

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caspase-3 (Homo sapiens (Human)) | BDBM50133876 ((S)-3-{(S)-3-Methyl-2-[2-(naphthalen-1-yloxy)-acet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound was tested against caspase-3 (Csp-3) | Bioorg Med Chem Lett 13: 3623-6 (2003) BindingDB Entry DOI: 10.7270/Q21835WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Mus musculus) | BDBM50133876 ((S)-3-{(S)-3-Methyl-2-[2-(naphthalen-1-yloxy)-acet...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound was tested against Murine caspase-1 (mCsp-1) | Bioorg Med Chem Lett 13: 3623-6 (2003) BindingDB Entry DOI: 10.7270/Q21835WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50133889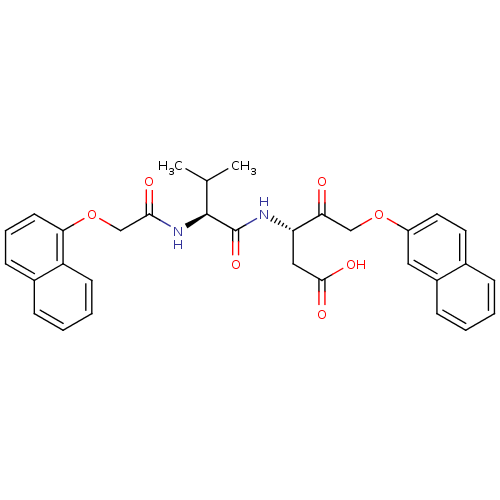 ((S)-3-{(S)-3-Methyl-2-[2-(naphthalen-1-yloxy)-acet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound was tested against caspase-3 (Csp-3) | Bioorg Med Chem Lett 13: 3623-6 (2003) BindingDB Entry DOI: 10.7270/Q21835WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50133879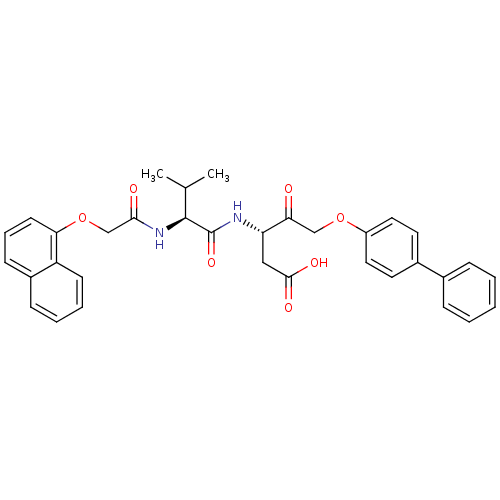 ((S)-5-(Biphenyl-4-yloxy)-3-{(S)-3-methyl-2-[2-(nap...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound was tested against caspase-3 (Csp-3) | Bioorg Med Chem Lett 13: 3623-6 (2003) BindingDB Entry DOI: 10.7270/Q21835WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Mus musculus) | BDBM50133879 ((S)-5-(Biphenyl-4-yloxy)-3-{(S)-3-methyl-2-[2-(nap...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound was tested against Murine caspase-1 (mCsp-1) | Bioorg Med Chem Lett 13: 3623-6 (2003) BindingDB Entry DOI: 10.7270/Q21835WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Mus musculus) | BDBM50133889 ((S)-3-{(S)-3-Methyl-2-[2-(naphthalen-1-yloxy)-acet...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound was tested against Murine caspase-1 (mCsp-1) | Bioorg Med Chem Lett 13: 3623-6 (2003) BindingDB Entry DOI: 10.7270/Q21835WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50532101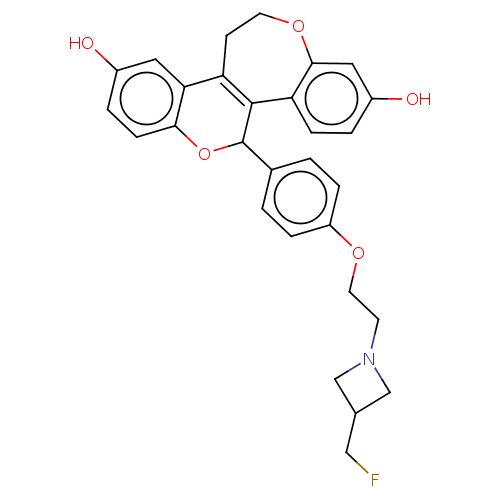 (CHEMBL4439408) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm... | Bioorg Med Chem Lett 29: 905-911 (2019) Article DOI: 10.1016/j.bmcl.2019.01.036 BindingDB Entry DOI: 10.7270/Q2Q52T3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50532101 (CHEMBL4439408) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm... | Bioorg Med Chem Lett 29: 905-911 (2019) Article DOI: 10.1016/j.bmcl.2019.01.036 BindingDB Entry DOI: 10.7270/Q2Q52T3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50206900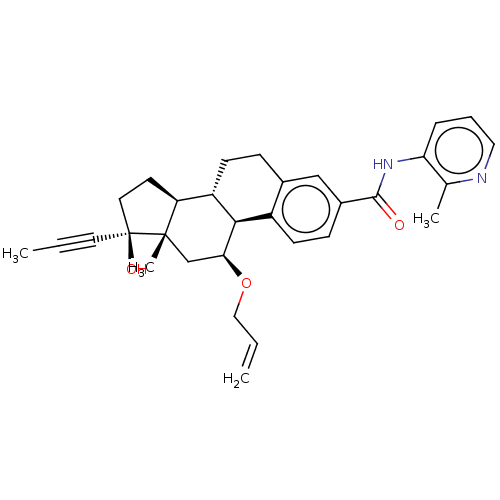 (CHEMBL3895181) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GR in human HepG2 cells assessed as inhibition of protein mediated-transcriptional activity by MMTV-promoter driven luciferase... | Bioorg Med Chem Lett 27: 347-353 (2017) Article DOI: 10.1016/j.bmcl.2016.11.007 BindingDB Entry DOI: 10.7270/Q24B339Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM368199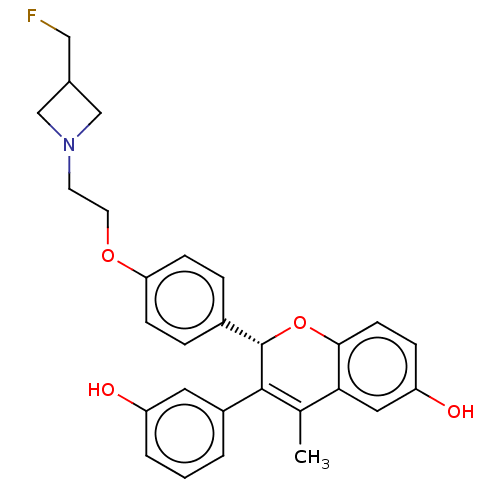 ((S)-2-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm... | Bioorg Med Chem Lett 29: 905-911 (2019) Article DOI: 10.1016/j.bmcl.2019.01.036 BindingDB Entry DOI: 10.7270/Q2Q52T3W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50532103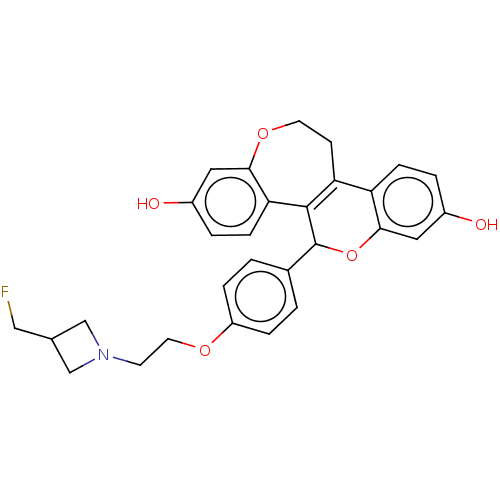 (CHEMBL4464224) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm... | Bioorg Med Chem Lett 29: 905-911 (2019) Article DOI: 10.1016/j.bmcl.2019.01.036 BindingDB Entry DOI: 10.7270/Q2Q52T3W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50532105 (CHEMBL4449709) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm... | Bioorg Med Chem Lett 29: 905-911 (2019) Article DOI: 10.1016/j.bmcl.2019.01.036 BindingDB Entry DOI: 10.7270/Q2Q52T3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50532102 (CHEMBL4437985) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm... | Bioorg Med Chem Lett 29: 905-911 (2019) Article DOI: 10.1016/j.bmcl.2019.01.036 BindingDB Entry DOI: 10.7270/Q2Q52T3W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50532098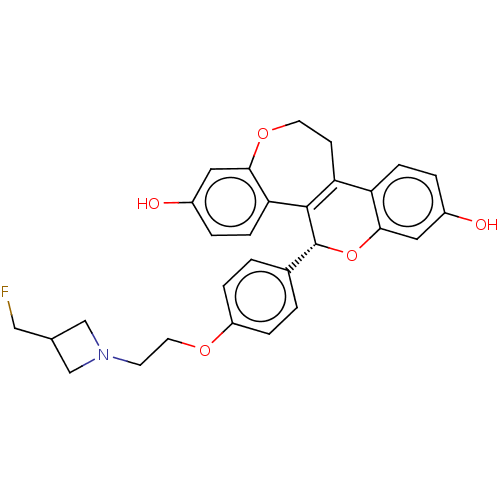 (CHEMBL4464058) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm... | Bioorg Med Chem Lett 29: 905-911 (2019) Article DOI: 10.1016/j.bmcl.2019.01.036 BindingDB Entry DOI: 10.7270/Q2Q52T3W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50206950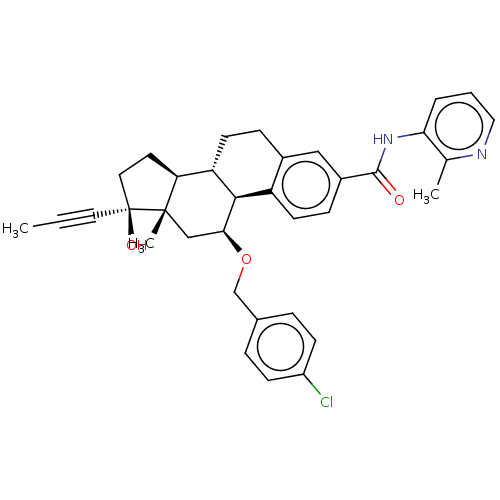 (CHEMBL3933702) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GR in human HepG2 cells assessed as inhibition of protein mediated-transcriptional activity by MMTV-promoter driven luciferase... | Bioorg Med Chem Lett 27: 347-353 (2017) Article DOI: 10.1016/j.bmcl.2016.11.007 BindingDB Entry DOI: 10.7270/Q24B339Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50532106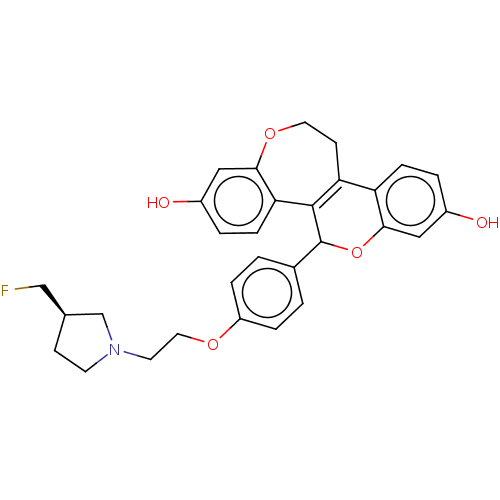 (CHEMBL4539802) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm... | Bioorg Med Chem Lett 29: 905-911 (2019) Article DOI: 10.1016/j.bmcl.2019.01.036 BindingDB Entry DOI: 10.7270/Q2Q52T3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50532104 (CHEMBL1087419) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm... | Bioorg Med Chem Lett 29: 905-911 (2019) Article DOI: 10.1016/j.bmcl.2019.01.036 BindingDB Entry DOI: 10.7270/Q2Q52T3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50206947 (CHEMBL3966490) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GR in human HepG2 cells assessed as inhibition of protein mediated-transcriptional activity by MMTV-promoter driven luciferase... | Bioorg Med Chem Lett 27: 347-353 (2017) Article DOI: 10.1016/j.bmcl.2016.11.007 BindingDB Entry DOI: 10.7270/Q24B339Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50206952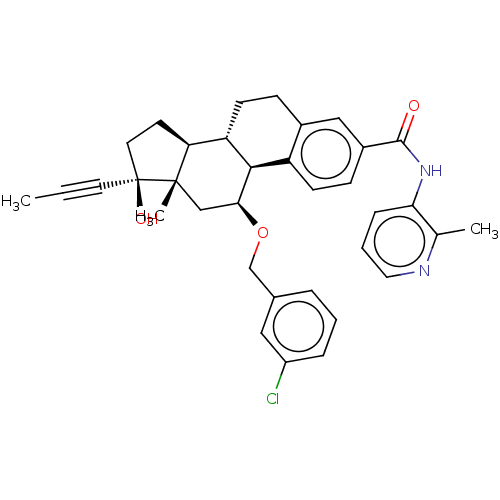 (CHEMBL3907029) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GR in human HepG2 cells assessed as inhibition of protein mediated-transcriptional activity by MMTV-promoter driven luciferase... | Bioorg Med Chem Lett 27: 347-353 (2017) Article DOI: 10.1016/j.bmcl.2016.11.007 BindingDB Entry DOI: 10.7270/Q24B339Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50206951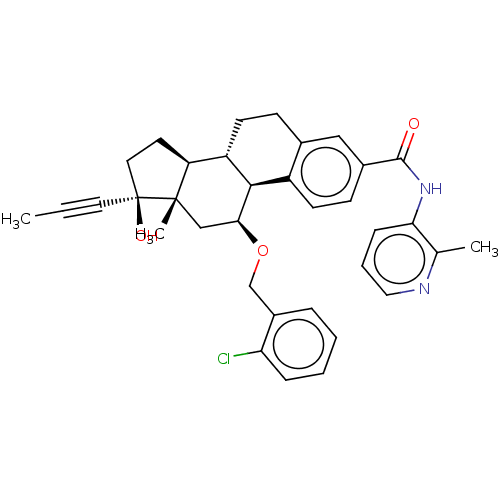 (CHEMBL3904106) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GR in human HepG2 cells assessed as inhibition of protein mediated-transcriptional activity by MMTV-promoter driven luciferase... | Bioorg Med Chem Lett 27: 347-353 (2017) Article DOI: 10.1016/j.bmcl.2016.11.007 BindingDB Entry DOI: 10.7270/Q24B339Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM84796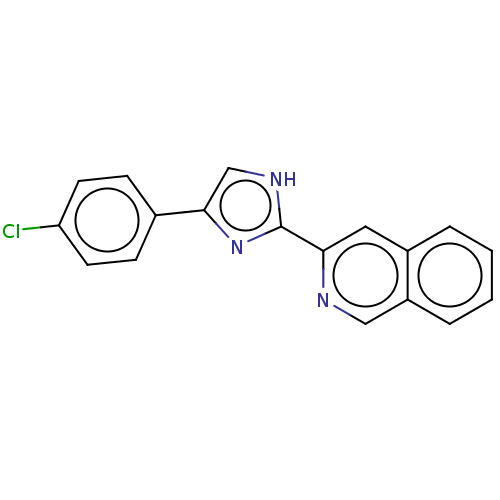 (PF-00215924) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc. | Assay Description Inhibition of active p38a MAP kinase by inhibitors was determinedusing a p38a cascade activity assay. A 30-lL reaction mixture wasprepared containing... | Chem Biol Drug Des 74: 547-59 (2009) Article DOI: 10.1111/j.1747-0285.2009.00884.x BindingDB Entry DOI: 10.7270/Q2930RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50119218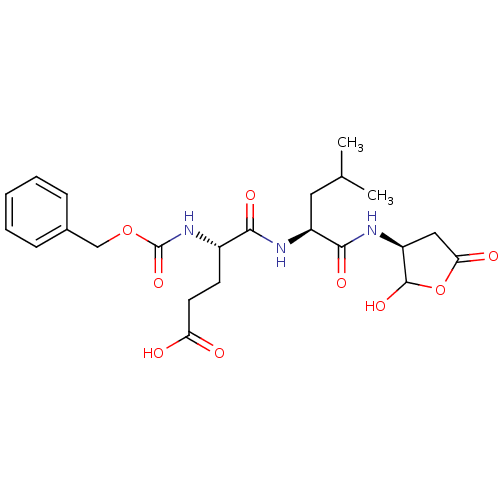 (4-Benzyloxycarbonylamino-4-[1-(2-hydroxy-5-oxo-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration of compound required against Caspase-3 enzyme compared to acylated dipeptides | Bioorg Med Chem Lett 12: 2973-5 (2002) BindingDB Entry DOI: 10.7270/Q24M93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50119218 (4-Benzyloxycarbonylamino-4-[1-(2-hydroxy-5-oxo-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration of compound required against Caspase-3 compared to acylated dipeptides | Bioorg Med Chem Lett 12: 2969-71 (2002) BindingDB Entry DOI: 10.7270/Q289157S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50532099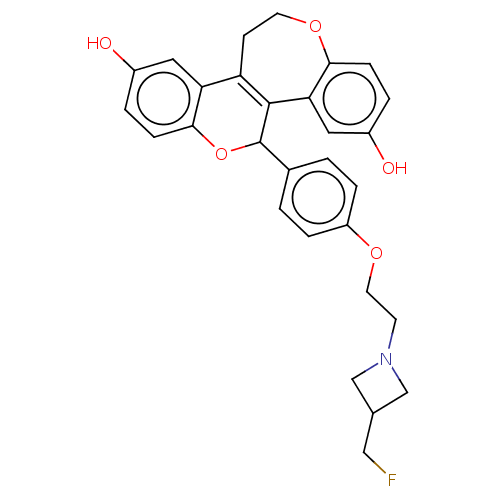 (CHEMBL4443826) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm... | Bioorg Med Chem Lett 29: 905-911 (2019) Article DOI: 10.1016/j.bmcl.2019.01.036 BindingDB Entry DOI: 10.7270/Q2Q52T3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50532099 (CHEMBL4443826) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm... | Bioorg Med Chem Lett 29: 905-911 (2019) Article DOI: 10.1016/j.bmcl.2019.01.036 BindingDB Entry DOI: 10.7270/Q2Q52T3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50072047 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-[1-((...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration of compound required against Caspase-3 enzyme compared to acylated dipeptides | Bioorg Med Chem Lett 12: 2973-5 (2002) BindingDB Entry DOI: 10.7270/Q24M93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-6 (Homo sapiens (Human)) | BDBM50119267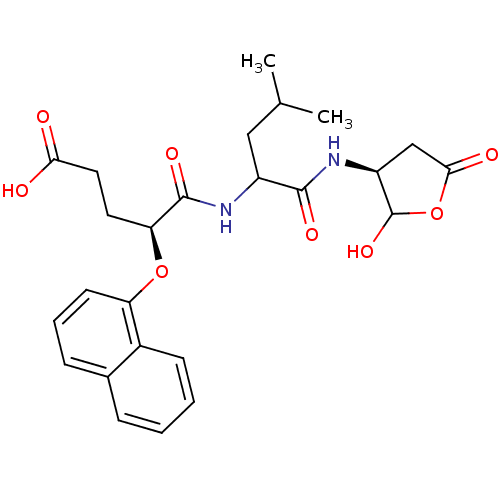 (4-[1-(2-Hydroxy-5-oxo-tetrahydro-furan-3-ylcarbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Caspase-6 | Bioorg Med Chem Lett 12: 2973-5 (2002) BindingDB Entry DOI: 10.7270/Q24M93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50119245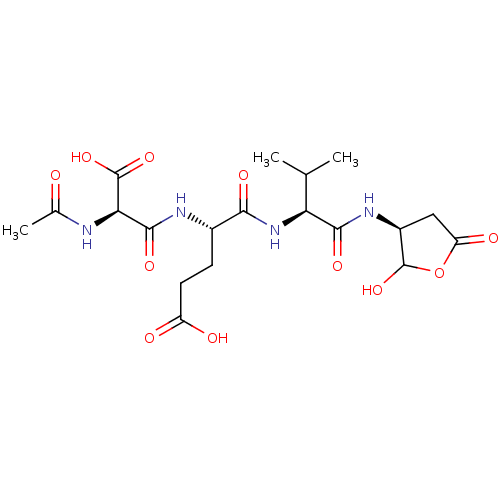 (4-(2-Acetylamino-2-carboxy-acetylamino)-4-[1-(2-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration of compound required against Caspase-3 compared to acylated dipeptides | Bioorg Med Chem Lett 12: 2969-71 (2002) BindingDB Entry DOI: 10.7270/Q289157S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50119267 (4-[1-(2-Hydroxy-5-oxo-tetrahydro-furan-3-ylcarbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Caspase-3 | Bioorg Med Chem Lett 12: 2973-5 (2002) BindingDB Entry DOI: 10.7270/Q24M93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50323438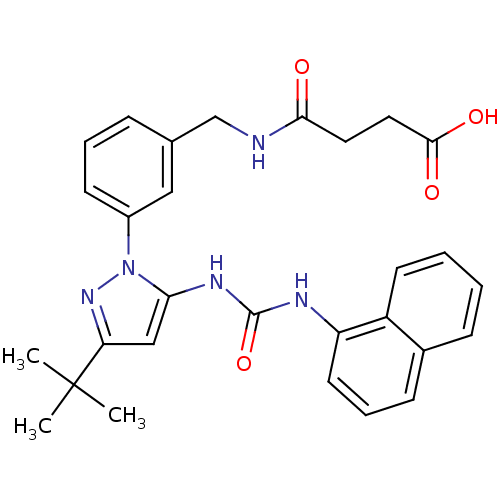 (4-(3-(3-tert-butyl-5-(3-naphthalen-1-ylureido)-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of unactive p38alpha | Bioorg Med Chem Lett 20: 4885-91 (2010) Article DOI: 10.1016/j.bmcl.2010.06.073 BindingDB Entry DOI: 10.7270/Q2Z31ZT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50323438 (4-(3-(3-tert-butyl-5-(3-naphthalen-1-ylureido)-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of active p38alpha | Bioorg Med Chem Lett 20: 4885-91 (2010) Article DOI: 10.1016/j.bmcl.2010.06.073 BindingDB Entry DOI: 10.7270/Q2Z31ZT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50005598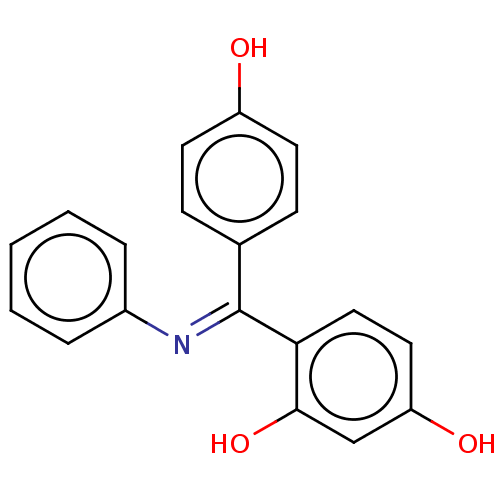 (CHEMBL3234626) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University) Curated by ChEMBL | Assay Description Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti... | J Med Chem 57: 3532-45 (2014) Article DOI: 10.1021/jm500268j BindingDB Entry DOI: 10.7270/Q2MS3V9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM84788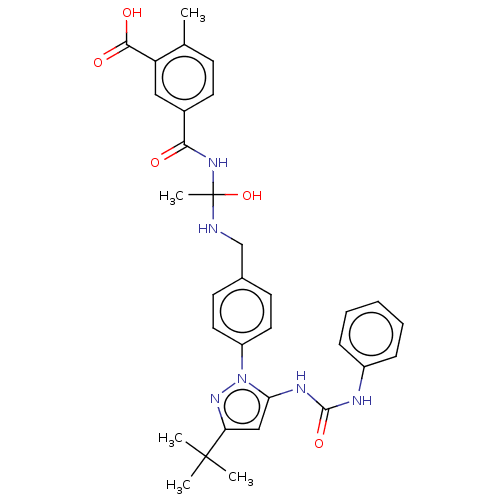 ((9a) BIRB796) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc. | Assay Description Binding of inhibitors to unactivated p38a that leads to decreasedphosphorylation of p38alpha by MKK6 was determined by measuringthe activated p38alph... | Chem Biol Drug Des 74: 547-59 (2009) Article DOI: 10.1111/j.1747-0285.2009.00884.x BindingDB Entry DOI: 10.7270/Q2930RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50005588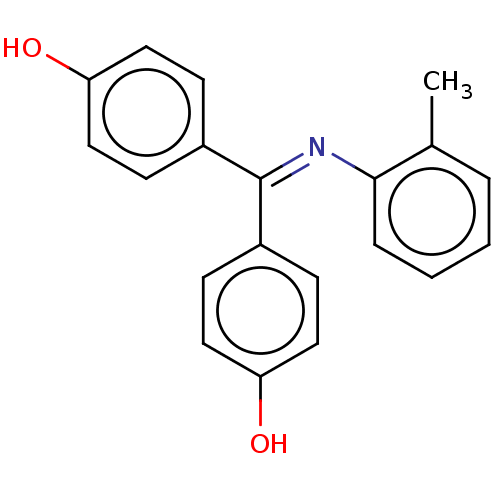 (CHEMBL140411 | CHEMBL3234614) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University) Curated by ChEMBL | Assay Description Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti... | J Med Chem 57: 3532-45 (2014) Article DOI: 10.1021/jm500268j BindingDB Entry DOI: 10.7270/Q2MS3V9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50005596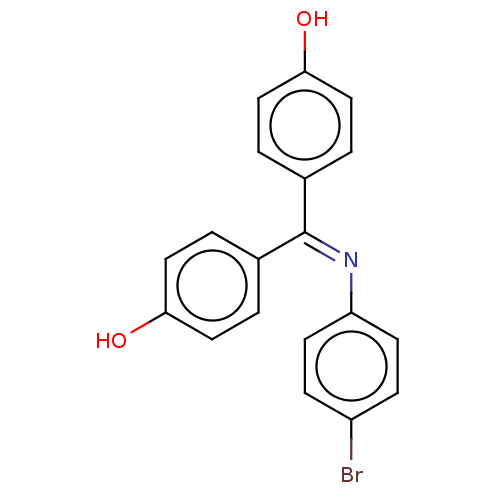 (CHEMBL3234622) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University) Curated by ChEMBL | Assay Description Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti... | J Med Chem 57: 3532-45 (2014) Article DOI: 10.1021/jm500268j BindingDB Entry DOI: 10.7270/Q2MS3V9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50005603 (CHEMBL3234630) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University) Curated by ChEMBL | Assay Description Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti... | J Med Chem 57: 3532-45 (2014) Article DOI: 10.1021/jm500268j BindingDB Entry DOI: 10.7270/Q2MS3V9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50323416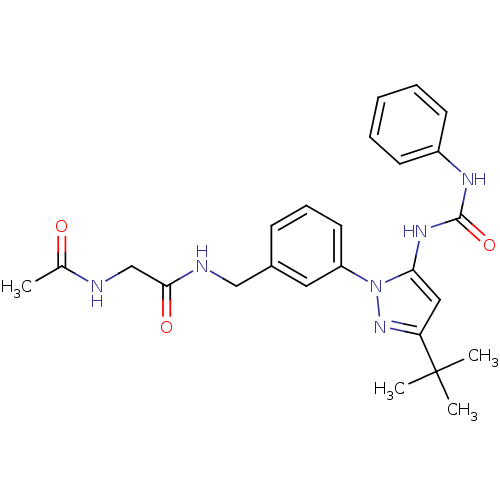 (2-acetamido-N-(3-(3-tert-butyl-5-(3-phenylureido)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of unactive p38alpha | Bioorg Med Chem Lett 20: 4885-91 (2010) Article DOI: 10.1016/j.bmcl.2010.06.073 BindingDB Entry DOI: 10.7270/Q2Z31ZT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50119218 (4-Benzyloxycarbonylamino-4-[1-(2-hydroxy-5-oxo-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration of compound required against Caspase-1 compared to acylated dipeptides | Bioorg Med Chem Lett 12: 2969-71 (2002) BindingDB Entry DOI: 10.7270/Q289157S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50119218 (4-Benzyloxycarbonylamino-4-[1-(2-hydroxy-5-oxo-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration of compound required against Caspase-1 enzyme compared to acylated dipeptides | Bioorg Med Chem Lett 12: 2973-5 (2002) BindingDB Entry DOI: 10.7270/Q24M93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM84786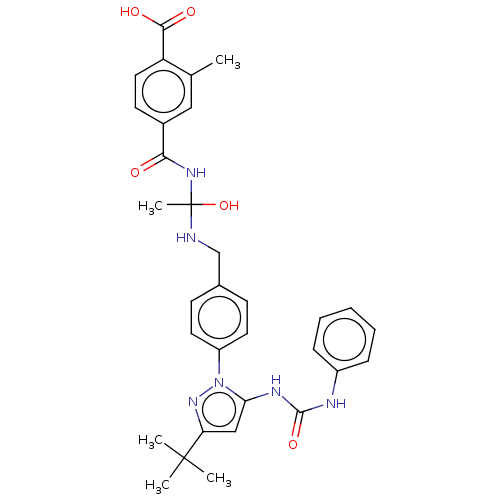 ((10a) BIRB796) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc. | Assay Description Binding of inhibitors to unactivated p38a that leads to decreasedphosphorylation of p38alpha by MKK6 was determined by measuringthe activated p38alph... | Chem Biol Drug Des 74: 547-59 (2009) Article DOI: 10.1111/j.1747-0285.2009.00884.x BindingDB Entry DOI: 10.7270/Q2930RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-8 (Homo sapiens (Human)) | BDBM50145622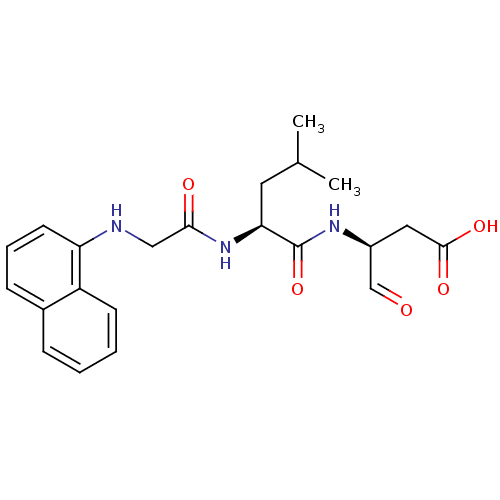 ((S)-3-{(S)-4-Methyl-2-[2-(naphthalen-1-ylamino)-ac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound towards human Caspase-8 | Bioorg Med Chem Lett 14: 2685-91 (2004) Article DOI: 10.1016/j.bmcl.2003.12.106 BindingDB Entry DOI: 10.7270/Q2BP027K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50005595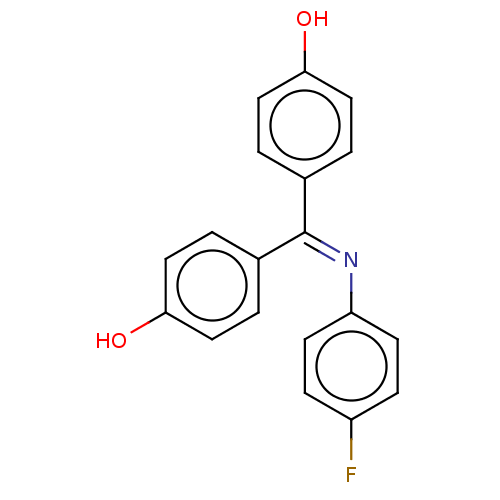 (CHEMBL3234621) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University) Curated by ChEMBL | Assay Description Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti... | J Med Chem 57: 3532-45 (2014) Article DOI: 10.1021/jm500268j BindingDB Entry DOI: 10.7270/Q2MS3V9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-8 (Homo sapiens (Human)) | BDBM50119262 (4-Methyl-2-[2-(naphthalen-1-ylamino)-acetylamino]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Caspase-8 enzyme | Bioorg Med Chem Lett 12: 2973-5 (2002) BindingDB Entry DOI: 10.7270/Q24M93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM84782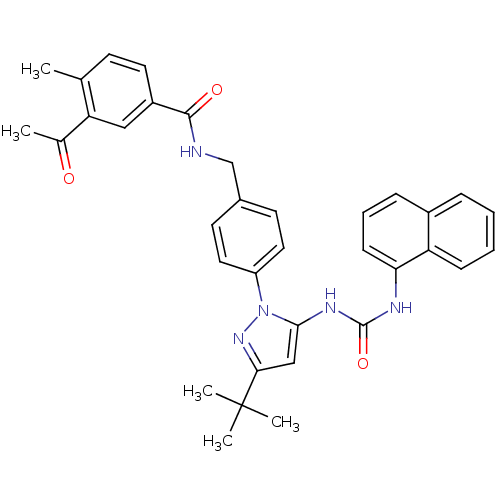 ((5a) BIRB796) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc. | Assay Description Binding of inhibitors to unactivated p38a that leads to decreasedphosphorylation of p38alpha by MKK6 was determined by measuringthe activated p38alph... | Chem Biol Drug Des 74: 547-59 (2009) Article DOI: 10.1111/j.1747-0285.2009.00884.x BindingDB Entry DOI: 10.7270/Q2930RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50054042 ((E)-6-(4-Hydroxy-7-methyl-3-oxo-6-vinyl-1,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Inhibition of human recombinant inosine monophosphate dehydrogenase type II . | J Med Chem 39: 4181-96 (1996) Article DOI: 10.1021/jm9603633 BindingDB Entry DOI: 10.7270/Q2668C8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50005593 (CHEMBL3234619) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University) Curated by ChEMBL | Assay Description Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti... | J Med Chem 57: 3532-45 (2014) Article DOI: 10.1021/jm500268j BindingDB Entry DOI: 10.7270/Q2MS3V9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50532100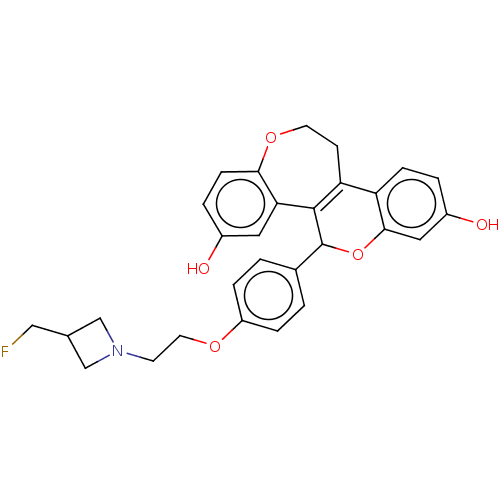 (CHEMBL4464146) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm... | Bioorg Med Chem Lett 29: 905-911 (2019) Article DOI: 10.1016/j.bmcl.2019.01.036 BindingDB Entry DOI: 10.7270/Q2Q52T3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50532100 (CHEMBL4464146) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm... | Bioorg Med Chem Lett 29: 905-911 (2019) Article DOI: 10.1016/j.bmcl.2019.01.036 BindingDB Entry DOI: 10.7270/Q2Q52T3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-7 (Homo sapiens (Human)) | BDBM50072047 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-[1-((...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration of compound required against Caspase-7 enzyme compared to acylated dipeptides | Bioorg Med Chem Lett 12: 2973-5 (2002) BindingDB Entry DOI: 10.7270/Q24M93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-7 (Homo sapiens (Human)) | BDBM50119218 (4-Benzyloxycarbonylamino-4-[1-(2-hydroxy-5-oxo-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration of compound required against Caspase-7 compared to acylated dipeptides | Bioorg Med Chem Lett 12: 2969-71 (2002) BindingDB Entry DOI: 10.7270/Q289157S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 754 total ) | Next | Last >> |