 Found 3677 hits with Last Name = 'ye' and Initial = 'x'
Found 3677 hits with Last Name = 'ye' and Initial = 'x' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50595097

(CHEMBL5170337)Show SMILES COC[C@H]1CCCN1c1cc(Nc2ccccn2)nc(n1)-n1cccn1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50507371
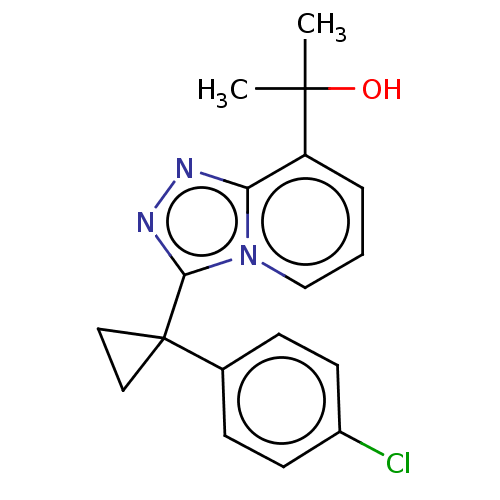
(BMS-823778)Show InChI InChI=1S/C18H18ClN3O/c1-17(2,23)14-4-3-11-22-15(14)20-21-16(22)18(9-10-18)12-5-7-13(19)8-6-12/h3-8,11,23H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 expressed in HEK293 cell microsomes using [3H]cortisone as substrate after 4 hrs by homogeneous immuno-ra... |
ACS Med Chem Lett 9: 1170-1174 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00307
BindingDB Entry DOI: 10.7270/Q20R9SP3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
WD repeat-containing protein 5
(Homo sapiens (Human)) | BDBM200712
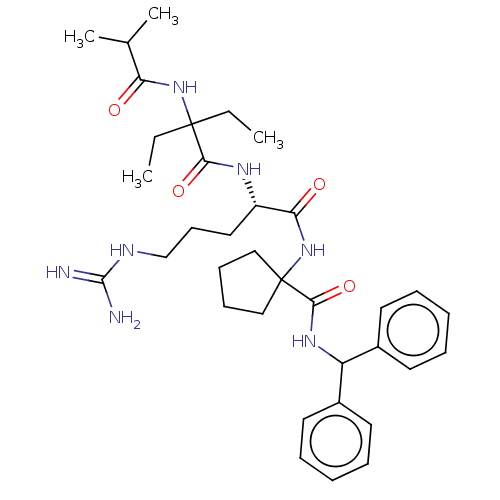
(US9233086, 10A)Show SMILES CCC(CC)(NC(=O)C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC1(CCCC1)C(=O)NC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C35H51N7O4/c1-5-34(6-2,41-29(43)24(3)4)31(45)39-27(20-15-23-38-33(36)37)30(44)42-35(21-13-14-22-35)32(46)40-28(25-16-9-7-10-17-25)26-18-11-8-12-19-26/h7-12,16-19,24,27-28H,5-6,13-15,20-23H2,1-4H3,(H,39,45)(H,40,46)(H,41,43)(H,42,44)(H4,36,37,38)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University
Curated by ChEMBL
| Assay Description
Displacement of 5-FAM labelled tracer from WDR5 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 29: 638-645 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.035
BindingDB Entry DOI: 10.7270/Q26W9FC5 |
More data for this
Ligand-Target Pair | |
WD repeat-containing protein 5
(Homo sapiens (Human)) | BDBM200723

(US9233086, 10L)Show SMILES CCC(CC)(NC(=O)C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC1(CCCC1)C(=O)NC(c1ccc(F)cc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C35H49F2N7O4/c1-5-34(6-2,43-29(45)22(3)4)31(47)41-27(10-9-21-40-33(38)39)30(46)44-35(19-7-8-20-35)32(48)42-28(23-11-15-25(36)16-12-23)24-13-17-26(37)18-14-24/h11-18,22,27-28H,5-10,19-21H2,1-4H3,(H,41,47)(H,42,48)(H,43,45)(H,44,46)(H4,38,39,40)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University
Curated by ChEMBL
| Assay Description
Displacement of 5-FAM labelled tracer from WDR5 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 29: 638-645 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.035
BindingDB Entry DOI: 10.7270/Q26W9FC5 |
More data for this
Ligand-Target Pair | |
WD repeat-containing protein 5
(Homo sapiens (Human)) | BDBM200722

(US9233086, 10K)Show SMILES CCC(CC)(NC(=O)C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC1(CCCC1)C(=O)NC(c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C35H49Cl2N7O4/c1-5-34(6-2,43-29(45)22(3)4)31(47)41-27(10-9-21-40-33(38)39)30(46)44-35(19-7-8-20-35)32(48)42-28(23-11-15-25(36)16-12-23)24-13-17-26(37)18-14-24/h11-18,22,27-28H,5-10,19-21H2,1-4H3,(H,41,47)(H,42,48)(H,43,45)(H,44,46)(H4,38,39,40)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University
Curated by ChEMBL
| Assay Description
Displacement of 5-FAM labelled tracer from WDR5 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 29: 638-645 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.035
BindingDB Entry DOI: 10.7270/Q26W9FC5 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50092173
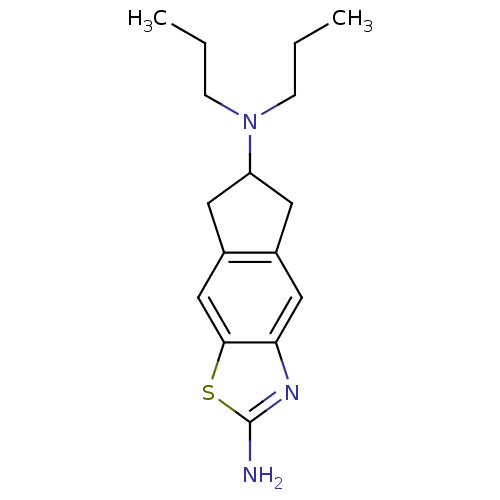
(CHEMBL325710 | N*6*,N*6*-Dipropyl-6,7-dihydro-5H-1...)Show InChI InChI=1S/C16H23N3S/c1-3-5-19(6-4-2)13-7-11-9-14-15(10-12(11)8-13)20-16(17)18-14/h9-10,13H,3-8H2,1-2H3,(H2,17,18) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50123627

((S)-6-Dipropylamino-5,6,7,8-tetrahydro-naphthalene...)Show InChI InChI=1S/C16H25NO2/c1-3-9-17(10-4-2)13-6-7-14-12(11-13)5-8-15(18)16(14)19/h5,8,13,18-19H,3-4,6-7,9-11H2,1-2H3/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50048466
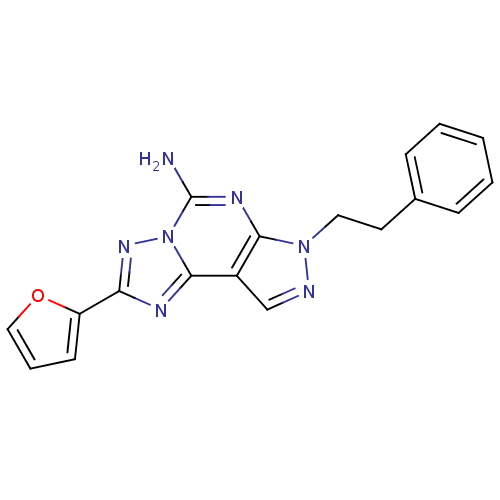
(2-(furan-2-yl)-7-phenethyl-7H-pyrazolo[4,3-e][1,2,...)Show InChI InChI=1S/C18H15N7O/c19-18-22-16-13(11-20-24(16)9-8-12-5-2-1-3-6-12)17-21-15(23-25(17)18)14-7-4-10-26-14/h1-7,10-11H,8-9H2,(H2,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131550
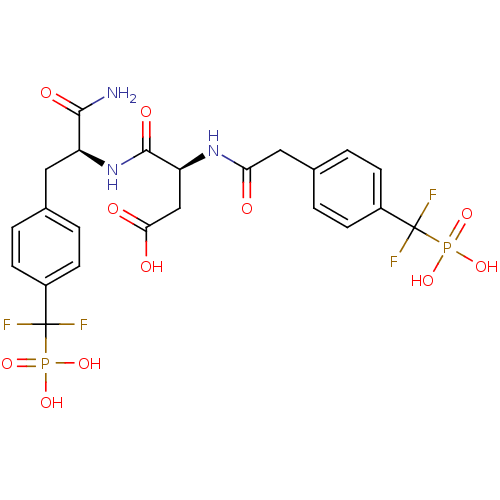
((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C23H25F4N3O11P2/c24-22(25,42(36,37)38)14-5-1-12(2-6-14)9-16(20(28)34)30-21(35)17(11-19(32)33)29-18(31)10-13-3-7-15(8-4-13)23(26,27)43(39,40)41/h1-8,16-17H,9-11H2,(H2,28,34)(H,29,31)(H,30,35)(H,32,33)(H2,36,37,38)(H2,39,40,41)/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 29: 2358-2363 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.011
BindingDB Entry DOI: 10.7270/Q2280C4B |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50212359
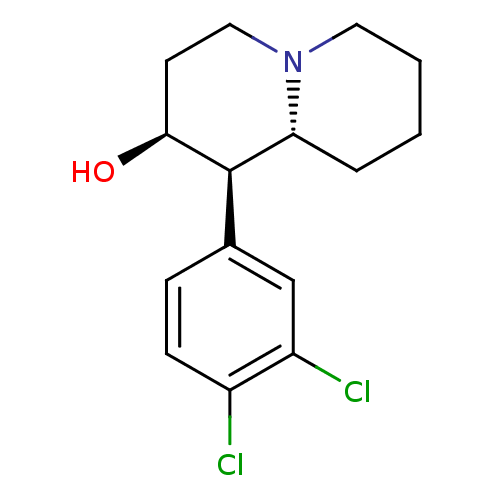
(CHEMBL558182 | threo-1-aza-4beta-hydroxy-5-(3,4-di...)Show SMILES O[C@H]1CCN2CCCC[C@@H]2[C@H]1c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C15H19Cl2NO/c16-11-5-4-10(9-12(11)17)15-13-3-1-2-7-18(13)8-6-14(15)19/h4-5,9,13-15,19H,1-3,6-8H2/t13-,14+,15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-35428 from DAT in Sprague-Dawley rat striatum |
J Med Chem 50: 2718-31 (2007)
Article DOI: 10.1021/jm061354p
BindingDB Entry DOI: 10.7270/Q2Q81DXB |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50123627

((S)-6-Dipropylamino-5,6,7,8-tetrahydro-naphthalene...)Show InChI InChI=1S/C16H25NO2/c1-3-9-17(10-4-2)13-6-7-14-12(11-13)5-8-15(18)16(14)19/h5,8,13,18-19H,3-4,6-7,9-11H2,1-2H3/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50212365
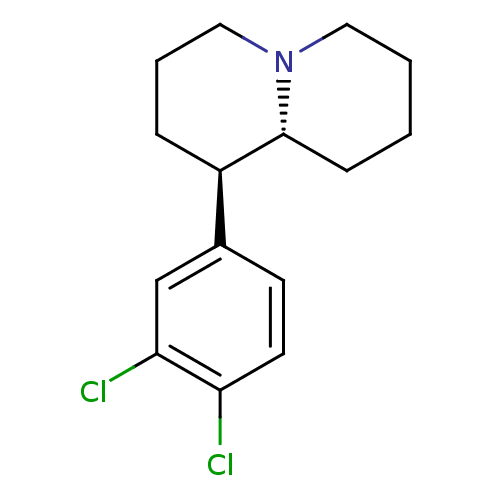
(CHEMBL556365 | threo-1-aza-5-(3,4-dichlorophenyl)[...)Show InChI InChI=1S/C15H19Cl2N/c16-13-7-6-11(10-14(13)17)12-4-3-9-18-8-2-1-5-15(12)18/h6-7,10,12,15H,1-5,8-9H2/t12-,15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-35428 from DAT in Sprague-Dawley rat striatum |
J Med Chem 50: 2718-31 (2007)
Article DOI: 10.1021/jm061354p
BindingDB Entry DOI: 10.7270/Q2Q81DXB |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50212361
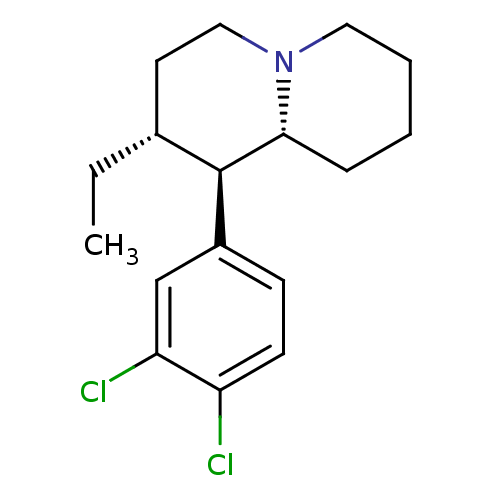
((1R,2R,9aR)-1-(3,4-dichlorophenyl)-2-ethyl-octahyd...)Show SMILES CC[C@@H]1CCN2CCCC[C@@H]2[C@H]1c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C17H23Cl2N/c1-2-12-8-10-20-9-4-3-5-16(20)17(12)13-6-7-14(18)15(19)11-13/h6-7,11-12,16-17H,2-5,8-10H2,1H3/t12-,16-,17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-35428 from DAT in Sprague-Dawley rat striatum |
J Med Chem 50: 2718-31 (2007)
Article DOI: 10.1021/jm061354p
BindingDB Entry DOI: 10.7270/Q2Q81DXB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239606

(CHEMBL4080667)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC1CC(C2)[C@H]3O)c1ccccc1 |r,wD:17.21,TLB:16:15:8.9.10:12,7:8:14.16.15:10.11.12,7:8:12:14.15.17,THB:16:9:12:14.15.17,18:17:8.9.10:12,17:15:8:10.11.12,17:11:8:14.16.15,19:8:14.16.15:10.11.12,19:8:12:14.15.17,(43.24,-21.55,;43.72,-20.08,;43.03,-18.71,;44.4,-18.01,;45.1,-19.38,;44.88,-16.54,;43.63,-15.64,;46.35,-16.07,;47.49,-17.11,;48.99,-16.68,;48.98,-15.1,;50.02,-13.87,;48.67,-14.34,;48.68,-15.83,;50,-16.32,;51.4,-15.97,;50.4,-17.25,;51.42,-14.44,;52.71,-13.59,;47.48,-18.64,;46.13,-19.39,;46.12,-20.93,;47.44,-21.71,;48.79,-20.95,;48.79,-19.41,)| Show InChI InChI=1S/C21H27NO3/c23-18-11-22(12-18)19(24)10-21(15-4-2-1-3-5-15)16-6-13-7-17(21)9-14(8-16)20(13)25/h1-5,13-14,16-18,20,23,25H,6-12H2/t13?,14?,16?,17?,20-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50212387
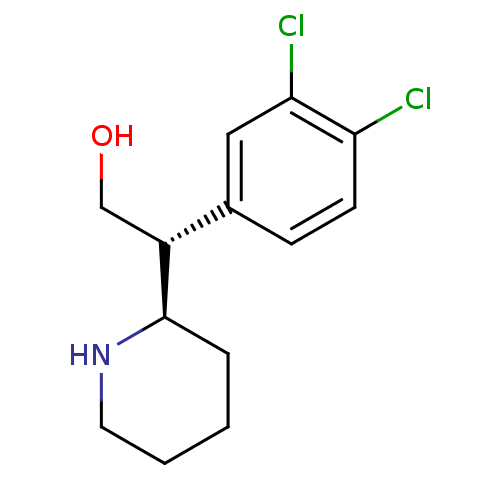
(CHEMBL537653 | threo-3,4-dichlororitalinol hydroch...)Show InChI InChI=1S/C13H17Cl2NO/c14-11-5-4-9(7-12(11)15)10(8-17)13-3-1-2-6-16-13/h4-5,7,10,13,16-17H,1-3,6,8H2/t10-,13-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-35428 from DAT in Sprague-Dawley rat striatum |
J Med Chem 50: 2718-31 (2007)
Article DOI: 10.1021/jm061354p
BindingDB Entry DOI: 10.7270/Q2Q81DXB |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50152240

(CHEMBL184309 | N*5*-{2-[4-(2,4-Difluoro-phenyl)-pi...)Show SMILES CN(CCN1CCN(CC1)c1ccc(F)cc1F)c1nc(N)n2nc(nc2n1)-c1ccco1 Show InChI InChI=1S/C21H23F2N9O/c1-29(6-7-30-8-10-31(11-9-30)16-5-4-14(22)13-15(16)23)20-26-19(24)32-21(27-20)25-18(28-32)17-3-2-12-33-17/h2-5,12-13H,6-11H2,1H3,(H2,24,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50212383
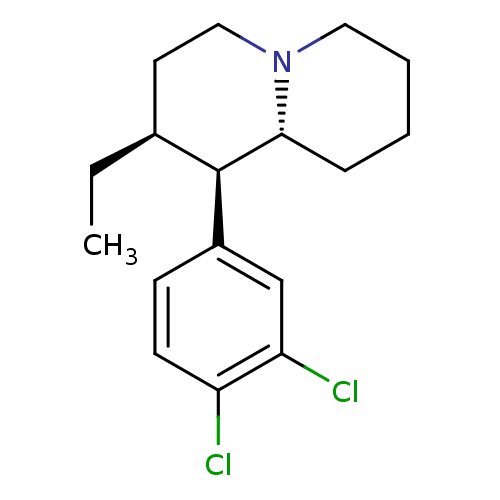
((1R,2S,9aR)-1-(3,4-dichlorophenyl)-2-ethyl-octahyd...)Show SMILES CC[C@H]1CCN2CCCC[C@@H]2[C@H]1c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C17H23Cl2N/c1-2-12-8-10-20-9-4-3-5-16(20)17(12)13-6-7-14(18)15(19)11-13/h6-7,11-12,16-17H,2-5,8-10H2,1H3/t12-,16+,17+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-35428 from DAT in Sprague-Dawley rat striatum |
J Med Chem 50: 2718-31 (2007)
Article DOI: 10.1021/jm061354p
BindingDB Entry DOI: 10.7270/Q2Q81DXB |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50142840
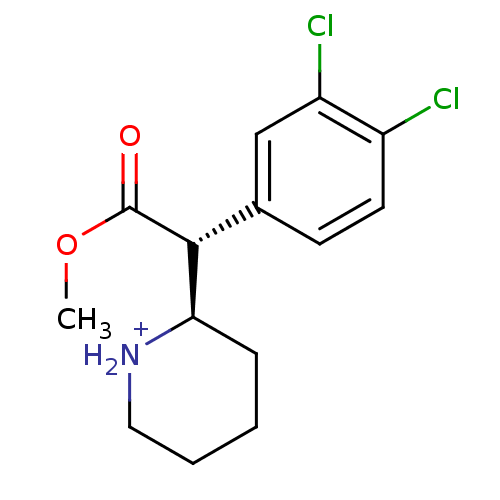
((R)-2-[(R)-(3,4-Dichloro-phenyl)-methoxycarbonyl-m...)Show SMILES COC(=O)[C@@H]([C@H]1CCCC[NH2+]1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C14H17Cl2NO2/c1-19-14(18)13(12-4-2-3-7-17-12)9-5-6-10(15)11(16)8-9/h5-6,8,12-13,17H,2-4,7H2,1H3/p+1/t12-,13-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-35428 from DAT in Sprague-Dawley rat striatum |
J Med Chem 50: 2718-31 (2007)
Article DOI: 10.1021/jm061354p
BindingDB Entry DOI: 10.7270/Q2Q81DXB |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50212373
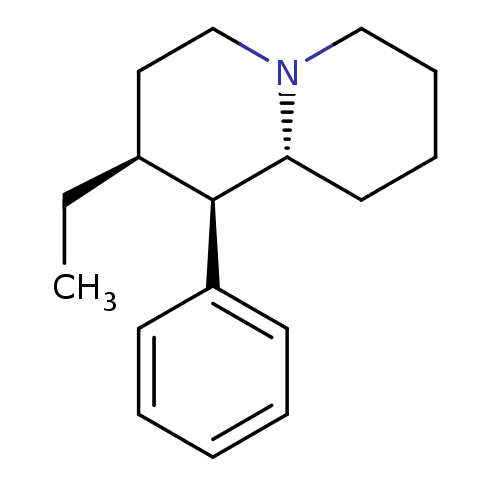
((1R,2S,9aR)-2-ethyl-1-phenyl-octahydro-1H-quinoliz...)Show InChI InChI=1S/C17H25N/c1-2-14-11-13-18-12-7-6-10-16(18)17(14)15-8-4-3-5-9-15/h3-5,8-9,14,16-17H,2,6-7,10-13H2,1H3/t14-,16+,17+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-35428 from DAT in Sprague-Dawley rat striatum |
J Med Chem 50: 2718-31 (2007)
Article DOI: 10.1021/jm061354p
BindingDB Entry DOI: 10.7270/Q2Q81DXB |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50499055
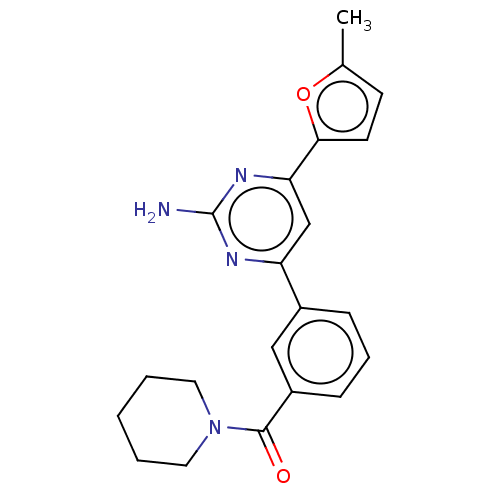
(CHEMBL3735985)Show SMILES Cc1ccc(o1)-c1cc(nc(N)n1)-c1cccc(c1)C(=O)N1CCCCC1 Show InChI InChI=1S/C21H22N4O2/c1-14-8-9-19(27-14)18-13-17(23-21(22)24-18)15-6-5-7-16(12-15)20(26)25-10-3-2-4-11-25/h5-9,12-13H,2-4,10-11H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202086
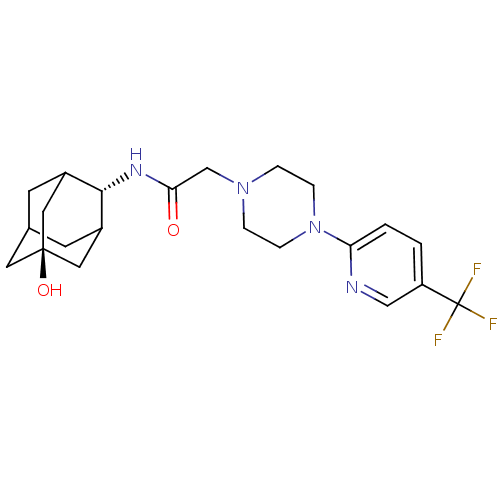
(CHEMBL219142 | N-[(E)-5-hydroxy-2-adamantyl]-2-{4-...)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CCN(CC1)c1ccc(cn1)C(F)(F)F)C(C3)C2 |r,wU:7.8,wD:1.0,TLB:4:3:30:6.5.7,4:5:2.3.29:30,THB:7:5:2:29.28.30,7:28:2:6.4.5,8:7:2.3.29:30,(1.8,-8.48,;3.15,-9.25,;1.93,-10.52,;3.44,-10.11,;4.84,-10.69,;5.87,-9.43,;4.47,-9.76,;5.9,-7.9,;7.19,-7.07,;8.56,-7.77,;8.64,-9.31,;9.85,-6.93,;11.23,-7.63,;11.25,-9.18,;12.59,-9.92,;13.92,-9.13,;13.88,-7.59,;12.53,-6.84,;15.23,-9.92,;15.2,-11.46,;16.52,-12.26,;17.87,-11.51,;17.89,-9.96,;16.57,-9.18,;19.19,-12.31,;20.51,-13.07,;18.4,-13.63,;19.98,-10.98,;4.51,-7.31,;3.45,-8.53,;3.16,-7.77,)| Show InChI InChI=1S/C22H29F3N4O2/c23-22(24,25)17-1-2-18(26-12-17)29-5-3-28(4-6-29)13-19(30)27-20-15-7-14-8-16(20)11-21(31,9-14)10-15/h1-2,12,14-16,20,31H,3-11,13H2,(H,27,30)/t14?,15?,16?,20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD-1 |
Bioorg Med Chem Lett 21: 6699-704 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.055
BindingDB Entry DOI: 10.7270/Q2Q240NV |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50102258
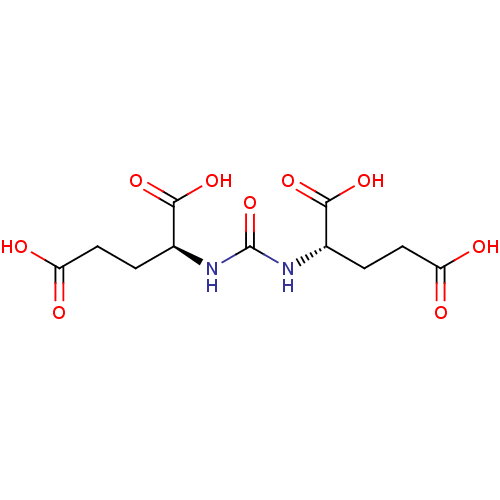
((S)-2-[3-((S)-3-Carboxy-1-carboxy-propyl)-ureido]-...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O Show InChI InChI=1S/C11H16N2O9/c14-7(15)3-1-5(9(18)19)12-11(22)13-6(10(20)21)2-4-8(16)17/h5-6H,1-4H2,(H,14,15)(H,16,17)(H,18,19)(H,20,21)(H2,12,13,22)/t5-,6-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity PSMA (unknown origin) |
J Med Chem 58: 3094-103 (2015)
Article DOI: 10.1021/jm5018384
BindingDB Entry DOI: 10.7270/Q2MG7R6G |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50499055

(CHEMBL3735985)Show SMILES Cc1ccc(o1)-c1cc(nc(N)n1)-c1cccc(c1)C(=O)N1CCCCC1 Show InChI InChI=1S/C21H22N4O2/c1-14-8-9-19(27-14)18-13-17(23-21(22)24-18)15-6-5-7-16(12-15)20(26)25-10-3-2-4-11-25/h5-9,12-13H,2-4,10-11H2,1H3,(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50152215

(7N-[1-(2-chloro-4-pyridylmethyl)-(2R)-tetrahydro-1...)Show SMILES Nc1nc(NC[C@H]2CCCN2Cc2ccnc(Cl)c2)nc2nc(nn12)-c1ccco1 Show InChI InChI=1S/C19H20ClN9O/c20-15-9-12(5-6-22-15)11-28-7-1-3-13(28)10-23-18-25-17(21)29-19(26-18)24-16(27-29)14-4-2-8-30-14/h2,4-6,8-9,13H,1,3,7,10-11H2,(H3,21,23,24,25,26,27)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50011294
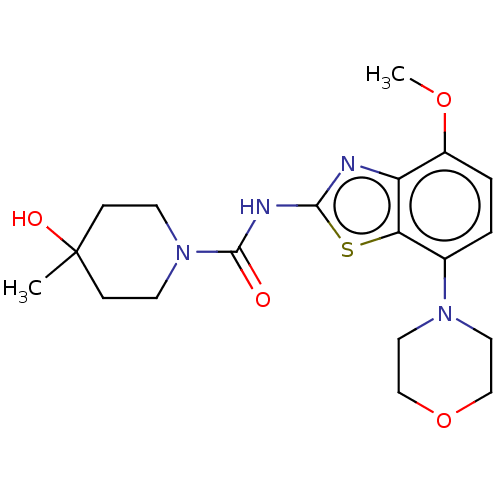
(A2A | Ro-4494351 | Ro-4494351-002 | Ro-4494351000 ...)Show SMILES COc1ccc(N2CCOCC2)c2sc(NC(=O)N3CCC(C)(O)CC3)nc12 Show InChI InChI=1S/C19H26N4O4S/c1-19(25)5-7-23(8-6-19)18(24)21-17-20-15-14(26-2)4-3-13(16(15)28-17)22-9-11-27-12-10-22/h3-4,25H,5-12H2,1-2H3,(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50212381
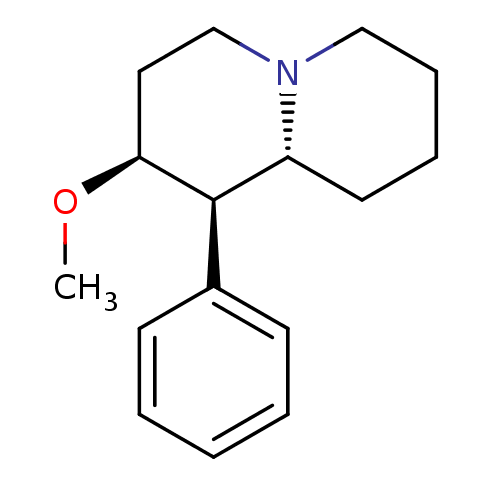
(CHEMBL537874 | threo-1-aza-4beta-methoxy-5-phenyl[...)Show InChI InChI=1S/C16H23NO/c1-18-15-10-12-17-11-6-5-9-14(17)16(15)13-7-3-2-4-8-13/h2-4,7-8,14-16H,5-6,9-12H2,1H3/t14-,15+,16-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-35428 from DAT in Sprague-Dawley rat striatum |
J Med Chem 50: 2718-31 (2007)
Article DOI: 10.1021/jm061354p
BindingDB Entry DOI: 10.7270/Q2Q81DXB |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50212385
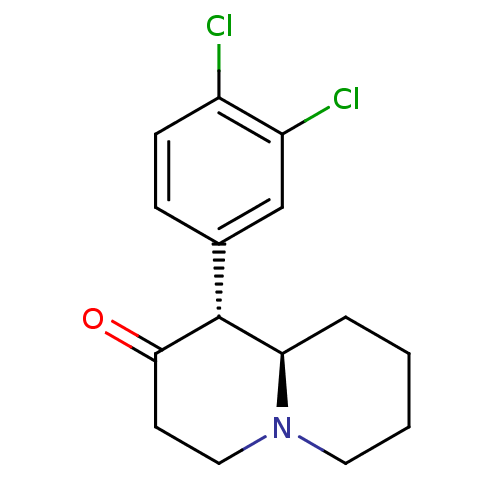
(CHEMBL558394 | threo-1-aza-4-oxo-5-(3,4-dichloroph...)Show InChI InChI=1S/C15H17Cl2NO/c16-11-5-4-10(9-12(11)17)15-13-3-1-2-7-18(13)8-6-14(15)19/h4-5,9,13,15H,1-3,6-8H2/t13-,15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-35428 from DAT in Sprague-Dawley rat striatum |
J Med Chem 50: 2718-31 (2007)
Article DOI: 10.1021/jm061354p
BindingDB Entry DOI: 10.7270/Q2Q81DXB |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50501643
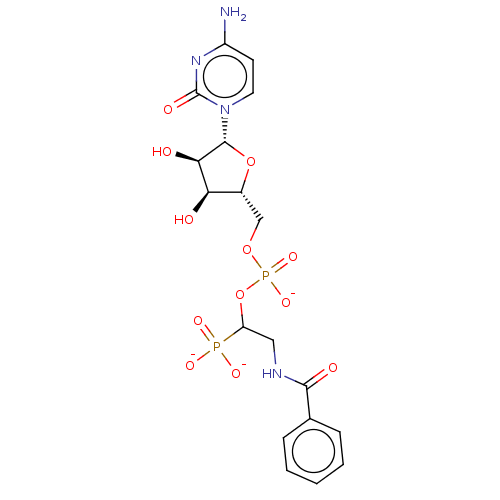
(CHEMBL4065191)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[#7]-c1ccn(-[#6@@H]-2-[#8]-[#6@H](-[#6]-[#8]P([#8-])(=O)[#8]-[#6](-[#6]-[#7]-[#6](=O)-c3ccccc3)P([#8-])([#8-])=O)-[#6@@H](-[#8])-[#6@H]-2-[#8])c(=O)n1 |r| Show InChI InChI=1S/C18H24N4O12P2.3Na/c19-12-6-7-22(18(26)21-12)17-15(24)14(23)11(33-17)9-32-36(30,31)34-13(35(27,28)29)8-20-16(25)10-4-2-1-3-5-10;;;/h1-7,11,13-15,17,23-24H,8-9H2,(H,20,25)(H,30,31)(H2,19,21,26)(H2,27,28,29);;;/q;3*+1/p-3/t11-,13?,14-,15-,17-;;;/m1.../s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method |
J Med Chem 60: 2135-2141 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01644
BindingDB Entry DOI: 10.7270/Q2XP77Z0 |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50501643

(CHEMBL4065191)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[#7]-c1ccn(-[#6@@H]-2-[#8]-[#6@H](-[#6]-[#8]P([#8-])(=O)[#8]-[#6](-[#6]-[#7]-[#6](=O)-c3ccccc3)P([#8-])([#8-])=O)-[#6@@H](-[#8])-[#6@H]-2-[#8])c(=O)n1 |r| Show InChI InChI=1S/C18H24N4O12P2.3Na/c19-12-6-7-22(18(26)21-12)17-15(24)14(23)11(33-17)9-32-36(30,31)34-13(35(27,28)29)8-20-16(25)10-4-2-1-3-5-10;;;/h1-7,11,13-15,17,23-24H,8-9H2,(H,20,25)(H,30,31)(H2,19,21,26)(H2,27,28,29);;;/q;3*+1/p-3/t11-,13?,14-,15-,17-;;;/m1.../s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method |
J Med Chem 60: 2135-2141 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01644
BindingDB Entry DOI: 10.7270/Q2XP77Z0 |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50501640
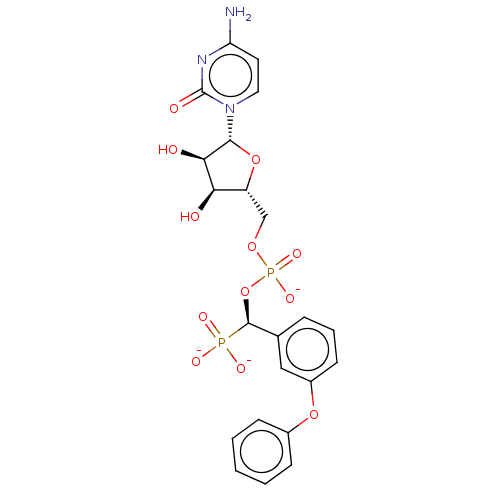
(CHEMBL4102509)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[#7]-c1ccn(-[#6@@H]-2-[#8]-[#6@H](-[#6]-[#8]P([#8-])(=O)[#8]-[#6@@H](-c3cccc(-[#8]-c4ccccc4)c3)P([#8-])([#8-])=O)-[#6@@H](-[#8])-[#6@H]-2-[#8])c(=O)n1 |r| Show InChI InChI=1S/C22H25N3O12P2.3Na/c23-17-9-10-25(22(28)24-17)20-19(27)18(26)16(36-20)12-34-39(32,33)37-21(38(29,30)31)13-5-4-8-15(11-13)35-14-6-2-1-3-7-14;;;/h1-11,16,18-21,26-27H,12H2,(H,32,33)(H2,23,24,28)(H2,29,30,31);;;/q;3*+1/p-3/t16-,18-,19-,20-,21-;;;/m1.../s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method |
J Med Chem 60: 2135-2141 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01644
BindingDB Entry DOI: 10.7270/Q2XP77Z0 |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
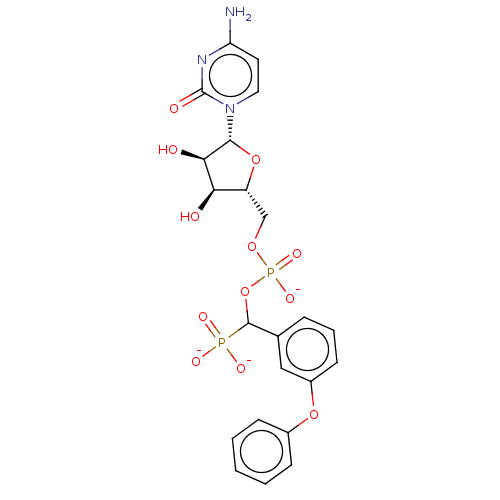
(Homo sapiens (Human)) | BDBM50127702
(CHEMBL3629697)Show SMILES [Na+].[Na+].[Na+].Nc1ccn([C@@H]2O[C@H](COP([O-])(=O)OC(c3cccc(Oc4ccccc4)c3)P([O-])([O-])=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C22H25N3O12P2.3Na/c23-17-9-10-25(22(28)24-17)20-19(27)18(26)16(36-20)12-34-39(32,33)37-21(38(29,30)31)13-5-4-8-15(11-13)35-14-6-2-1-3-7-14;;;/h1-11,16,18-21,26-27H,12H2,(H,32,33)(H2,23,24,28)(H2,29,30,31);;;/q;3*+1/p-3/t16-,18-,19-,20-,21?;;;/m1.../s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant ST6Gal-1 using CMP-Neu5Ac and p-nitrophenyl LacNAc as donar and acceptor by Lineweaver-Burk double recipr... |
J Med Chem 58: 7972-90 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01181
BindingDB Entry DOI: 10.7270/Q20V8FMQ |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50501638

(CHEMBL4091389)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[#7]-c1ccn(-[#6@@H]-2-[#8]-[#6@H](-[#6]-[#8]P([#8-])(=O)[#8]-[#6](-[#6](=O)-[#7]-3-[#6]-[#6]-[#6]-[#6]-[#6]-3)P([#8-])([#8-])=O)-[#6@@H](-[#8])-[#6@H]-2-[#8])c(=O)n1 |r| Show InChI InChI=1S/C16H26N4O12P2.3Na/c17-10-4-7-20(16(24)18-10)14-12(22)11(21)9(31-14)8-30-34(28,29)32-15(33(25,26)27)13(23)19-5-2-1-3-6-19;;;/h4,7,9,11-12,14-15,21-22H,1-3,5-6,8H2,(H,28,29)(H2,17,18,24)(H2,25,26,27);;;/q;3*+1/p-3/t9-,11-,12-,14-,15?;;;/m1.../s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method |
J Med Chem 60: 2135-2141 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01644
BindingDB Entry DOI: 10.7270/Q2XP77Z0 |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50501638

(CHEMBL4091389)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[#7]-c1ccn(-[#6@@H]-2-[#8]-[#6@H](-[#6]-[#8]P([#8-])(=O)[#8]-[#6](-[#6](=O)-[#7]-3-[#6]-[#6]-[#6]-[#6]-[#6]-3)P([#8-])([#8-])=O)-[#6@@H](-[#8])-[#6@H]-2-[#8])c(=O)n1 |r| Show InChI InChI=1S/C16H26N4O12P2.3Na/c17-10-4-7-20(16(24)18-10)14-12(22)11(21)9(31-14)8-30-34(28,29)32-15(33(25,26)27)13(23)19-5-2-1-3-6-19;;;/h4,7,9,11-12,14-15,21-22H,1-3,5-6,8H2,(H,28,29)(H2,17,18,24)(H2,25,26,27);;;/q;3*+1/p-3/t9-,11-,12-,14-,15?;;;/m1.../s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method |
J Med Chem 60: 2135-2141 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01644
BindingDB Entry DOI: 10.7270/Q2XP77Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50131550

((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C23H25F4N3O11P2/c24-22(25,42(36,37)38)14-5-1-12(2-6-14)9-16(20(28)34)30-21(35)17(11-19(32)33)29-18(31)10-13-3-7-15(8-4-13)23(26,27)43(39,40)41/h1-8,16-17H,9-11H2,(H2,28,34)(H,29,31)(H,30,35)(H,32,33)(H2,36,37,38)(H2,39,40,41)/t16-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) |
Bioorg Med Chem Lett 29: 2358-2363 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.011
BindingDB Entry DOI: 10.7270/Q2280C4B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50092173

(CHEMBL325710 | N*6*,N*6*-Dipropyl-6,7-dihydro-5H-1...)Show InChI InChI=1S/C16H23N3S/c1-3-5-19(6-4-2)13-7-11-9-14-15(10-12(11)8-13)20-16(17)18-14/h9-10,13H,3-8H2,1-2H3,(H2,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50092312
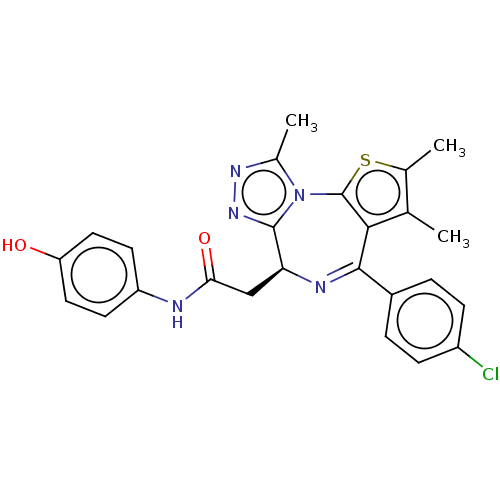
(Birabresib | MK-8628 | OTX-015)Show SMILES [H][C@@]1(CC(=O)Nc2ccc(O)cc2)N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1 |r,c:14| Show InChI InChI=1S/C25H22ClN5O2S/c1-13-14(2)34-25-22(13)23(16-4-6-17(26)7-5-16)28-20(24-30-29-15(3)31(24)25)12-21(33)27-18-8-10-19(32)11-9-18/h4-11,20,32H,12H2,1-3H3,(H,27,33)/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114423
BindingDB Entry DOI: 10.7270/Q2S46WZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50127703
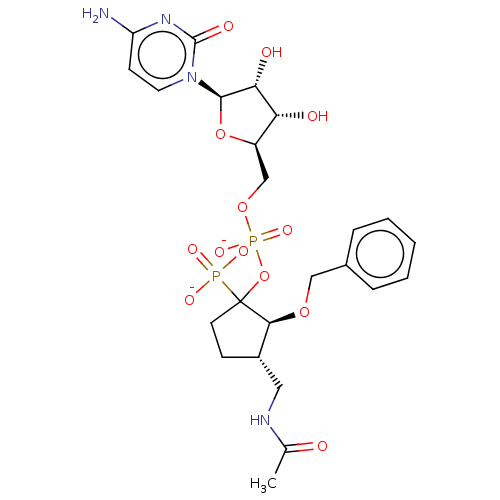
(CHEMBL3629696)Show SMILES [Na+].[Na+].[Na+].CC(=O)NC[C@@H]1CCC(OP([O-])(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2ccc(N)nc2=O)([C@H]1OCc1ccccc1)P([O-])([O-])=O |r| Show InChI InChI=1S/C24H34N4O13P2.3Na/c1-14(29)26-11-16-7-9-24(42(33,34)35,21(16)38-12-15-5-3-2-4-6-15)41-43(36,37)39-13-17-19(30)20(31)22(40-17)28-10-8-18(25)27-23(28)32;;;/h2-6,8,10,16-17,19-22,30-31H,7,9,11-13H2,1H3,(H,26,29)(H,36,37)(H2,25,27,32)(H2,33,34,35);;;/q;3*+1/p-3/t16-,17+,19+,20+,21-,22+,24?;;;/m0.../s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method |
J Med Chem 60: 2135-2141 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01644
BindingDB Entry DOI: 10.7270/Q2XP77Z0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50212371

((1R,2R,9aR)-2-ethyl-1-phenyl-octahydro-1H-quinoliz...)Show InChI InChI=1S/C17H25N/c1-2-14-11-13-18-12-7-6-10-16(18)17(14)15-8-4-3-5-9-15/h3-5,8-9,14,16-17H,2,6-7,10-13H2,1H3/t14-,16-,17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-35428 from DAT in Sprague-Dawley rat striatum |
J Med Chem 50: 2718-31 (2007)
Article DOI: 10.1021/jm061354p
BindingDB Entry DOI: 10.7270/Q2Q81DXB |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50127703

(CHEMBL3629696)Show SMILES [Na+].[Na+].[Na+].CC(=O)NC[C@@H]1CCC(OP([O-])(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2ccc(N)nc2=O)([C@H]1OCc1ccccc1)P([O-])([O-])=O |r| Show InChI InChI=1S/C24H34N4O13P2.3Na/c1-14(29)26-11-16-7-9-24(42(33,34)35,21(16)38-12-15-5-3-2-4-6-15)41-43(36,37)39-13-17-19(30)20(31)22(40-17)28-10-8-18(25)27-23(28)32;;;/h2-6,8,10,16-17,19-22,30-31H,7,9,11-13H2,1H3,(H,26,29)(H,36,37)(H2,25,27,32)(H2,33,34,35);;;/q;3*+1/p-3/t16-,17+,19+,20+,21-,22+,24?;;;/m0.../s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant ST6Gal-1 using CMP-Neu5Ac and p-nitrophenyl LacNAc as donar and acceptor by Lineweaver-Burk double recipr... |
J Med Chem 58: 7972-90 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01181
BindingDB Entry DOI: 10.7270/Q20V8FMQ |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50127703

(CHEMBL3629696)Show SMILES [Na+].[Na+].[Na+].CC(=O)NC[C@@H]1CCC(OP([O-])(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2ccc(N)nc2=O)([C@H]1OCc1ccccc1)P([O-])([O-])=O |r| Show InChI InChI=1S/C24H34N4O13P2.3Na/c1-14(29)26-11-16-7-9-24(42(33,34)35,21(16)38-12-15-5-3-2-4-6-15)41-43(36,37)39-13-17-19(30)20(31)22(40-17)28-10-8-18(25)27-23(28)32;;;/h2-6,8,10,16-17,19-22,30-31H,7,9,11-13H2,1H3,(H,26,29)(H,36,37)(H2,25,27,32)(H2,33,34,35);;;/q;3*+1/p-3/t16-,17+,19+,20+,21-,22+,24?;;;/m0.../s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method |
J Med Chem 60: 2135-2141 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01644
BindingDB Entry DOI: 10.7270/Q2XP77Z0 |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus) | BDBM50212365

(CHEMBL556365 | threo-1-aza-5-(3,4-dichlorophenyl)[...)Show InChI InChI=1S/C15H19Cl2N/c16-13-7-6-11(10-14(13)17)12-4-3-9-18-8-2-1-5-15(12)18/h6-7,10,12,15H,1-5,8-9H2/t12-,15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from NET in Sprague-Dawley rat cortical tissue |
J Med Chem 50: 2718-31 (2007)
Article DOI: 10.1021/jm061354p
BindingDB Entry DOI: 10.7270/Q2Q81DXB |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50127703

(CHEMBL3629696)Show SMILES [Na+].[Na+].[Na+].CC(=O)NC[C@@H]1CCC(OP([O-])(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2ccc(N)nc2=O)([C@H]1OCc1ccccc1)P([O-])([O-])=O |r| Show InChI InChI=1S/C24H34N4O13P2.3Na/c1-14(29)26-11-16-7-9-24(42(33,34)35,21(16)38-12-15-5-3-2-4-6-15)41-43(36,37)39-13-17-19(30)20(31)22(40-17)28-10-8-18(25)27-23(28)32;;;/h2-6,8,10,16-17,19-22,30-31H,7,9,11-13H2,1H3,(H,26,29)(H,36,37)(H2,25,27,32)(H2,33,34,35);;;/q;3*+1/p-3/t16-,17+,19+,20+,21-,22+,24?;;;/m0.../s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant ST6Gal-1 using CMP-Neu5Ac and p-nitrophenyl LacNAc as donar and acceptor by Lineweaver-Burk double recipr... |
J Med Chem 58: 7972-90 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01181
BindingDB Entry DOI: 10.7270/Q20V8FMQ |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus) | BDBM50212359

(CHEMBL558182 | threo-1-aza-4beta-hydroxy-5-(3,4-di...)Show SMILES O[C@H]1CCN2CCCC[C@@H]2[C@H]1c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C15H19Cl2NO/c16-11-5-4-10(9-12(11)17)15-13-3-1-2-7-18(13)8-6-14(15)19/h4-5,9,13-15,19H,1-3,6-8H2/t13-,14+,15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from NET in Sprague-Dawley rat cortical tissue |
J Med Chem 50: 2718-31 (2007)
Article DOI: 10.1021/jm061354p
BindingDB Entry DOI: 10.7270/Q2Q81DXB |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50501642
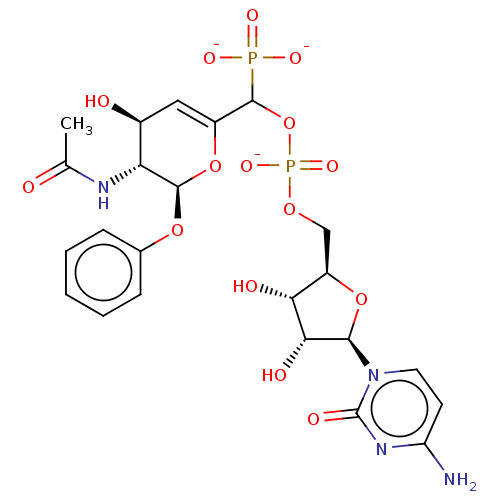
(CHEMBL3629698)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@@H](-[#8])-[#6]=[#6](-[#8]-[#6@H]-1-[#8]-c1ccccc1)-[#6](-[#8]P([#8-])(=O)[#8]-[#6]-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1ccc(-[#7])nc1=O)P([#8-])([#8-])=O |r,c:7| Show InChI InChI=1S/C23H30N4O15P2.3Na/c1-11(28)25-17-13(29)9-14(41-21(17)39-12-5-3-2-4-6-12)22(43(33,34)35)42-44(36,37)38-10-15-18(30)19(31)20(40-15)27-8-7-16(24)26-23(27)32;;;/h2-9,13,15,17-22,29-31H,10H2,1H3,(H,25,28)(H,36,37)(H2,24,26,32)(H2,33,34,35);;;/q;3*+1/p-3/t13-,15+,17+,18+,19+,20+,21+,22?;;;/m0.../s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method |
J Med Chem 60: 2135-2141 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01644
BindingDB Entry DOI: 10.7270/Q2XP77Z0 |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50501642

(CHEMBL3629698)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@@H](-[#8])-[#6]=[#6](-[#8]-[#6@H]-1-[#8]-c1ccccc1)-[#6](-[#8]P([#8-])(=O)[#8]-[#6]-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1ccc(-[#7])nc1=O)P([#8-])([#8-])=O |r,c:7| Show InChI InChI=1S/C23H30N4O15P2.3Na/c1-11(28)25-17-13(29)9-14(41-21(17)39-12-5-3-2-4-6-12)22(43(33,34)35)42-44(36,37)38-10-15-18(30)19(31)20(40-15)27-8-7-16(24)26-23(27)32;;;/h2-9,13,15,17-22,29-31H,10H2,1H3,(H,25,28)(H,36,37)(H2,24,26,32)(H2,33,34,35);;;/q;3*+1/p-3/t13-,15+,17+,18+,19+,20+,21+,22?;;;/m0.../s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method |
J Med Chem 60: 2135-2141 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01644
BindingDB Entry DOI: 10.7270/Q2XP77Z0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50212363
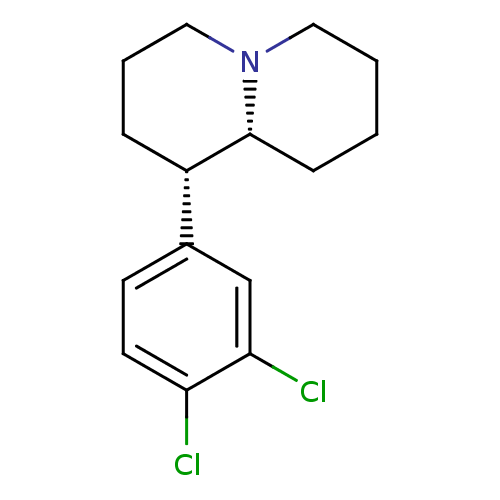
(CHEMBL559186 | erythro-1-aza-5-(3,4-dichlorophenyl...)Show InChI InChI=1S/C15H19Cl2N/c16-13-7-6-11(10-14(13)17)12-4-3-9-18-8-2-1-5-15(12)18/h6-7,10,12,15H,1-5,8-9H2/t12-,15+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-35428 from DAT in Sprague-Dawley rat striatum |
J Med Chem 50: 2718-31 (2007)
Article DOI: 10.1021/jm061354p
BindingDB Entry DOI: 10.7270/Q2Q81DXB |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
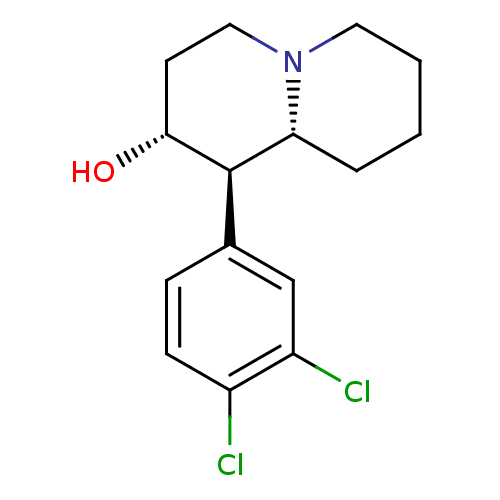
(Rattus norvegicus (rat)) | BDBM50212382
(CHEMBL537652 | threo-1-aza-4alpha-hydroxy-5-(3,4-d...)Show SMILES O[C@@H]1CCN2CCCC[C@@H]2[C@H]1c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C15H19Cl2NO/c16-11-5-4-10(9-12(11)17)15-13-3-1-2-7-18(13)8-6-14(15)19/h4-5,9,13-15,19H,1-3,6-8H2/t13-,14-,15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-35428 from DAT in Sprague-Dawley rat striatum |
J Med Chem 50: 2718-31 (2007)
Article DOI: 10.1021/jm061354p
BindingDB Entry DOI: 10.7270/Q2Q81DXB |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50594647

(CHEMBL5182020)Show SMILES Cc1cccc(Nc2nc(NCCCO)nc3n(ncc23)-c2ccccc2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114378
BindingDB Entry DOI: 10.7270/Q2377DQ0 |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50501637
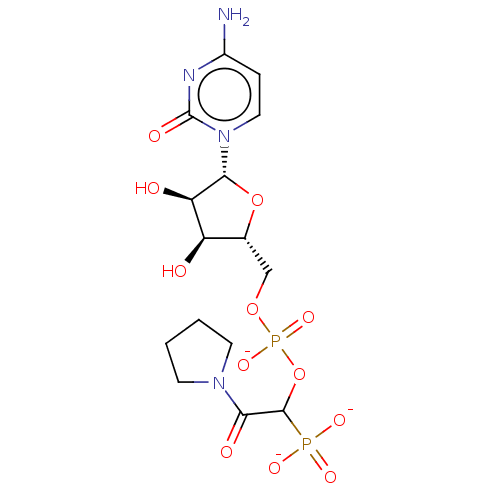
(CHEMBL4063545)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[#7]-c1ccn(-[#6@@H]-2-[#8]-[#6@H](-[#6]-[#8]P([#8-])(=O)[#8]-[#6](-[#6](=O)-[#7]-3-[#6]-[#6]-[#6]-[#6]-3)P([#8-])([#8-])=O)-[#6@@H](-[#8])-[#6@H]-2-[#8])c(=O)n1 |r| Show InChI InChI=1S/C15H24N4O12P2.3Na/c16-9-3-6-19(15(23)17-9)13-11(21)10(20)8(30-13)7-29-33(27,28)31-14(32(24,25)26)12(22)18-4-1-2-5-18;;;/h3,6,8,10-11,13-14,20-21H,1-2,4-5,7H2,(H,27,28)(H2,16,17,23)(H2,24,25,26);;;/q;3*+1/p-3/t8-,10-,11-,13-,14?;;;/m1.../s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method |
J Med Chem 60: 2135-2141 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01644
BindingDB Entry DOI: 10.7270/Q2XP77Z0 |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50501637

(CHEMBL4063545)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[#7]-c1ccn(-[#6@@H]-2-[#8]-[#6@H](-[#6]-[#8]P([#8-])(=O)[#8]-[#6](-[#6](=O)-[#7]-3-[#6]-[#6]-[#6]-[#6]-3)P([#8-])([#8-])=O)-[#6@@H](-[#8])-[#6@H]-2-[#8])c(=O)n1 |r| Show InChI InChI=1S/C15H24N4O12P2.3Na/c16-9-3-6-19(15(23)17-9)13-11(21)10(20)8(30-13)7-29-33(27,28)31-14(32(24,25)26)12(22)18-4-1-2-5-18;;;/h3,6,8,10-11,13-14,20-21H,1-2,4-5,7H2,(H,27,28)(H2,16,17,23)(H2,24,25,26);;;/q;3*+1/p-3/t8-,10-,11-,13-,14?;;;/m1.../s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method |
J Med Chem 60: 2135-2141 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01644
BindingDB Entry DOI: 10.7270/Q2XP77Z0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data