured afte... | J Med Chem 63: 6784-6801 (2020) Article DOI: 10.1021/acs.jmedchem.9b01803 BindingDB Entry DOI: 10.7270/Q2QF8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Rattus norvegicus) | BDBM50254387 (CHEMBL4062193) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Medicinal Chemistry, Neuroscience and Pain Research Unit, Portway Building, Granta Park, Great Abington, Cambridgeshire CB21 6GS, U.K. Curated by ChEMBL | Assay Description Inhibition of full length rat TRPA1 at a holding potential of 15 mV measured after 1 min by PatchXpress electrophysiology assay | ACS Med Chem Lett 8: 666-671 (2017) Article DOI: 10.1021/acsmedchemlett.7b00140 BindingDB Entry DOI: 10.7270/Q2C24ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50240267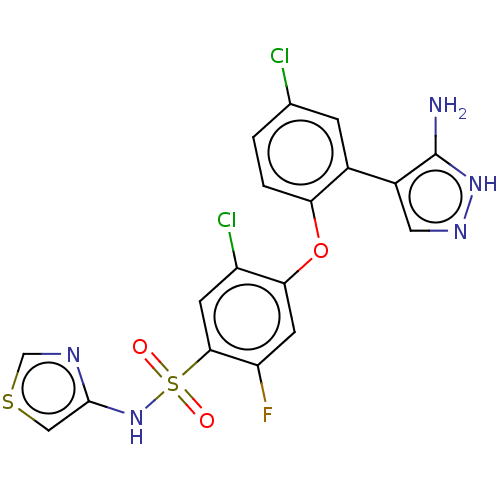 (CHEMBL2325014) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257211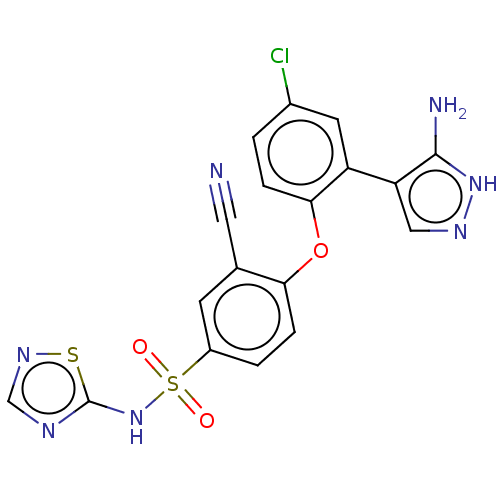 (CHEMBL2325038) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase type 1B (Homo sapiens (Human)) | BDBM50545576 (CHEMBL4640712 | US11530193, Example 50) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of human CAMK1B using KKALRRQETVDAL as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay | J Med Chem 63: 6784-6801 (2020) Article DOI: 10.1021/acs.jmedchem.9b01803 BindingDB Entry DOI: 10.7270/Q2QF8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50005480 ((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of MDR1 (unknown origin) transfected in human SKOV3 cells assessed as growth inhibition after 48 hrs by SRB assay | Bioorg Med Chem 21: 891-902 (2013) Article DOI: 10.1016/j.bmc.2012.12.010 BindingDB Entry DOI: 10.7270/Q2XP7692 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257212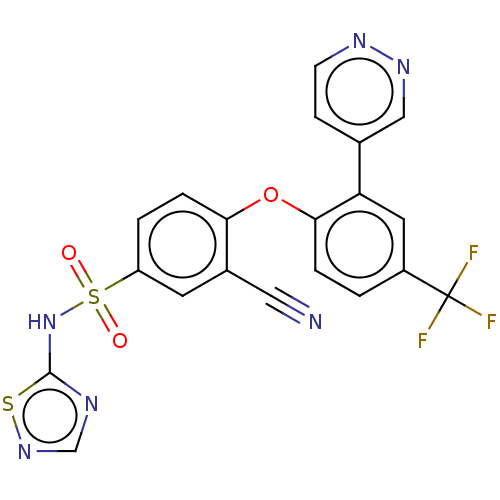 (CHEMBL2325021) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042681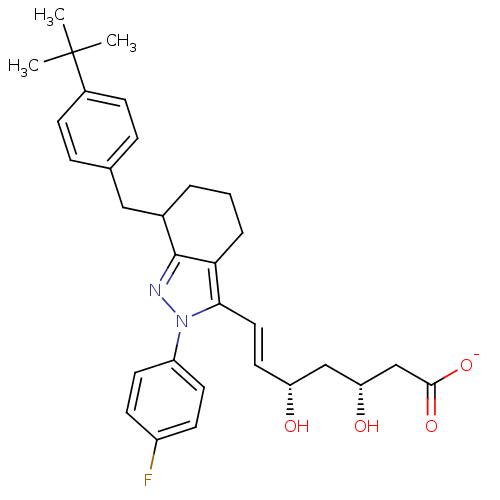 (CHEMBL123826 | Sodium; 7-[7-(4-tert-butyl-benzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against partially purified rat liver HMG-CoA reductase in vitro; 0.0040-0.053 | J Med Chem 36: 3674-85 (1994) BindingDB Entry DOI: 10.7270/Q2R78FVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50301972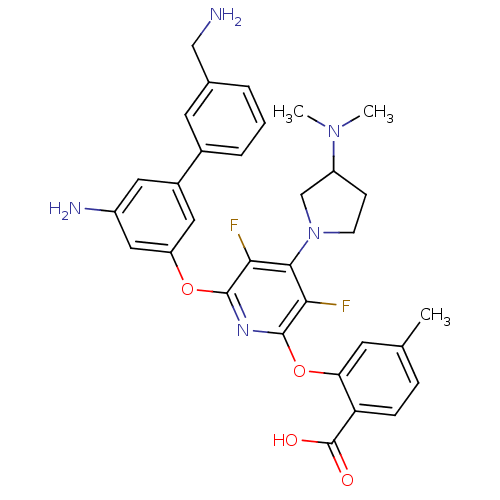 (2-(6-(5-amino-3'-(aminomethyl)biphenyl-3-yloxy)-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibition of uPA | Bioorg Med Chem Lett 19: 5712-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.008 BindingDB Entry DOI: 10.7270/Q2JQ113Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50228272 (CHEMBL411997) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory concentration against specific binding of [125 I]Ang II to rat pituitary membranes (Angiotensin II receptor, type 1) in presence of 0.2% b... | J Med Chem 40: 903-19 (1997) Article DOI: 10.1021/jm960553d BindingDB Entry DOI: 10.7270/Q28S4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase type 1D (Homo sapiens (Human)) | BDBM50545587 (CHEMBL4633229 | US11530193, Example 135) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of HA-tagged CAMK1D (unknown origin) expressed in human MDA-MB-231 cells assessed as reduction in CAMK1D autophosphorylation measured afte... | J Med Chem 63: 6784-6801 (2020) Article DOI: 10.1021/acs.jmedchem.9b01803 BindingDB Entry DOI: 10.7270/Q2QF8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50254387 (CHEMBL4062193) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Medicinal Chemistry, Neuroscience and Pain Research Unit, Portway Building, Granta Park, Great Abington, Cambridgeshire CB21 6GS, U.K. Curated by ChEMBL | Assay Description Antagonist activity at human TRPA1 by calcium flux based FLIPR assay | ACS Med Chem Lett 8: 666-671 (2017) Article DOI: 10.1021/acsmedchemlett.7b00140 BindingDB Entry DOI: 10.7270/Q2C24ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257183 (CHEMBL2324755) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 601 total ) | Next | Last >> |