 Found 669 hits with Last Name = 'witte' and Initial = 'dg'
Found 669 hits with Last Name = 'witte' and Initial = 'dg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine receptor H3
(Dog) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50074629

(4-[(1R,2R)-2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl...)Show InChI InChI=1S/C14H20N2/c1-14(2,3)7-5-4-6-11-8-12(11)13-9-15-10-16-13/h9-12H,5,7-8H2,1-3H3,(H,15,16)/t11-,12-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27210

((2R)-N,3-dimethyl-2-(methylamino)-N-[(1R,2S,5S,6S,...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@H](NC)C(C)C)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C29H49N3O/c1-18(2)26(30-5)27(33)32(7)21-12-14-28(4)20(16-21)8-9-22-24(28)13-15-29-17-31(6)19(3)23(29)10-11-25(22)29/h8,18-19,21-26,30H,9-17H2,1-7H3/t19-,21-,22+,23+,24-,25-,26+,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha methyl histamine from human cloned histamine H3 receptor expressed in C6 cells |
J Med Chem 52: 4640-9 (2009)
Article DOI: 10.1021/jm900480x
BindingDB Entry DOI: 10.7270/Q2Z31ZNT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27210

((2R)-N,3-dimethyl-2-(methylamino)-N-[(1R,2S,5S,6S,...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@H](NC)C(C)C)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C29H49N3O/c1-18(2)26(30-5)27(33)32(7)21-12-14-28(4)20(16-21)8-9-22-24(28)13-15-29-17-31(6)19(3)23(29)10-11-25(22)29/h8,18-19,21-26,30H,9-17H2,1-7H3/t19-,21-,22+,23+,24-,25-,26+,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27208
((2S,3S)-2-amino-N,3-dimethyl-N-[(1R,2S,5S,6S,9R,12...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@@H](N)[C@@H](C)CC)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C29H49N3O/c1-7-18(2)26(30)27(33)32(6)21-12-14-28(4)20(16-21)8-9-22-24(28)13-15-29-17-31(5)19(3)23(29)10-11-25(22)29/h8,18-19,21-26H,7,9-17,30H2,1-6H3/t18-,19-,21-,22+,23+,24-,25-,26-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50074629

(4-[(1R,2R)-2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl...)Show InChI InChI=1S/C14H20N2/c1-14(2,3)7-5-4-6-11-8-12(11)13-9-15-10-16-13/h9-12H,5,7-8H2,1-3H3,(H,15,16)/t11-,12-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27209

((2R,3R)-2-amino-N,3-dimethyl-N-[(1R,2S,5S,6S,9R,12...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@H](N)[C@H](C)CC)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C29H49N3O/c1-7-18(2)26(30)27(33)32(6)21-12-14-28(4)20(16-21)8-9-22-24(28)13-15-29-17-31(5)19(3)23(29)10-11-25(22)29/h8,18-19,21-26H,7,9-17,30H2,1-6H3/t18-,19+,21+,22-,23-,24+,25+,26-,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.370 | -53.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(GUINEA PIG) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 39: 346-51 (1991)
BindingDB Entry DOI: 10.7270/Q2222S8S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158595
((4'-(3-((2R,5R)-2,5-dimethylpyrrolidin-1-yl)propox...)Show SMILES C[C@@H]1CC[C@@H](C)N1CCCOc1ccc(cc1)-c1ccc(cc1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H34N2O3/c1-20-4-5-21(2)28(20)14-3-17-31-25-12-10-23(11-13-25)22-6-8-24(9-7-22)26(29)27-15-18-30-19-16-27/h6-13,20-21H,3-5,14-19H2,1-2H3/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158595

((4'-(3-((2R,5R)-2,5-dimethylpyrrolidin-1-yl)propox...)Show SMILES C[C@@H]1CC[C@@H](C)N1CCCOc1ccc(cc1)-c1ccc(cc1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H34N2O3/c1-20-4-5-21(2)28(20)14-3-17-31-25-12-10-23(11-13-25)22-6-8-24(9-7-22)26(29)27-15-18-30-19-16-27/h6-13,20-21H,3-5,14-19H2,1-2H3/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50139391
((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C22H22N2O/c1-16-3-2-11-24(16)12-10-21-14-20-13-19(8-9-22(20)25-21)18-6-4-17(15-23)5-7-18/h4-9,13-14,16H,2-3,10-12H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha methyl histamine from human cloned histamine H3 receptor expressed in C6 cells |
J Med Chem 52: 4640-9 (2009)
Article DOI: 10.1021/jm900480x
BindingDB Entry DOI: 10.7270/Q2Z31ZNT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50139391

((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C22H22N2O/c1-16-3-2-11-24(16)12-10-21-14-20-13-19(8-9-22(20)25-21)18-6-4-17(15-23)5-7-18/h4-9,13-14,16H,2-3,10-12H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM27208

((2S,3S)-2-amino-N,3-dimethyl-N-[(1R,2S,5S,6S,9R,12...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@@H](N)[C@@H](C)CC)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C29H49N3O/c1-7-18(2)26(30)27(33)32(6)21-12-14-28(4)20(16-21)8-9-22-24(28)13-15-29-17-31(5)19(3)23(29)10-11-25(22)29/h8,18-19,21-26H,7,9-17,30H2,1-6H3/t18-,19-,21-,22+,23+,24-,25-,26-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50294063
((3aR,6aR)-4'-(5-Methyl-hexahydro-pyrrolo[3,4-b]pyr...)Show SMILES CN1C[C@H]2CCN([C@H]2C1)c1ccc(cc1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C20H21N3/c1-22-13-18-10-11-23(20(18)14-22)19-8-6-17(7-9-19)16-4-2-15(12-21)3-5-16/h2-9,18,20H,10-11,13-14H2,1H3/t18-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha methyl histamine from human cloned histamine H3 receptor expressed in C6 cells |
J Med Chem 52: 4640-9 (2009)
Article DOI: 10.1021/jm900480x
BindingDB Entry DOI: 10.7270/Q2Z31ZNT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50158595

((4'-(3-((2R,5R)-2,5-dimethylpyrrolidin-1-yl)propox...)Show SMILES C[C@@H]1CC[C@@H](C)N1CCCOc1ccc(cc1)-c1ccc(cc1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H34N2O3/c1-20-4-5-21(2)28(20)14-3-17-31-25-12-10-23(11-13-25)22-6-8-24(9-7-22)26(29)27-15-18-30-19-16-27/h6-13,20-21H,3-5,14-19H2,1-2H3/t20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50074629

(4-[(1R,2R)-2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl...)Show InChI InChI=1S/C14H20N2/c1-14(2,3)7-5-4-6-11-8-12(11)13-9-15-10-16-13/h9-12H,5,7-8H2,1-3H3,(H,15,16)/t11-,12-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.654 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50158601
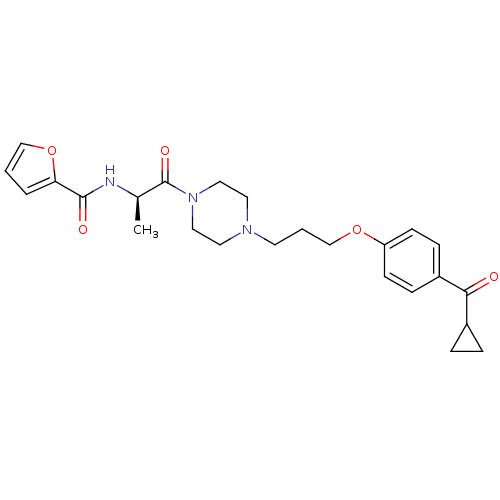
(A-317920 | CHEMBL361355 | Furan-2-carboxylic acid ...)Show SMILES C[C@@H](NC(=O)c1ccco1)C(=O)N1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)CC1 Show InChI InChI=1S/C25H31N3O5/c1-18(26-24(30)22-4-2-16-33-22)25(31)28-14-12-27(13-15-28)11-3-17-32-21-9-7-20(8-10-21)23(29)19-5-6-19/h2,4,7-10,16,18-19H,3,5-6,11-15,17H2,1H3,(H,26,30)/t18-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50158601

(A-317920 | CHEMBL361355 | Furan-2-carboxylic acid ...)Show SMILES C[C@@H](NC(=O)c1ccco1)C(=O)N1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)CC1 Show InChI InChI=1S/C25H31N3O5/c1-18(26-24(30)22-4-2-16-33-22)25(31)28-14-12-27(13-15-28)11-3-17-32-21-9-7-20(8-10-21)23(29)19-5-6-19/h2,4,7-10,16,18-19H,3,5-6,11-15,17H2,1H3,(H,26,30)/t18-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27212

((2S)-2-acetamido-N-methyl-3-phenyl-N-[(1R,2S,5S,6S...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@H](Cc4ccccc4)NC(C)=O)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C34H49N3O2/c1-22-28-13-14-30-27-12-11-25-20-26(15-17-33(25,3)29(27)16-18-34(28,30)21-36(22)4)37(5)32(39)31(35-23(2)38)19-24-9-7-6-8-10-24/h6-11,22,26-31H,12-21H2,1-5H3,(H,35,38)/t22-,26-,27+,28+,29-,30-,31-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.15 | -51.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Dog) | BDBM50158595

((4'-(3-((2R,5R)-2,5-dimethylpyrrolidin-1-yl)propox...)Show SMILES C[C@@H]1CC[C@@H](C)N1CCCOc1ccc(cc1)-c1ccc(cc1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H34N2O3/c1-20-4-5-21(2)28(20)14-3-17-31-25-12-10-23(11-13-25)22-6-8-24(9-7-22)26(29)27-15-18-30-19-16-27/h6-13,20-21H,3-5,14-19H2,1-2H3/t20-,21-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50139391

((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C22H22N2O/c1-16-3-2-11-24(16)12-10-21-14-20-13-19(8-9-22(20)25-21)18-6-4-17(15-23)5-7-18/h4-9,13-14,16H,2-3,10-12H2,1H3/t16-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM86061
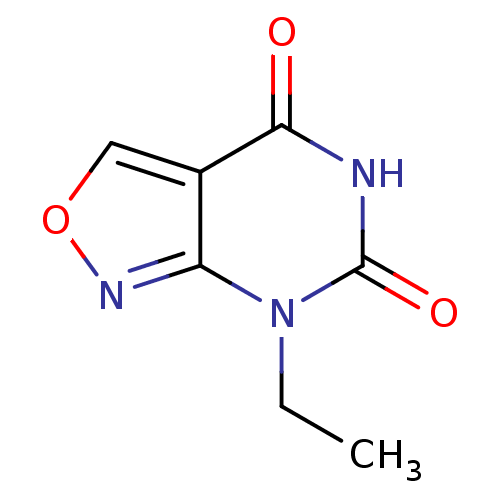
(A-304121)Show InChI InChI=1S/C7H7N3O3/c1-2-10-5-4(3-13-9-5)6(11)8-7(10)12/h3H,2H2,1H3,(H,8,11,12) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Dog) | BDBM50074629

(4-[(1R,2R)-2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl...)Show InChI InChI=1S/C14H20N2/c1-14(2,3)7-5-4-6-11-8-12(11)13-9-15-10-16-13/h9-12H,5,7-8H2,1-3H3,(H,15,16)/t11-,12-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Rattus norvegicus (rat)) | BDBM26399
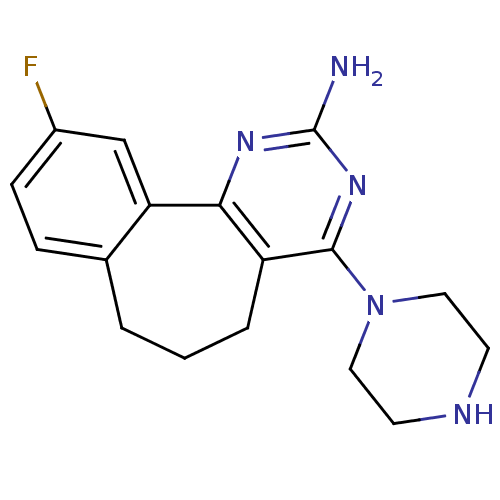
(14-fluoro-6-(piperazin-1-yl)-3,5-diazatricyclo[9.4...)Show InChI InChI=1S/C17H20FN5/c18-12-5-4-11-2-1-3-13-15(14(11)10-12)21-17(19)22-16(13)23-8-6-20-7-9-23/h4-5,10,20H,1-3,6-9H2,(H2,19,21,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.45 | -50.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 6547-57 (2008)
Article DOI: 10.1021/jm800670r
BindingDB Entry DOI: 10.7270/Q2KH0KM5 |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50158595

((4'-(3-((2R,5R)-2,5-dimethylpyrrolidin-1-yl)propox...)Show SMILES C[C@@H]1CC[C@@H](C)N1CCCOc1ccc(cc1)-c1ccc(cc1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H34N2O3/c1-20-4-5-21(2)28(20)14-3-17-31-25-12-10-23(11-13-25)22-6-8-24(9-7-22)26(29)27-15-18-30-19-16-27/h6-13,20-21H,3-5,14-19H2,1-2H3/t20-,21-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Rattus norvegicus (rat)) | BDBM50415770
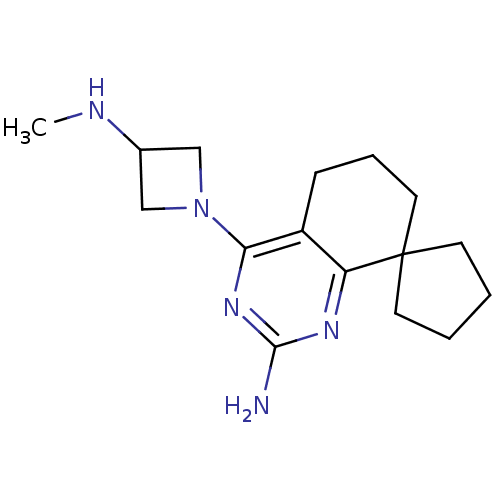
(CHEMBL1079516)Show InChI InChI=1S/C16H25N5/c1-18-11-9-21(10-11)14-12-5-4-8-16(6-2-3-7-16)13(12)19-15(17)20-14/h11,18H,2-10H2,1H3,(H2,17,19,20) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from rat histamine H4 receptor after 1 hr by liquid scintillation counting |
Bioorg Med Chem Lett 20: 1900-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.131
BindingDB Entry DOI: 10.7270/Q21V5G6X |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50294089

((3aR,6aR)-1-(4'-Methoxy-biphenyl-4-yl)-5-methyl-oc...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)N1CC[C@@H]2CN(C)C[C@H]12 |r| Show InChI InChI=1S/C20H24N2O/c1-21-13-17-11-12-22(20(17)14-21)18-7-3-15(4-8-18)16-5-9-19(23-2)10-6-16/h3-10,17,20H,11-14H2,1-2H3/t17-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha methyl histamine from human cloned histamine H3 receptor expressed in C6 cells |
J Med Chem 52: 4640-9 (2009)
Article DOI: 10.1021/jm900480x
BindingDB Entry DOI: 10.7270/Q2Z31ZNT |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50415770

(CHEMBL1079516)Show InChI InChI=1S/C16H25N5/c1-18-11-9-21(10-11)14-12-5-4-8-16(6-2-3-7-16)13(12)19-15(17)20-14/h11,18H,2-10H2,1H3,(H2,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from human histamine H4 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting |
Bioorg Med Chem Lett 20: 1900-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.131
BindingDB Entry DOI: 10.7270/Q21V5G6X |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27211

((2S)-2-acetamido-N,4-dimethyl-N-[(1R,2S,5S,6S,9R,1...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@H](CC(C)C)NC(C)=O)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C31H51N3O2/c1-19(2)16-28(32-21(4)35)29(36)34(7)23-12-14-30(5)22(17-23)8-9-24-26(30)13-15-31-18-33(6)20(3)25(31)10-11-27(24)31/h8,19-20,23-28H,9-18H2,1-7H3,(H,32,35)/t20-,23-,24+,25+,26-,27-,28-,30-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.62 | -50.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27201
(4-{methyl[(1R,2S,5S,6S,9R,12S,13R,16S)-6,7,13-trim...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)c4ccc(cc4)C#N)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C30H41N3/c1-20-26-11-12-28-25-10-7-22-17-24(33(4)23-8-5-21(18-31)6-9-23)13-15-29(22,2)27(25)14-16-30(26,28)19-32(20)3/h5-9,20,24-28H,10-17,19H2,1-4H3/t20-,24-,25+,26+,27-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.66 | -50.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50158595

((4'-(3-((2R,5R)-2,5-dimethylpyrrolidin-1-yl)propox...)Show SMILES C[C@@H]1CC[C@@H](C)N1CCCOc1ccc(cc1)-c1ccc(cc1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H34N2O3/c1-20-4-5-21(2)28(20)14-3-17-31-25-12-10-23(11-13-25)22-6-8-24(9-7-22)26(29)27-15-18-30-19-16-27/h6-13,20-21H,3-5,14-19H2,1-2H3/t20-,21-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50415769
(CHEMBL1089390)Show SMILES CN[C@@H]1CCN(C1)c1nc(N)nc2c1CCCC21CCCC1 |r| Show InChI InChI=1S/C17H27N5/c1-19-12-6-10-22(11-12)15-13-5-4-9-17(7-2-3-8-17)14(13)20-16(18)21-15/h12,19H,2-11H2,1H3,(H2,18,20,21)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from human histamine H4 receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting |
Bioorg Med Chem Lett 20: 1900-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.131
BindingDB Entry DOI: 10.7270/Q21V5G6X |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM27212

((2S)-2-acetamido-N-methyl-3-phenyl-N-[(1R,2S,5S,6S...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@H](Cc4ccccc4)NC(C)=O)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C34H49N3O2/c1-22-28-13-14-30-27-12-11-25-20-26(15-17-33(25,3)29(27)16-18-34(28,30)21-36(22)4)37(5)32(39)31(35-23(2)38)19-24-9-7-6-8-10-24/h6-11,22,26-31H,12-21H2,1-5H3,(H,35,38)/t22-,26-,27+,28+,29-,30-,31-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50158601

(A-317920 | CHEMBL361355 | Furan-2-carboxylic acid ...)Show SMILES C[C@@H](NC(=O)c1ccco1)C(=O)N1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)CC1 Show InChI InChI=1S/C25H31N3O5/c1-18(26-24(30)22-4-2-16-33-22)25(31)28-14-12-27(13-15-28)11-3-17-32-21-9-7-20(8-10-21)23(29)19-5-6-19/h2,4,7-10,16,18-19H,3,5-6,11-15,17H2,1H3,(H,26,30)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM86061

(A-304121)Show InChI InChI=1S/C7H7N3O3/c1-2-10-5-4(3-13-9-5)6(11)8-7(10)12/h3H,2H2,1H3,(H,8,11,12) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data