

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069559 (Biphalin Analogue | CHEMBL2371057) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards mu opioid receptor in guinea pig brain homogenates using [3H]-CTOP as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069560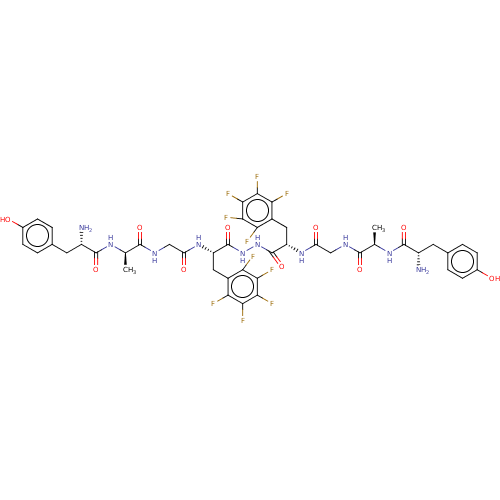 (Biphalin Analogue | CHEMBL2371080) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards mu opioid receptor in guinea pig brain homogenates using [3H]-CTOP as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069562 (2-Amino-N-((S)-1-{[((R)-1-{N'-[(R)-2-(2-{(S)-2-[2-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards mu opioid receptor in guinea pig brain homogenates using [3H]-CTOP as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069563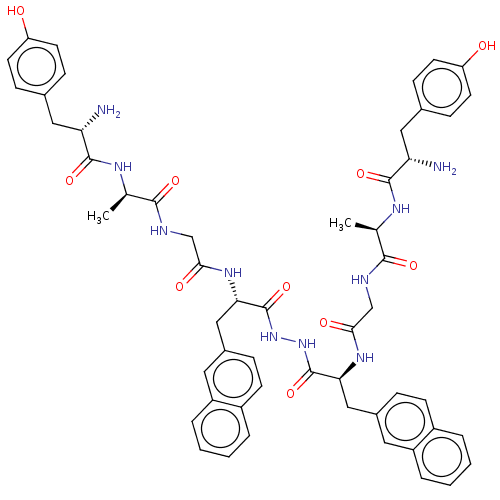 (Biphalin Analogue | CHEMBL2371079) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards mu opioid receptor in guinea pig brain homogenates using [3H]-CTOP as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069558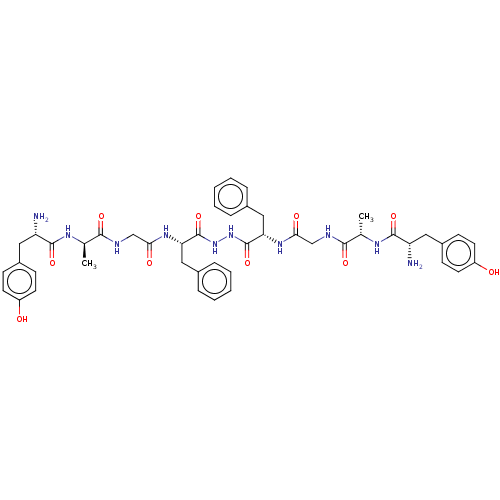 (2-Amino-N-((S)-1-{[(2-{N'-[2-(2-{(S)-2-[2-amino-3-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards mu opioid receptor in guinea pig brain homogenates using [3H]-CTOP as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069561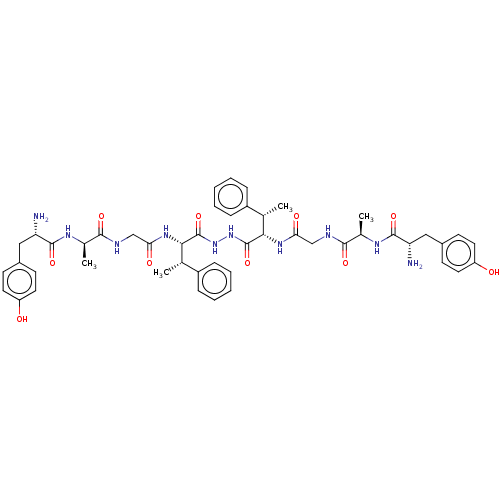 (2-Amino-N-((S)-1-{[((S)-1-{N'-[(S)-2-(2-{(S)-2-[2-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards mu opioid receptor in guinea pig brain homogenates using [3H]-CTOP as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50069558 (2-Amino-N-((S)-1-{[(2-{N'-[2-(2-{(S)-2-[2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor delta 1 in guinea pig brain homogenates using [3H]-[p-Cl-Phe]-DPDPE as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50069559 (Biphalin Analogue | CHEMBL2371057) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor delta 1 in guinea pig brain homogenates using [3H]-[p-Cl-Phe]-DPDPE as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50069563 (Biphalin Analogue | CHEMBL2371079) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor delta 1 in guinea pig brain homogenates using [3H]-[p-Cl-Phe]-DPDPE as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50069560 (Biphalin Analogue | CHEMBL2371080) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor delta 1 in guinea pig brain homogenates using [3H]-[p-Cl-Phe]-DPDPE as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50069561 (2-Amino-N-((S)-1-{[((S)-1-{N'-[(S)-2-(2-{(S)-2-[2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor delta 1 in guinea pig brain homogenates using [3H]-[p-Cl-Phe]-DPDPE as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50069562 (2-Amino-N-((S)-1-{[((R)-1-{N'-[(R)-2-(2-{(S)-2-[2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor delta 1 in guinea pig brain homogenates using [3H]-[p-Cl-Phe]-DPDPE as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513250 (CHEMBL4475689) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.327 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50010704 (CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of binding [3H]U-69,539 at Opioid receptor kappa 1 of guinea pig brain membrane (GPB) homogenates. | J Med Chem 39: 2456-60 (1996) Article DOI: 10.1021/jm950655o BindingDB Entry DOI: 10.7270/Q2C828D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50051599 (CHEMBL415224 | Dynorphin A analogues) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of binding [3H]U-69,539 at Opioid receptor kappa 1 of guinea pig brain membrane (GPB) homogenates. | J Med Chem 39: 2456-60 (1996) Article DOI: 10.1021/jm950655o BindingDB Entry DOI: 10.7270/Q2C828D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513228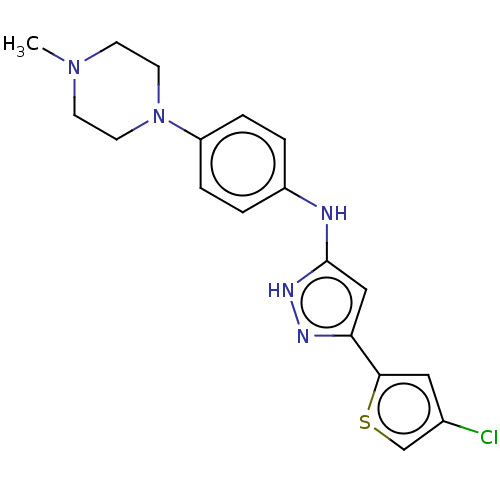 (CHEMBL4571743) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.799 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50051595 (CHEMBL413228 | Dynorphin A analogues) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of binding [3H]U-69,539 at Opioid receptor kappa 1 of guinea pig brain membrane (GPB) homogenates. | J Med Chem 39: 2456-60 (1996) Article DOI: 10.1021/jm950655o BindingDB Entry DOI: 10.7270/Q2C828D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50507645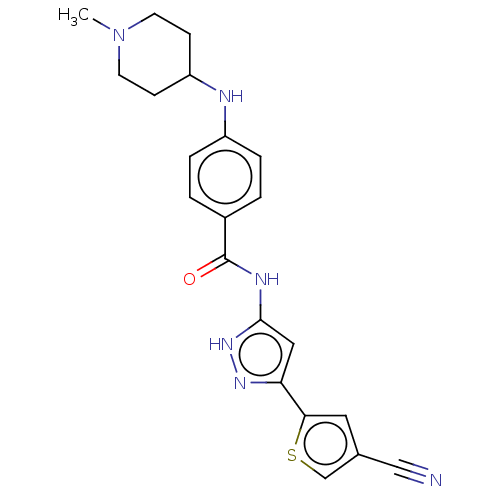 (CHEMBL4473820) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50051594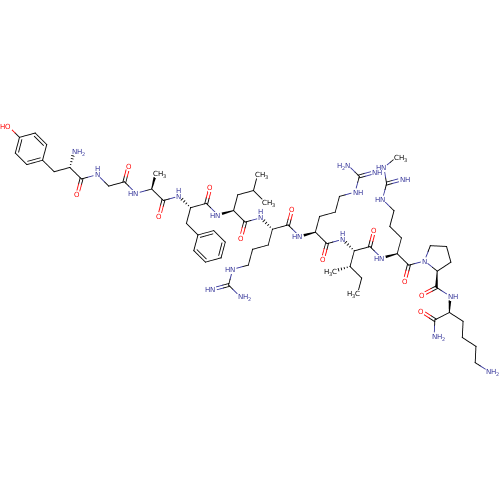 (CHEMBL407303 | Dynorphin A analogues) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of binding [3H]U-69,539 at Opioid receptor kappa 1 of guinea pig brain membrane (GPB) homogenates. | J Med Chem 39: 2456-60 (1996) Article DOI: 10.1021/jm950655o BindingDB Entry DOI: 10.7270/Q2C828D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50004169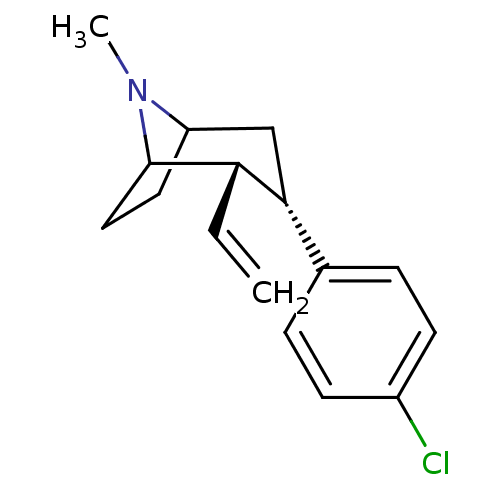 ((2S,3S)-3-(4-Chloro-phenyl)-8-methyl-2-vinyl-8-aza...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Education and Research Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]mazindol binding from rat striatal membranes | J Med Chem 35: 4764-6 (1993) BindingDB Entry DOI: 10.7270/Q2QR4XQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513230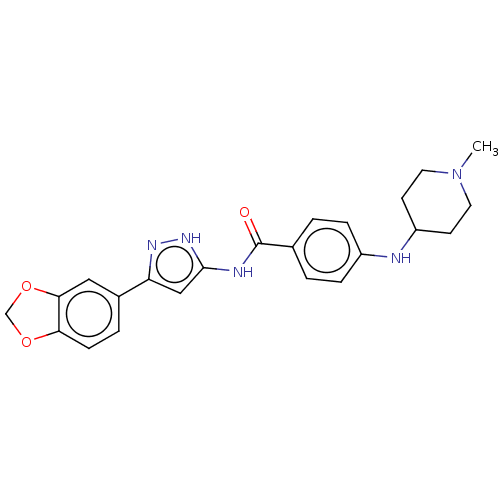 (CHEMBL4456471) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513235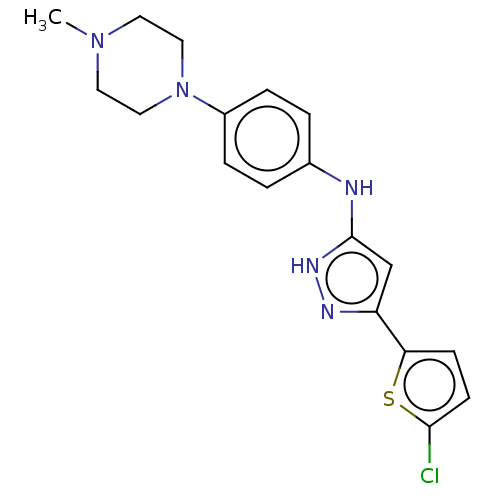 (CHEMBL4461271) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513240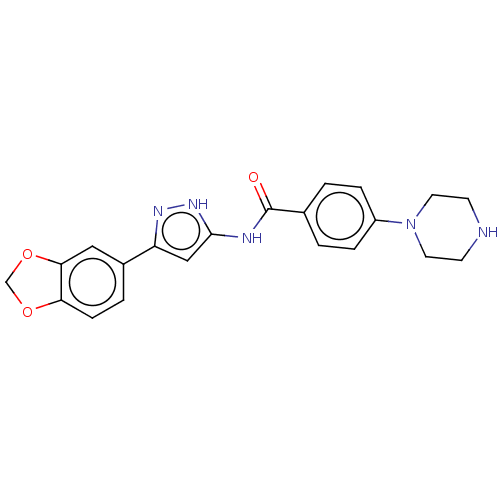 (CHEMBL4459133) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50035738 ((R)-3-(4-Chloro-phenyl)-8-methyl-8-aza-bicyclo[3.2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Education and Research Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]mazindol binding from rat striatal membranes | J Med Chem 35: 4764-6 (1993) BindingDB Entry DOI: 10.7270/Q2QR4XQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069559 (Biphalin Analogue | CHEMBL2371057) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibitory concentration required against biological activity of mu opioid receptor from guinea pig ileum (GPI) | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513234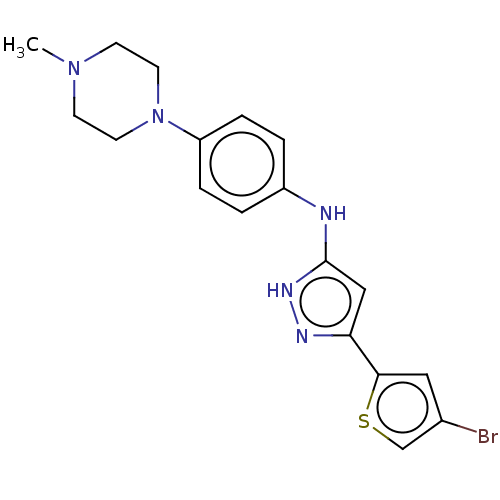 (CHEMBL4513662) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513236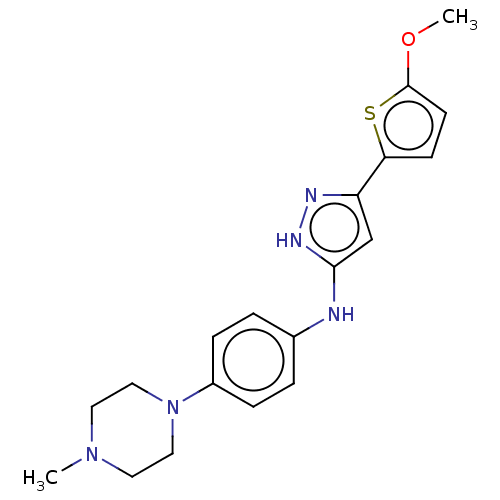 (CHEMBL4522701) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513253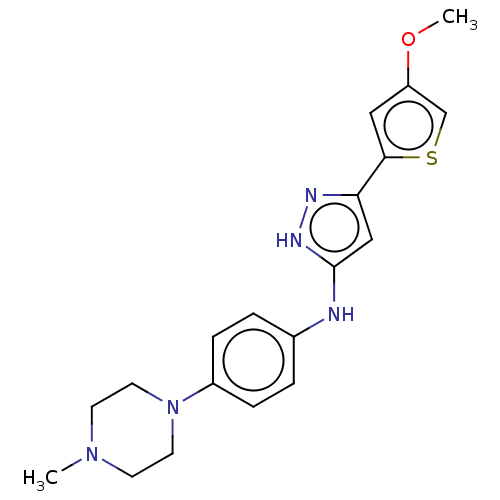 (CHEMBL4435445) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513245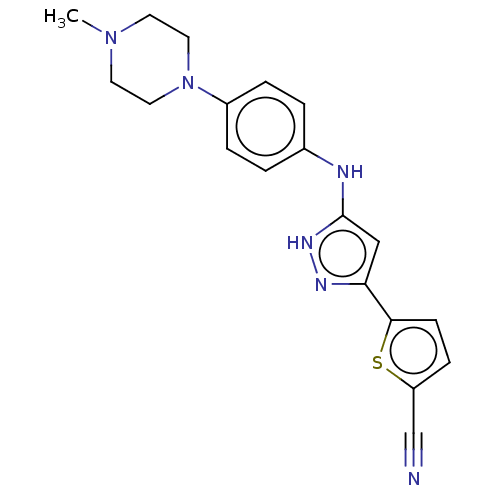 (CHEMBL4457695) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069563 (Biphalin Analogue | CHEMBL2371079) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibitory concentration required against biological activity of mu opioid receptor from guinea pig ileum (GPI) | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513241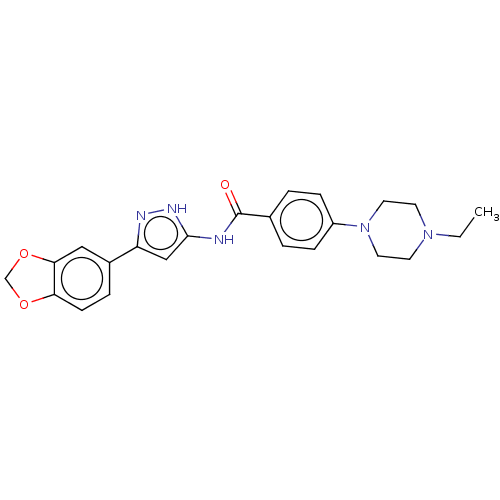 (CHEMBL4469023) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513233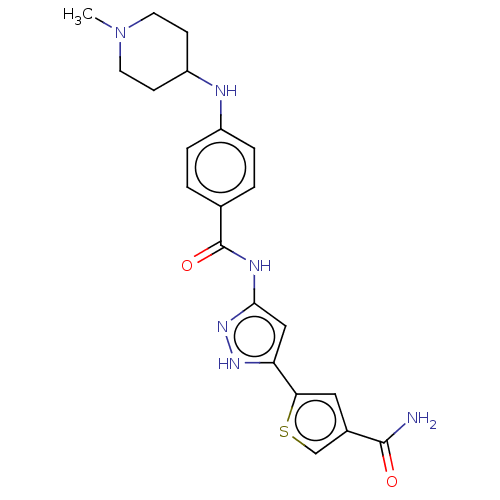 (CHEMBL4519177) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513237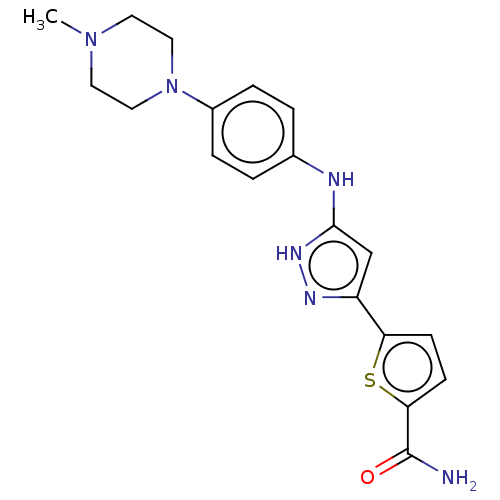 (CHEMBL4469054) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513254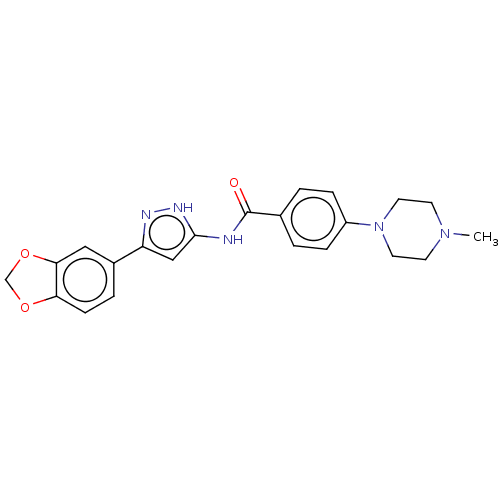 (CHEMBL4533224) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50051603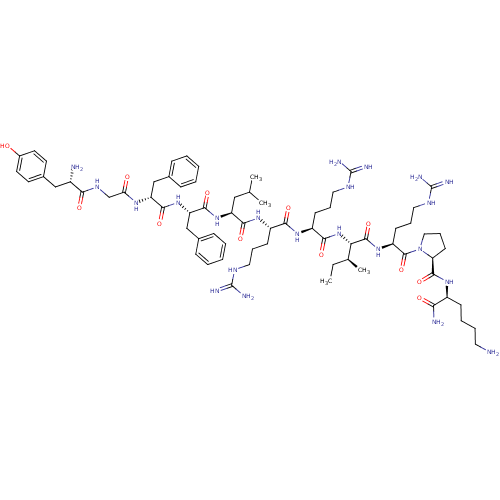 (CHEMBL412792 | Dynorphin A analogues) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of binding [3H]U-69,539 at Opioid receptor kappa 1 of guinea pig brain membrane (GPB) homogenates. | J Med Chem 39: 2456-60 (1996) Article DOI: 10.1021/jm950655o BindingDB Entry DOI: 10.7270/Q2C828D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50051592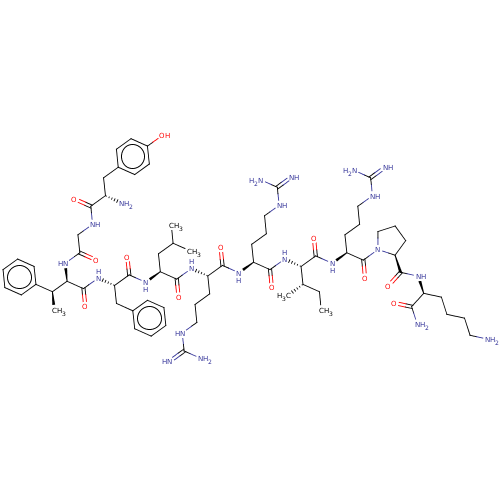 (CHEMBL2369902 | [(2S,3R)-beta-MePhe3] Dynorphin A ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of binding [3H]U-69,539 at Opioid receptor kappa 1 of guinea pig brain membrane (GPB) homogenates. | J Med Chem 39: 2456-60 (1996) Article DOI: 10.1021/jm950655o BindingDB Entry DOI: 10.7270/Q2C828D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50051592 (CHEMBL2369902 | [(2S,3R)-beta-MePhe3] Dynorphin A ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonistic activity towards Opioid receptor delta 1 was determined by evaluating the inhibitory activity towards electrically stimulated mouse vas def... | J Med Chem 39: 2456-60 (1996) Article DOI: 10.1021/jm950655o BindingDB Entry DOI: 10.7270/Q2C828D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513230 (CHEMBL4456471) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 R595_E596insEY mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513252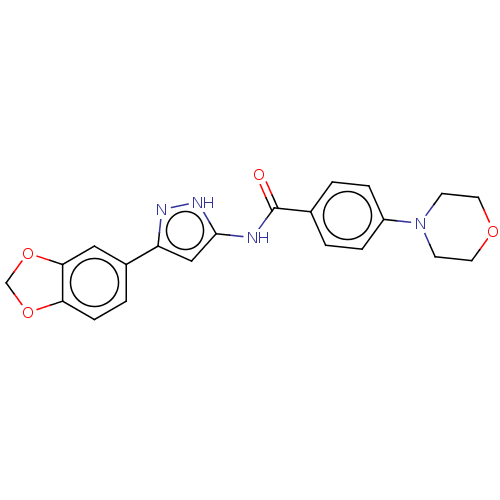 (CHEMBL4575589) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069558 (2-Amino-N-((S)-1-{[(2-{N'-[2-(2-{(S)-2-[2-amino-3-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibitory concentration required against biological activity of mu opioid receptor from guinea pig ileum (GPI) | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069560 (Biphalin Analogue | CHEMBL2371080) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibitory concentration required against biological activity of mu opioid receptor from guinea pig ileum (GPI) | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50454203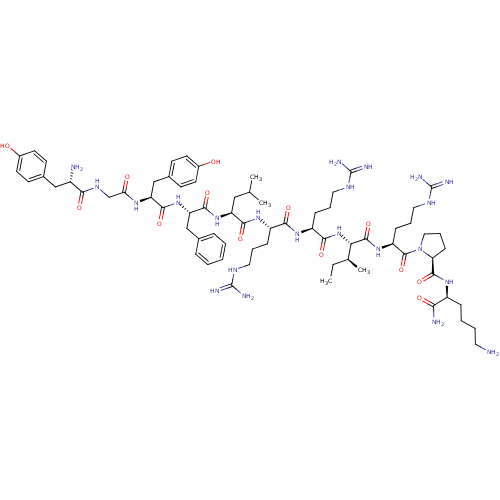 (CHEMBL2369896) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of binding [3H]U-69,539 at Opioid receptor kappa 1 of guinea pig brain membrane (GPB) homogenates. | J Med Chem 39: 2456-60 (1996) Article DOI: 10.1021/jm950655o BindingDB Entry DOI: 10.7270/Q2C828D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50069560 (Biphalin Analogue | CHEMBL2371080) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibitory concentration required against biological activity of Opioid receptor delta 1 from mouse vas deferens (MVD) | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513232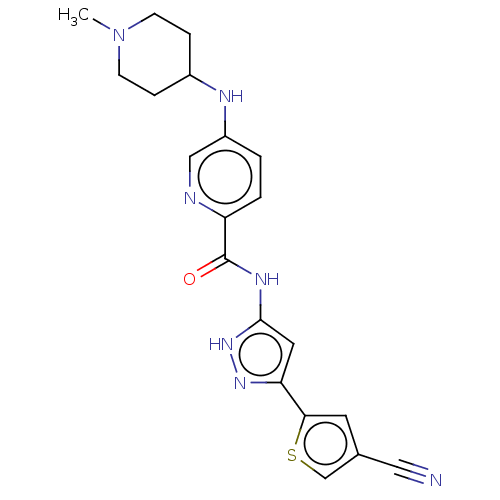 (CHEMBL4528422) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50069563 (Biphalin Analogue | CHEMBL2371079) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibitory concentration required against biological activity of Opioid receptor delta 1 from mouse vas deferens (MVD) | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50010704 (CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of binding [3H]-DAMGO at Opioid receptor mu 1 of guinea pig brain membrane (GPB) homogenates. | J Med Chem 39: 2456-60 (1996) Article DOI: 10.1021/jm950655o BindingDB Entry DOI: 10.7270/Q2C828D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513230 (CHEMBL4456471) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 F594_R595insREY mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513238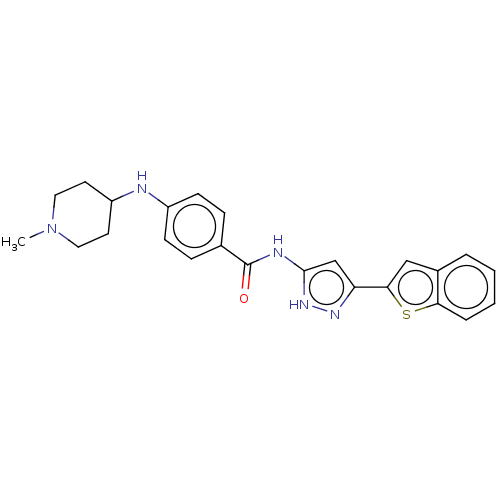 (CHEMBL4571608) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 ITD mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50513230 (CHEMBL4456471) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human FLT3 F594_R595insR mutant using EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Eur J Med Chem 176: 248-267 (2019) Article DOI: 10.1016/j.ejmech.2019.05.021 BindingDB Entry DOI: 10.7270/Q2FR00ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 159 total ) | Next | Last >> |