 Found 203 hits with Last Name = 'whipple' and Initial = 'da'
Found 203 hits with Last Name = 'whipple' and Initial = 'da' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286335
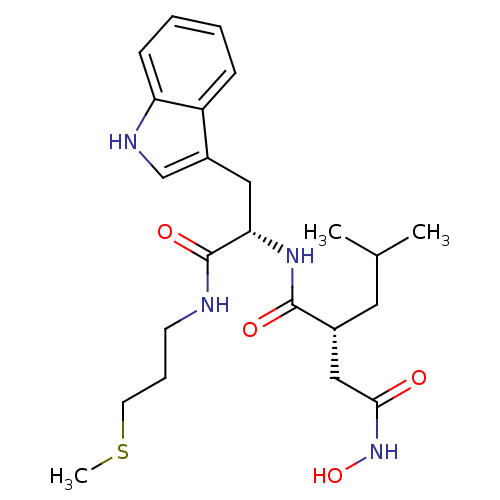
((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(3-...)Show SMILES CSCCCNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C23H34N4O4S/c1-15(2)11-16(13-21(28)27-31)22(29)26-20(23(30)24-9-6-10-32-3)12-17-14-25-19-8-5-4-7-18(17)19/h4-5,7-8,14-16,20,25,31H,6,9-13H2,1-3H3,(H,24,30)(H,26,29)(H,27,28)/t16-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286344
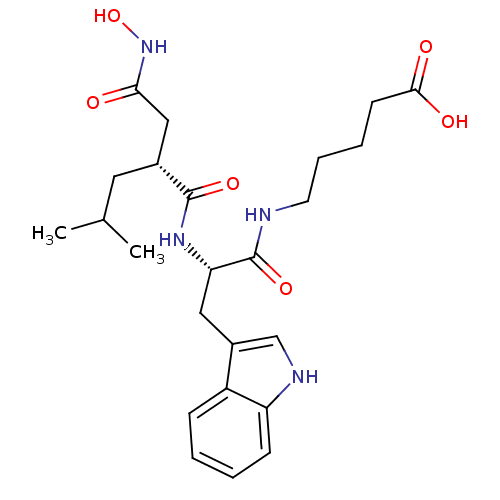
(5-[(S)-2-((R)-2-Hydroxycarbamoylmethyl-4-methyl-pe...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCCCC(O)=O Show InChI InChI=1S/C24H34N4O6/c1-15(2)11-16(13-21(29)28-34)23(32)27-20(24(33)25-10-6-5-9-22(30)31)12-17-14-26-19-8-4-3-7-18(17)19/h3-4,7-8,14-16,20,26,34H,5-6,9-13H2,1-2H3,(H,25,33)(H,27,32)(H,28,29)(H,30,31)/t16-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286340
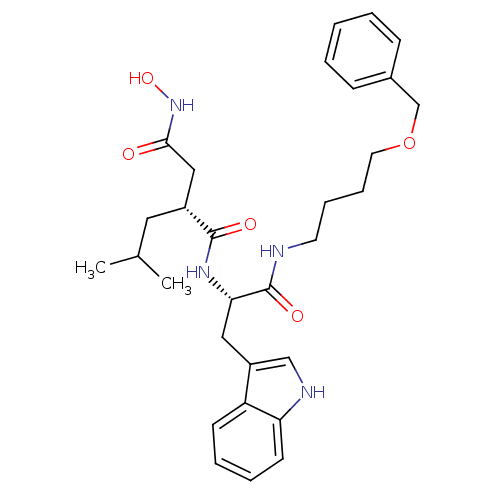
((R)-N*1*-[(S)-1-(4-Benzyloxy-butylcarbamoyl)-2-(1H...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCCCOCc1ccccc1 Show InChI InChI=1S/C30H40N4O5/c1-21(2)16-23(18-28(35)34-38)29(36)33-27(17-24-19-32-26-13-7-6-12-25(24)26)30(37)31-14-8-9-15-39-20-22-10-4-3-5-11-22/h3-7,10-13,19,21,23,27,32,38H,8-9,14-18,20H2,1-2H3,(H,31,37)(H,33,36)(H,34,35)/t23-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50062351
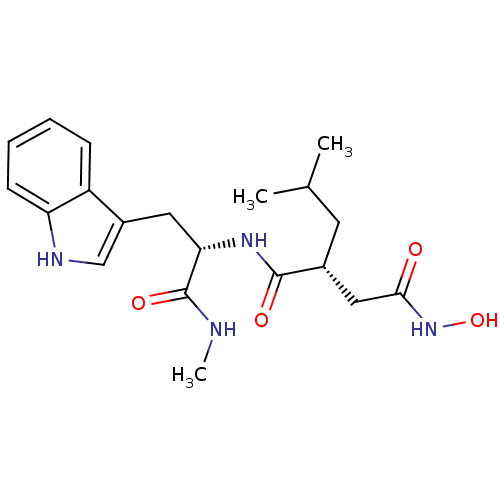
((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286337
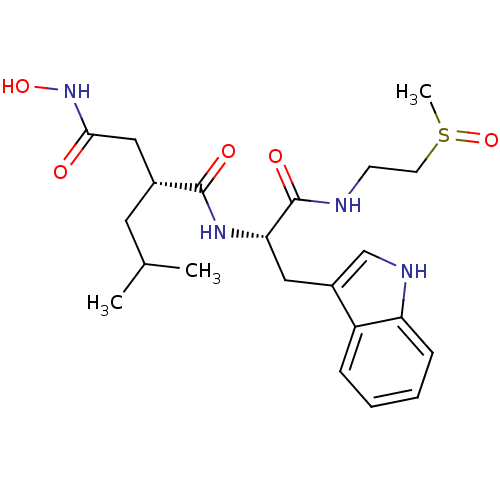
((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(2-...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCS(C)=O Show InChI InChI=1S/C22H32N4O5S/c1-14(2)10-15(12-20(27)26-30)21(28)25-19(22(29)23-8-9-32(3)31)11-16-13-24-18-7-5-4-6-17(16)18/h4-7,13-15,19,24,30H,8-12H2,1-3H3,(H,23,29)(H,25,28)(H,26,27)/t15-,19+,32?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
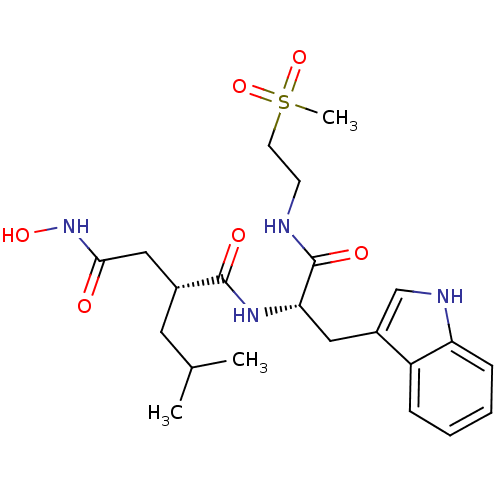
(Homo sapiens (Human)) | BDBM50286334
((R)-N*4*-Hydroxy-N*1*-{(S)-2-(1H-indol-3-yl)-1-[5-...)Show SMILES COc1ccc(cc1)C(=O)CCCCNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C31H40N4O6/c1-20(2)16-22(18-29(37)35-40)30(38)34-27(17-23-19-33-26-9-5-4-8-25(23)26)31(39)32-15-7-6-10-28(36)21-11-13-24(41-3)14-12-21/h4-5,8-9,11-14,19-20,22,27,33,40H,6-7,10,15-18H2,1-3H3,(H,32,39)(H,34,38)(H,35,37)/t22-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286350
((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(2-...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCS(C)(=O)=O Show InChI InChI=1S/C22H32N4O6S/c1-14(2)10-15(12-20(27)26-30)21(28)25-19(22(29)23-8-9-33(3,31)32)11-16-13-24-18-7-5-4-6-17(16)18/h4-7,13-15,19,24,30H,8-12H2,1-3H3,(H,23,29)(H,25,28)(H,26,27)/t15-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286339
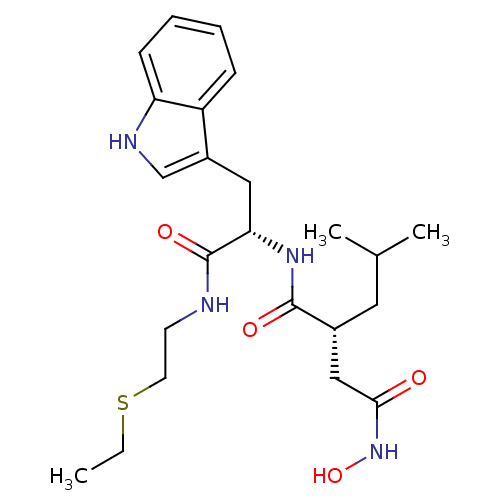
((R)-N*1*-[(S)-1-(2-Ethylsulfanyl-ethylcarbamoyl)-2...)Show SMILES CCSCCNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C23H34N4O4S/c1-4-32-10-9-24-23(30)20(12-17-14-25-19-8-6-5-7-18(17)19)26-22(29)16(11-15(2)3)13-21(28)27-31/h5-8,14-16,20,25,31H,4,9-13H2,1-3H3,(H,24,30)(H,26,29)(H,27,28)/t16-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286349
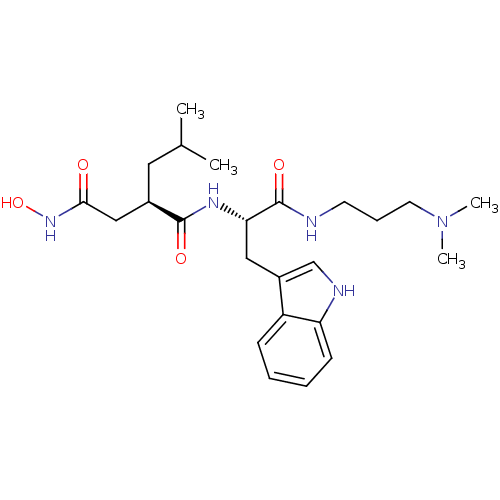
((R)-N*1*-[(S)-1-(3-Dimethylamino-propylcarbamoyl)-...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCCN(C)C Show InChI InChI=1S/C24H37N5O4/c1-16(2)12-17(14-22(30)28-33)23(31)27-21(24(32)25-10-7-11-29(3)4)13-18-15-26-20-9-6-5-8-19(18)20/h5-6,8-9,15-17,21,26,33H,7,10-14H2,1-4H3,(H,25,32)(H,27,31)(H,28,30)/t17-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286347
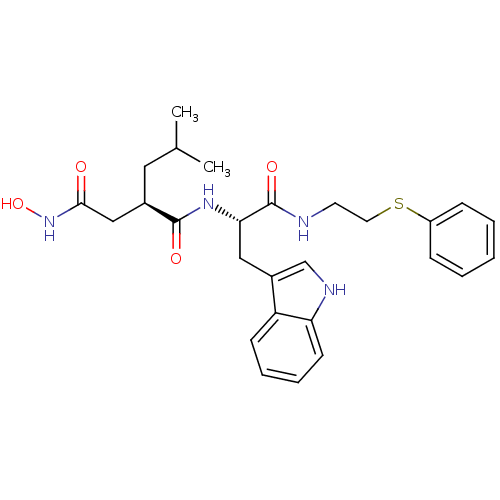
((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(2-...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCSc1ccccc1 Show InChI InChI=1S/C27H34N4O4S/c1-18(2)14-19(16-25(32)31-35)26(33)30-24(15-20-17-29-23-11-7-6-10-22(20)23)27(34)28-12-13-36-21-8-4-3-5-9-21/h3-11,17-19,24,29,35H,12-16H2,1-2H3,(H,28,34)(H,30,33)(H,31,32)/t19-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286345
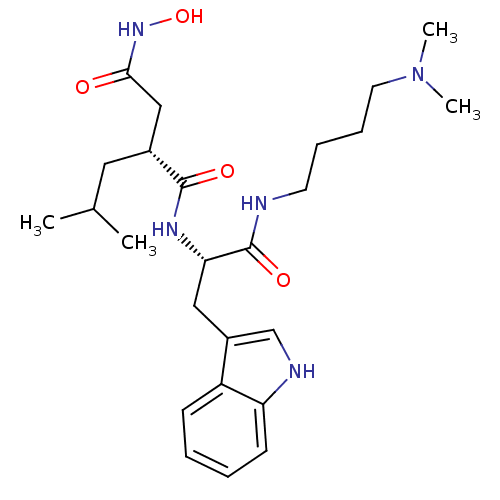
((R)-N*1*-[(S)-1-(4-Dimethylamino-butylcarbamoyl)-2...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCCCN(C)C Show InChI InChI=1S/C25H39N5O4/c1-17(2)13-18(15-23(31)29-34)24(32)28-22(25(33)26-11-7-8-12-30(3)4)14-19-16-27-21-10-6-5-9-20(19)21/h5-6,9-10,16-18,22,27,34H,7-8,11-15H2,1-4H3,(H,26,33)(H,28,32)(H,29,31)/t18-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286343

((R)-N*1*-[(S)-1-(4-Dimethylaminomethyl-benzylcarba...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1ccc(CN(C)C)cc1 Show InChI InChI=1S/C29H39N5O4/c1-19(2)13-22(15-27(35)33-38)28(36)32-26(14-23-17-30-25-8-6-5-7-24(23)25)29(37)31-16-20-9-11-21(12-10-20)18-34(3)4/h5-12,17,19,22,26,30,38H,13-16,18H2,1-4H3,(H,31,37)(H,32,36)(H,33,35)/t22-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286346
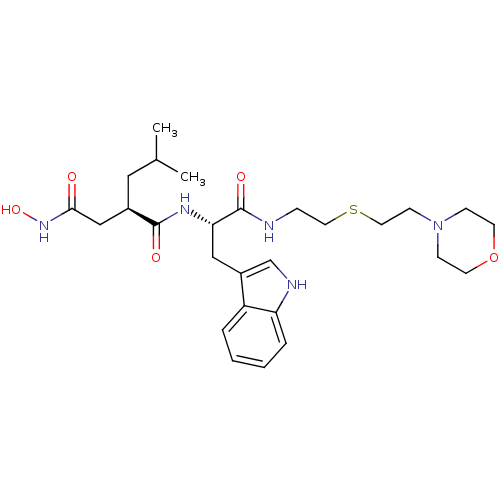
((R)-N*4*-Hydroxy-N*1*-{(S)-2-(1H-indol-3-yl)-1-[2-...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCSCCN1CCOCC1 Show InChI InChI=1S/C27H41N5O5S/c1-19(2)15-20(17-25(33)31-36)26(34)30-24(16-21-18-29-23-6-4-3-5-22(21)23)27(35)28-7-13-38-14-10-32-8-11-37-12-9-32/h3-6,18-20,24,29,36H,7-17H2,1-2H3,(H,28,35)(H,30,34)(H,31,33)/t20-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286336
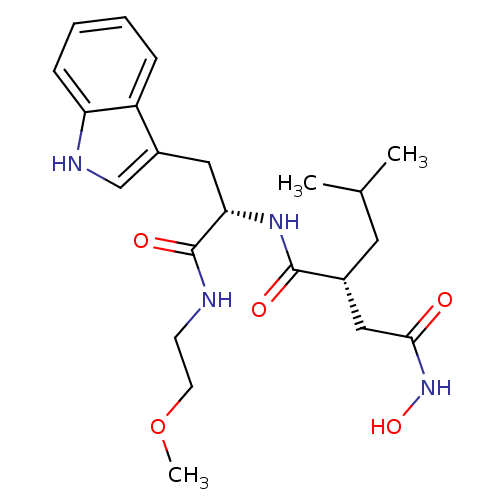
((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(2-...)Show SMILES COCCNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C22H32N4O5/c1-14(2)10-15(12-20(27)26-30)21(28)25-19(22(29)23-8-9-31-3)11-16-13-24-18-7-5-4-6-17(16)18/h4-7,13-15,19,24,30H,8-12H2,1-3H3,(H,23,29)(H,25,28)(H,26,27)/t15-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286348
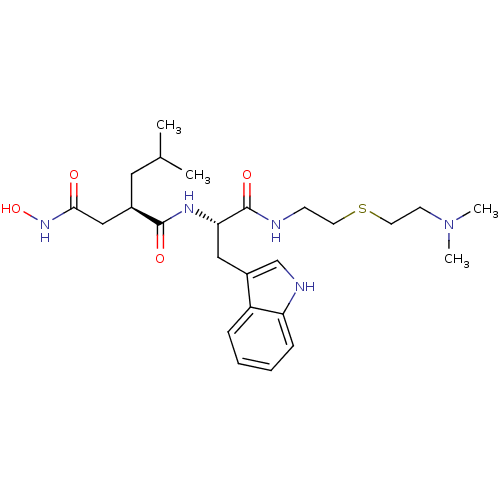
((R)-N*1*-[(S)-1-[2-(2-Dimethylamino-ethylsulfanyl)...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCSCCN(C)C Show InChI InChI=1S/C25H39N5O4S/c1-17(2)13-18(15-23(31)29-34)24(32)28-22(25(33)26-9-11-35-12-10-30(3)4)14-19-16-27-21-8-6-5-7-20(19)21/h5-8,16-18,22,27,34H,9-15H2,1-4H3,(H,26,33)(H,28,32)(H,29,31)/t18-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286342
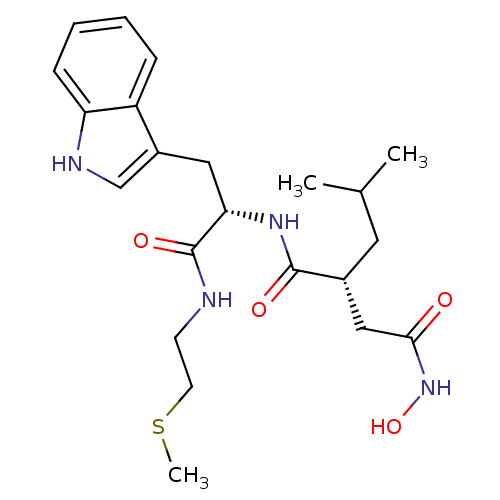
((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(2-...)Show SMILES CSCCNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C22H32N4O4S/c1-14(2)10-15(12-20(27)26-30)21(28)25-19(22(29)23-8-9-31-3)11-16-13-24-18-7-5-4-6-17(16)18/h4-7,13-15,19,24,30H,8-12H2,1-3H3,(H,23,29)(H,25,28)(H,26,27)/t15-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50104969
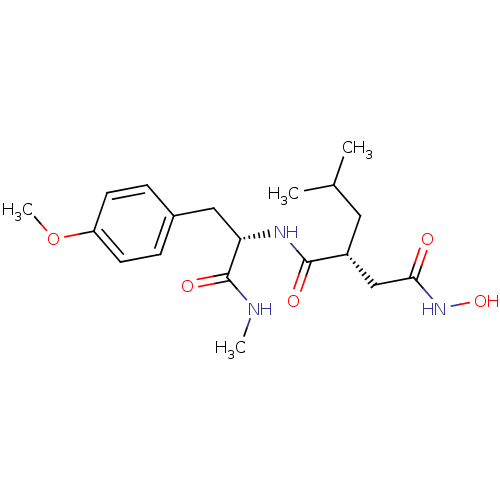
((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-2-(4-methoxy...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C19H29N3O5/c1-12(2)9-14(11-17(23)22-26)18(24)21-16(19(25)20-3)10-13-5-7-15(27-4)8-6-13/h5-8,12,14,16,26H,9-11H2,1-4H3,(H,20,25)(H,21,24)(H,22,23)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286338
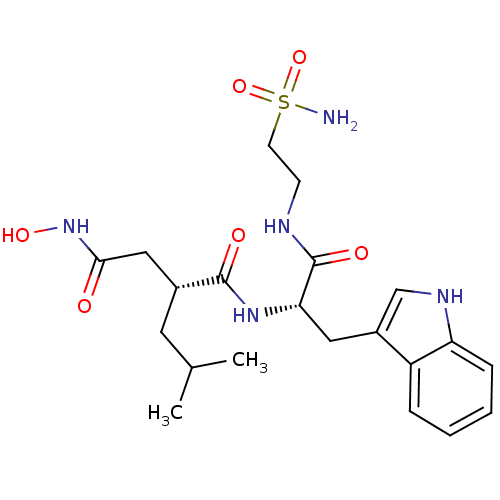
((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(2-...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCS(N)(=O)=O Show InChI InChI=1S/C21H31N5O6S/c1-13(2)9-14(11-19(27)26-30)20(28)25-18(21(29)23-7-8-33(22,31)32)10-15-12-24-17-6-4-3-5-16(15)17/h3-6,12-14,18,24,30H,7-11H2,1-2H3,(H,23,29)(H,25,28)(H,26,27)(H2,22,31,32)/t14-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286341
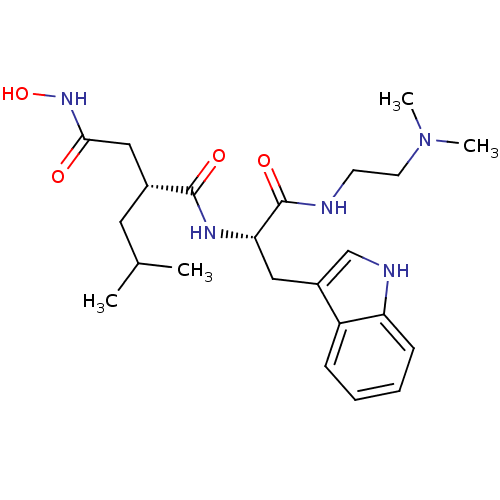
((R)-N*1*-[(S)-1-(2-Dimethylamino-ethylcarbamoyl)-2...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCN(C)C Show InChI InChI=1S/C23H35N5O4/c1-15(2)11-16(13-21(29)27-32)22(30)26-20(23(31)24-9-10-28(3)4)12-17-14-25-19-8-6-5-7-18(17)19/h5-8,14-16,20,25,32H,9-13H2,1-4H3,(H,24,31)(H,26,30)(H,27,29)/t16-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50097699
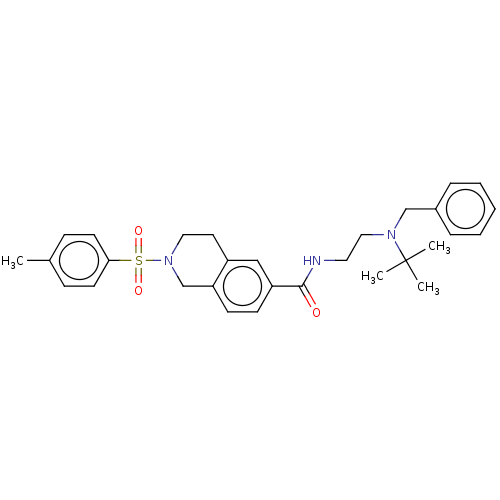
(CHEMBL3590195)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCc2cc(ccc2C1)C(=O)NCCN(Cc1ccccc1)C(C)(C)C Show InChI InChI=1S/C35H49FN6O5S/c1-3-4-5-6-7-8-9-10-11-12-18-40(2)32(45)25-42-23-29(33(46)39-35(42)48-26-27-13-15-30(36)16-14-27)20-28-21-38-34(47)41(22-28)24-31(44)37-17-19-43/h13-16,21-23,43H,3-12,17-20,24-26H2,1-2H3,(H,37,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Displacement of radio-labeled U69,593 from rat kappa opioid receptor expressed in HEK293 cell membranes |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM54817
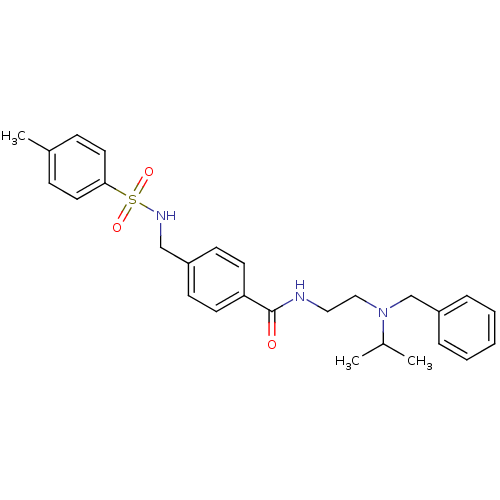
(4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[2-[(p...)Show SMILES CC(C)N(CCNC(=O)c1ccc(CNS(=O)(=O)c2ccc(C)cc2)cc1)Cc1ccccc1 Show InChI InChI=1S/C27H33N3O3S/c1-21(2)30(20-24-7-5-4-6-8-24)18-17-28-27(31)25-13-11-23(12-14-25)19-29-34(32,33)26-15-9-22(3)10-16-26/h4-16,21,29H,17-20H2,1-3H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Displacement of radio-labeled U69,593 from rat kappa opioid receptor expressed in HEK293 cell membranes |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50097703
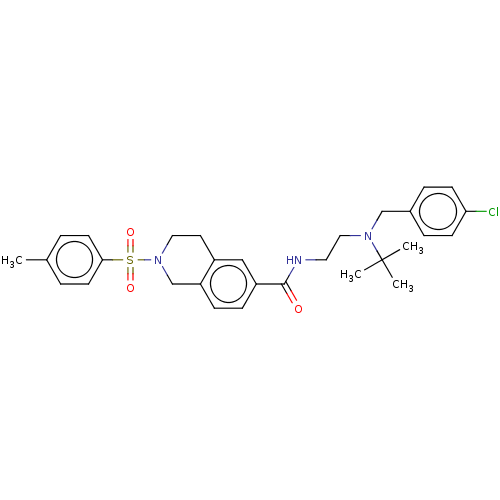
(CHEMBL3590199)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCc2cc(ccc2C1)C(=O)NCCN(Cc1ccc(Cl)cc1)C(C)(C)C Show InChI InChI=1S/C29H38FN5O3S/c1-4-6-7-8-9-10-15-34(3)26(36)20-35-19-24(16-23-17-31-28(32-18-23)38-5-2)27(37)33-29(35)39-21-22-11-13-25(30)14-12-22/h11-14,17-19H,4-10,15-16,20-21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Displacement of radio-labeled U69,593 from rat kappa opioid receptor expressed in HEK293 cell membranes |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50097697
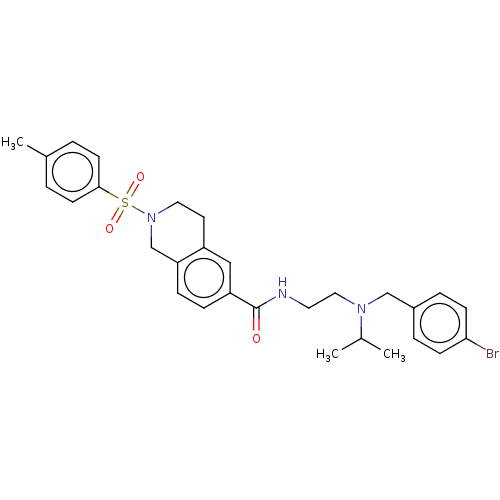
(CHEMBL3590193)Show SMILES CC(C)N(CCNC(=O)c1ccc2CN(CCc2c1)S(=O)(=O)c1ccc(C)cc1)Cc1ccc(Br)cc1 Show InChI InChI=1S/C34H46FN7O4S/c1-4-5-6-7-8-9-14-39(3)30(43)23-42-22-28(32(45)37-34(42)47-25-26-10-12-29(35)13-11-26)19-27-20-36-33(46)41(21-27)24-31(44)40-17-15-38(2)16-18-40/h10-13,20-22H,4-9,14-19,23-25H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Displacement of radio-labeled U69,593 from rat kappa opioid receptor expressed in HEK293 cell membranes |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM54817

(4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[2-[(p...)Show SMILES CC(C)N(CCNC(=O)c1ccc(CNS(=O)(=O)c2ccc(C)cc2)cc1)Cc1ccccc1 Show InChI InChI=1S/C27H33N3O3S/c1-21(2)30(20-24-7-5-4-6-8-24)18-17-28-27(31)25-13-11-23(12-14-25)19-29-34(32,33)26-15-9-22(3)10-16-26/h4-16,21,29H,17-20H2,1-3H3,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 306 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor (unknown origin) |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM54817

(4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[2-[(p...)Show SMILES CC(C)N(CCNC(=O)c1ccc(CNS(=O)(=O)c2ccc(C)cc2)cc1)Cc1ccccc1 Show InChI InChI=1S/C27H33N3O3S/c1-21(2)30(20-24-7-5-4-6-8-24)18-17-28-27(31)25-13-11-23(12-14-25)19-29-34(32,33)26-15-9-22(3)10-16-26/h4-16,21,29H,17-20H2,1-3H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Binding affinity to delta opioid receptor (unknown origin) |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50097699

(CHEMBL3590195)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCc2cc(ccc2C1)C(=O)NCCN(Cc1ccccc1)C(C)(C)C Show InChI InChI=1S/C35H49FN6O5S/c1-3-4-5-6-7-8-9-10-11-12-18-40(2)32(45)25-42-23-29(33(46)39-35(42)48-26-27-13-15-30(36)16-14-27)20-28-21-38-34(47)41(22-28)24-31(44)37-17-19-43/h13-16,21-23,43H,3-12,17-20,24-26H2,1-2H3,(H,37,44) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor (unknown origin) |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50097699

(CHEMBL3590195)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCc2cc(ccc2C1)C(=O)NCCN(Cc1ccccc1)C(C)(C)C Show InChI InChI=1S/C35H49FN6O5S/c1-3-4-5-6-7-8-9-10-11-12-18-40(2)32(45)25-42-23-29(33(46)39-35(42)48-26-27-13-15-30(36)16-14-27)20-28-21-38-34(47)41(22-28)24-31(44)37-17-19-43/h13-16,21-23,43H,3-12,17-20,24-26H2,1-2H3,(H,37,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Binding affinity to delta opioid receptor (unknown origin) |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50097697

(CHEMBL3590193)Show SMILES CC(C)N(CCNC(=O)c1ccc2CN(CCc2c1)S(=O)(=O)c1ccc(C)cc1)Cc1ccc(Br)cc1 Show InChI InChI=1S/C34H46FN7O4S/c1-4-5-6-7-8-9-14-39(3)30(43)23-42-22-28(32(45)37-34(42)47-25-26-10-12-29(35)13-11-26)19-27-20-36-33(46)41(21-27)24-31(44)40-17-15-38(2)16-18-40/h10-13,20-22H,4-9,14-19,23-25H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Binding affinity to delta opioid receptor (unknown origin) |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50097697

(CHEMBL3590193)Show SMILES CC(C)N(CCNC(=O)c1ccc2CN(CCc2c1)S(=O)(=O)c1ccc(C)cc1)Cc1ccc(Br)cc1 Show InChI InChI=1S/C34H46FN7O4S/c1-4-5-6-7-8-9-14-39(3)30(43)23-42-22-28(32(45)37-34(42)47-25-26-10-12-29(35)13-11-26)19-27-20-36-33(46)41(21-27)24-31(44)40-17-15-38(2)16-18-40/h10-13,20-22H,4-9,14-19,23-25H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor (unknown origin) |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50097703

(CHEMBL3590199)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCc2cc(ccc2C1)C(=O)NCCN(Cc1ccc(Cl)cc1)C(C)(C)C Show InChI InChI=1S/C29H38FN5O3S/c1-4-6-7-8-9-10-15-34(3)26(36)20-35-19-24(16-23-17-31-28(32-18-23)38-5-2)27(37)33-29(35)39-21-22-11-13-25(30)14-12-22/h11-14,17-19H,4-10,15-16,20-21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Binding affinity to delta opioid receptor (unknown origin) |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50097703

(CHEMBL3590199)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCc2cc(ccc2C1)C(=O)NCCN(Cc1ccc(Cl)cc1)C(C)(C)C Show InChI InChI=1S/C29H38FN5O3S/c1-4-6-7-8-9-10-15-34(3)26(36)20-35-19-24(16-23-17-31-28(32-18-23)38-5-2)27(37)33-29(35)39-21-22-11-13-25(30)14-12-22/h11-14,17-19H,4-10,15-16,20-21H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor (unknown origin) |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82551
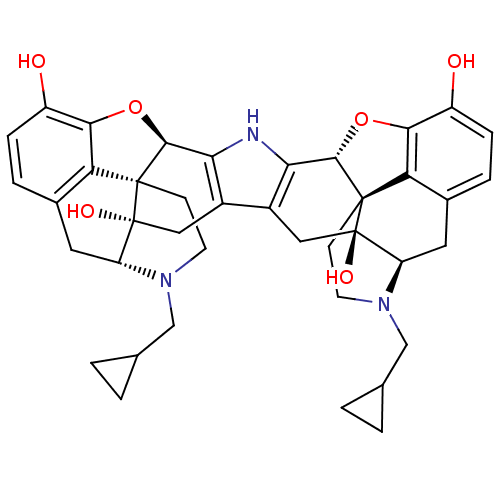
(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Antagonist activity against HA-tagged human recombinant kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by [35S]GTPgammaS bi... |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human JAK3 catalytic domain expressed in Sf9 cells by ELISA |
J Med Chem 53: 8468-84 (2010)
Article DOI: 10.1021/jm1004286
BindingDB Entry DOI: 10.7270/Q2154H9D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human JAK3 catalytic domain expressed in Sf9 cells by ELISA |
J Med Chem 53: 8468-84 (2010)
Article DOI: 10.1021/jm1004286
BindingDB Entry DOI: 10.7270/Q2154H9D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by solution phase kinase assay |
J Med Chem 53: 8468-84 (2010)
Article DOI: 10.1021/jm1004286
BindingDB Entry DOI: 10.7270/Q2154H9D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50097703

(CHEMBL3590199)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCc2cc(ccc2C1)C(=O)NCCN(Cc1ccc(Cl)cc1)C(C)(C)C Show InChI InChI=1S/C29H38FN5O3S/c1-4-6-7-8-9-10-15-34(3)26(36)20-35-19-24(16-23-17-31-28(32-18-23)38-5-2)27(37)33-29(35)39-21-22-11-13-25(30)14-12-22/h11-14,17-19H,4-10,15-16,20-21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Antagonist activity against HA-tagged human recombinant kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by [35S]GTPgammaS bi... |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50332622
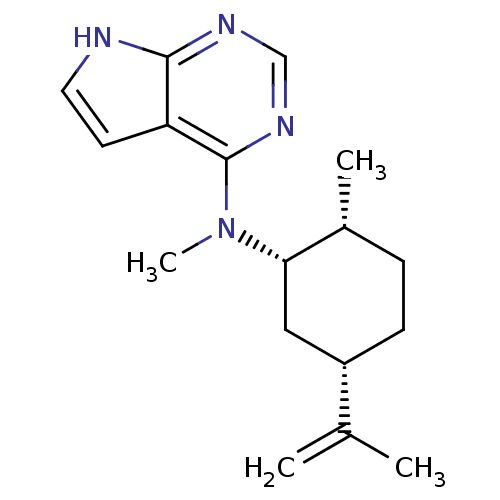
(CHEMBL1630782 | N-Methyl-N-((1S,2R,5S)-2-methyl-5-...)Show SMILES C[C@@H]1CC[C@@H](C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(C)=C |r| Show InChI InChI=1S/C17H24N4/c1-11(2)13-6-5-12(3)15(9-13)21(4)17-14-7-8-18-16(14)19-10-20-17/h7-8,10,12-13,15H,1,5-6,9H2,2-4H3,(H,18,19,20)/t12-,13+,15+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human JAK3 catalytic domain expressed in Sf9 cells by ELISA |
J Med Chem 53: 8468-84 (2010)
Article DOI: 10.1021/jm1004286
BindingDB Entry DOI: 10.7270/Q2154H9D |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82551

(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Antagonist activity against human kappa opioid receptor expressed in human U2OS cells co-expressing beta-arrestin2 pre-treated for 15 mins before U69... |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50097704

(CHEMBL3590200)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCc2cc(ccc2C1)C(=O)NCCN(Cc1ccc(Br)cc1)C(C)(C)C Show InChI InChI=1S/C37H51FN6O5S/c1-3-4-5-6-7-8-9-10-11-12-17-41(2)33(45)26-44-25-31(35(47)40-37(44)50-28-29-13-15-32(38)16-14-29)22-30-23-39-36(48)43(24-30)27-34(46)42-18-20-49-21-19-42/h13-16,23-25H,3-12,17-22,26-28H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Antagonist activity against HA-tagged human recombinant kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by [35S]GTPgammaS bi... |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 by solution phase kinase assay |
J Med Chem 53: 8468-84 (2010)
Article DOI: 10.1021/jm1004286
BindingDB Entry DOI: 10.7270/Q2154H9D |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50097700
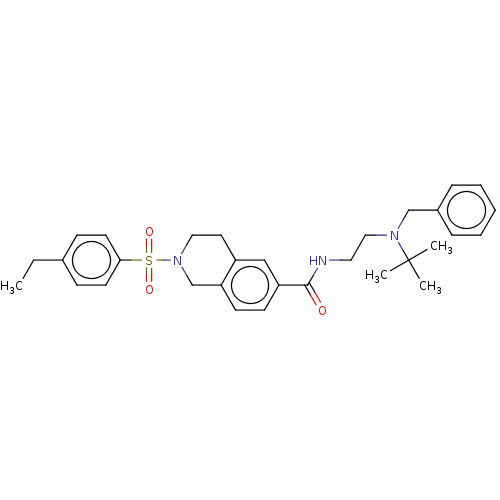
(CHEMBL3590196)Show SMILES CCc1ccc(cc1)S(=O)(=O)N1CCc2cc(ccc2C1)C(=O)NCCN(Cc1ccccc1)C(C)(C)C Show InChI InChI=1S/C28H36FN5O3S/c1-4-5-6-7-8-9-14-33(2)25(35)19-34-18-23(15-22-16-30-27(37-3)31-17-22)26(36)32-28(34)38-20-21-10-12-24(29)13-11-21/h10-13,16-18H,4-9,14-15,19-20H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Antagonist activity against HA-tagged human recombinant kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by [35S]GTPgammaS bi... |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50097701

(CHEMBL3590197)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCc2cc(ccc2C1)C(=O)NCCN(Cc1ccc(F)cc1)C(C)(C)C Show InChI InChI=1S/C31H40FN5O5S/c1-4-6-7-8-9-10-15-35(3)27(38)20-37-19-25(16-24-17-33-30(41)36(18-24)21-28(39)42-5-2)29(40)34-31(37)43-22-23-11-13-26(32)14-12-23/h11-14,17-19H,4-10,15-16,20-22H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Antagonist activity against HA-tagged human recombinant kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by [35S]GTPgammaS bi... |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50332622

(CHEMBL1630782 | N-Methyl-N-((1S,2R,5S)-2-methyl-5-...)Show SMILES C[C@@H]1CC[C@@H](C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(C)=C |r| Show InChI InChI=1S/C17H24N4/c1-11(2)13-6-5-12(3)15(9-13)21(4)17-14-7-8-18-16(14)19-10-20-17/h7-8,10,12-13,15H,1,5-6,9H2,2-4H3,(H,18,19,20)/t12-,13+,15+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human JAK3 catalytic domain expressed in Sf9 cells by ELISA |
J Med Chem 53: 8468-84 (2010)
Article DOI: 10.1021/jm1004286
BindingDB Entry DOI: 10.7270/Q2154H9D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 by solution phase kinase assay |
J Med Chem 53: 8468-84 (2010)
Article DOI: 10.1021/jm1004286
BindingDB Entry DOI: 10.7270/Q2154H9D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82551

(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Antagonist activity against HA-tagged human recombinant kappa opioid receptor expressed in CHO cells assessed as inhibition of U69,593-induced ERK ph... |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50097699

(CHEMBL3590195)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCc2cc(ccc2C1)C(=O)NCCN(Cc1ccccc1)C(C)(C)C Show InChI InChI=1S/C35H49FN6O5S/c1-3-4-5-6-7-8-9-10-11-12-18-40(2)32(45)25-42-23-29(33(46)39-35(42)48-26-27-13-15-30(36)16-14-27)20-28-21-38-34(47)41(22-28)24-31(44)37-17-19-43/h13-16,21-23,43H,3-12,17-20,24-26H2,1-2H3,(H,37,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Antagonist activity against HA-tagged human recombinant kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by [35S]GTPgammaS bi... |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50097696

(CHEMBL3590192)Show SMILES CC(C)N(CCNC(=O)c1ccc2CN(CCc2c1)S(=O)(=O)c1ccc(C)cc1)Cc1ccc(Cl)cc1 Show InChI InChI=1S/C29H34ClN3O3S/c1-21(2)32(19-23-6-10-27(30)11-7-23)17-15-31-29(34)25-8-9-26-20-33(16-14-24(26)18-25)37(35,36)28-12-4-22(3)5-13-28/h4-13,18,21H,14-17,19-20H2,1-3H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Antagonist activity against HA-tagged human recombinant kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by [35S]GTPgammaS bi... |
Bioorg Med Chem 23: 3948-56 (2015)
Article DOI: 10.1016/j.bmc.2014.12.033
BindingDB Entry DOI: 10.7270/Q2R49SJX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50332620

(2-Methyl-3-(4-methyl-3-(methyl(7H-pyrrolo[2,3-d]py...)Show SMILES CC(C#N)C(=O)N1CCC(C)C(C1)N(C)c1ncnc2[nH]ccc12 Show InChI InChI=1S/C17H22N6O/c1-11-5-7-23(17(24)12(2)8-18)9-14(11)22(3)16-13-4-6-19-15(13)20-10-21-16/h4,6,10-12,14H,5,7,9H2,1-3H3,(H,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human JAK3 catalytic domain expressed in Sf9 cells by ELISA |
J Med Chem 53: 8468-84 (2010)
Article DOI: 10.1021/jm1004286
BindingDB Entry DOI: 10.7270/Q2154H9D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50332623

(CHEMBL1630786 | N-Benzyl-2-((R)-2-((1S,3S,4R)-4-me...)Show SMILES C[C@@H](COCC(=O)NCc1ccccc1)[C@H]1CC[C@@H](C)[C@H](C1)N(C)c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C26H35N5O2/c1-18-9-10-21(13-23(18)31(3)26-22-11-12-27-25(22)29-17-30-26)19(2)15-33-16-24(32)28-14-20-7-5-4-6-8-20/h4-8,11-12,17-19,21,23H,9-10,13-16H2,1-3H3,(H,28,32)(H,27,29,30)/t18-,19+,21+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human JAK3 catalytic domain expressed in Sf9 cells by ELISA |
J Med Chem 53: 8468-84 (2010)
Article DOI: 10.1021/jm1004286
BindingDB Entry DOI: 10.7270/Q2154H9D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50332604
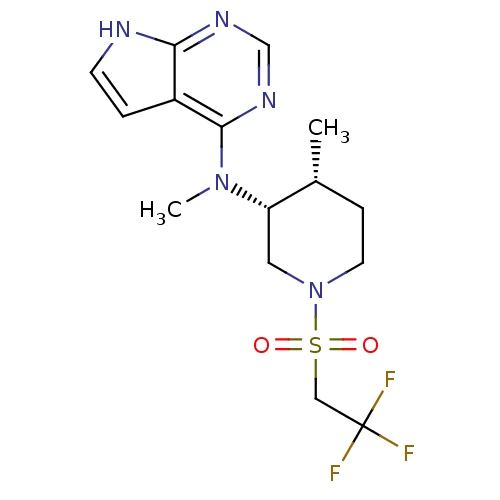
(CHEMBL1630788 | cis-N-Methyl-N-(4-methyl-1-(2,2,2-...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)S(=O)(=O)CC(F)(F)F |r| Show InChI InChI=1S/C15H20F3N5O2S/c1-10-4-6-23(26(24,25)8-15(16,17)18)7-12(10)22(2)14-11-3-5-19-13(11)20-9-21-14/h3,5,9-10,12H,4,6-8H2,1-2H3,(H,19,20,21)/t10-,12+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human JAK3 catalytic domain expressed in Sf9 cells by ELISA |
J Med Chem 53: 8468-84 (2010)
Article DOI: 10.1021/jm1004286
BindingDB Entry DOI: 10.7270/Q2154H9D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data