 Found 667 hits with Last Name = 'zaller' and Initial = 'd'
Found 667 hits with Last Name = 'zaller' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301603
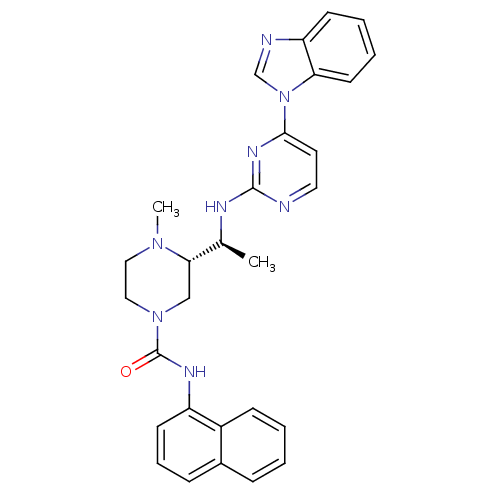
((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2ccccc12)[C@@H]1CN(CCN1C)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C29H30N8O/c1-20(32-28-30-15-14-27(34-28)37-19-31-24-11-5-6-13-25(24)37)26-18-36(17-16-35(26)2)29(38)33-23-12-7-9-21-8-3-4-10-22(21)23/h3-15,19-20,26H,16-18H2,1-2H3,(H,33,38)(H,30,32,34)/t20-,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301604
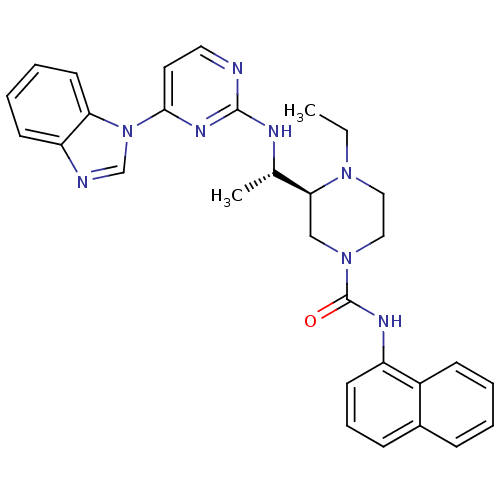
((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...)Show SMILES CCN1CCN(C[C@H]1[C@H](C)Nc1nccc(n1)-n1cnc2ccccc12)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C30H32N8O/c1-3-36-17-18-37(30(39)34-24-13-8-10-22-9-4-5-11-23(22)24)19-27(36)21(2)33-29-31-16-15-28(35-29)38-20-32-25-12-6-7-14-26(25)38/h4-16,20-21,27H,3,17-19H2,1-2H3,(H,34,39)(H,31,33,35)/t21-,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301619
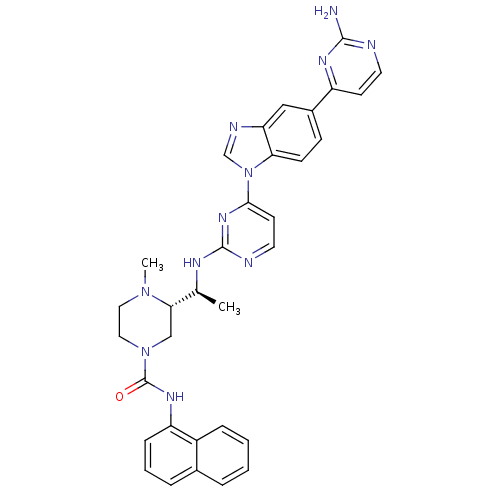
((S)-3-((S)-1-(4-(5-(2-aminopyrimidin-4-yl)-1H-benz...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2cc(ccc12)-c1ccnc(N)n1)[C@@H]1CN(CCN1C)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C33H33N11O/c1-21(29-19-43(17-16-42(29)2)33(45)40-26-9-5-7-22-6-3-4-8-24(22)26)38-32-36-15-13-30(41-32)44-20-37-27-18-23(10-11-28(27)44)25-12-14-35-31(34)39-25/h3-15,18,20-21,29H,16-17,19H2,1-2H3,(H,40,45)(H2,34,35,39)(H,36,38,41)/t21-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175745
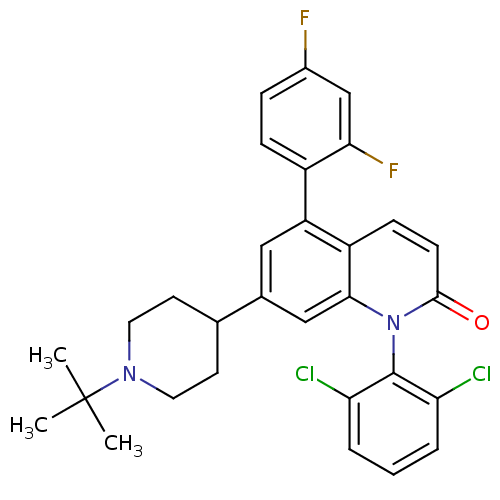
(7-(1-tert-butylpiperidin-4-yl)-1-(2,6-dichlorophen...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccc(F)cc2F)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(5.3,.54,;3.97,-.23,;4.75,-1.56,;3.2,1.1,;2.64,-1,;2.64,-2.54,;1.31,-3.31,;-.03,-2.54,;-.04,-1.01,;1.3,-.23,;-1.36,-3.31,;-1.35,-4.86,;-2.69,-5.64,;-2.69,-7.17,;-4.03,-7.94,;-4.03,-9.48,;-2.69,-10.25,;-2.69,-11.79,;-1.35,-9.47,;-1.36,-7.94,;-.03,-7.16,;-4.03,-4.86,;-5.36,-5.63,;-6.69,-4.85,;-6.69,-3.31,;-8.02,-2.53,;-5.34,-2.55,;-5.33,-1.01,;-6.66,-.24,;-8,-1,;-6.66,1.3,;-5.31,2.07,;-3.98,1.29,;-3.99,-.25,;-2.67,-1.03,;-4.02,-3.32,;-2.69,-2.55,)| Show InChI InChI=1S/C30H28Cl2F2N2O/c1-30(2,3)35-13-11-18(12-14-35)19-15-23(21-8-7-20(33)17-26(21)34)22-9-10-28(37)36(27(22)16-19)29-24(31)5-4-6-25(29)32/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of wild type p38alpha (unknown origin) |
J Biol Chem 282: 34663-71 (2007)
Article DOI: 10.1074/jbc.M704236200
BindingDB Entry DOI: 10.7270/Q2G44Q2F |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122386
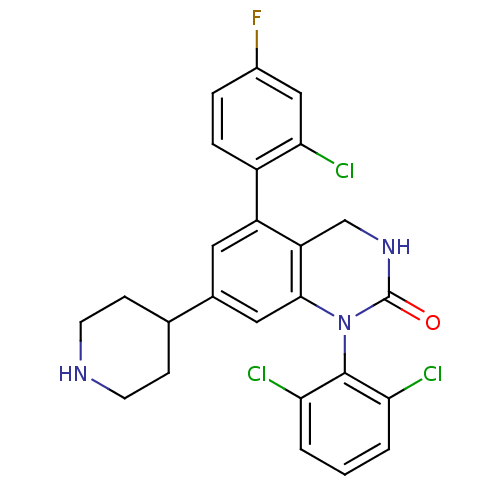
(5-(2-Chloro-4-fluoro-phenyl)-1-(2,6-dichloro-pheny...)Show SMILES Fc1ccc(c(Cl)c1)-c1cc(cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl)C1CCNCC1 Show InChI InChI=1S/C25H21Cl3FN3O/c26-20-2-1-3-21(27)24(20)32-23-11-15(14-6-8-30-9-7-14)10-18(19(23)13-31-25(32)33)17-5-4-16(29)12-22(17)28/h1-5,10-12,14,30H,6-9,13H2,(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM15459

(4-[1-methyl-2-(piperidin-4-yl)-4-[3-(trifluorometh...)Show SMILES C[C@H](Nc1cc(ccn1)-c1c(nc(C2CCNCC2)n1C)-c1cccc(c1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C29H30F3N5/c1-19(20-7-4-3-5-8-20)35-25-18-23(13-16-34-25)27-26(22-9-6-10-24(17-22)29(30,31)32)36-28(37(27)2)21-11-14-33-15-12-21/h3-10,13,16-19,21,33H,11-12,14-15H2,1-2H3,(H,34,35)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of wild type p38alpha (unknown origin) |
J Biol Chem 282: 34663-71 (2007)
Article DOI: 10.1074/jbc.M704236200
BindingDB Entry DOI: 10.7270/Q2G44Q2F |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122387

(5-(2-Chloro-4-fluoro-phenyl)-1-(2,6-dichloro-pheny...)Show SMILES Fc1ccc(c(Cl)c1)-c1cc(OC2CCNCC2)cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl Show InChI InChI=1S/C25H21Cl3FN3O2/c26-20-2-1-3-21(27)24(20)32-23-12-16(34-15-6-8-30-9-7-15)11-18(19(23)13-31-25(32)33)17-5-4-14(29)10-22(17)28/h1-5,10-12,15,30H,6-9,13H2,(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14 [G110A]
(Mus musculus (mouse)) | BDBM15240
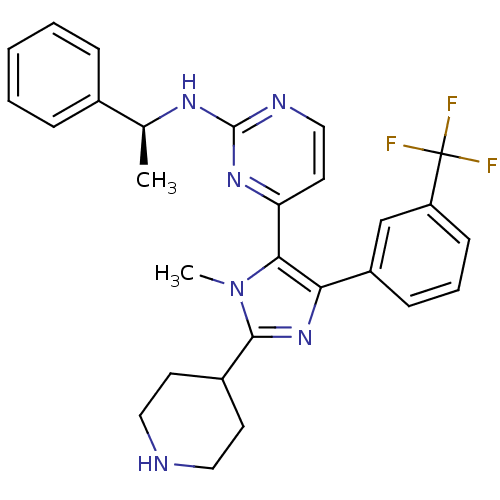
(4-[1-methyl-2-(piperidin-4-yl)-4-[3-(trifluorometh...)Show SMILES C[C@H](Nc1nccc(n1)-c1c(nc(C2CCNCC2)n1C)-c1cccc(c1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C28H29F3N6/c1-18(19-7-4-3-5-8-19)34-27-33-16-13-23(35-27)25-24(21-9-6-10-22(17-21)28(29,30)31)36-26(37(25)2)20-11-14-32-15-12-20/h3-10,13,16-18,20,32H,11-12,14-15H2,1-2H3,(H,33,34,35)/t18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with wild-type or mutant p38alpha enzymes, and substrates in the presence ATP/[gam... |
Nat Struct Biol 10: 764-9 (2003)
Article DOI: 10.1038/nsb949
BindingDB Entry DOI: 10.7270/Q2SX6BF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175747
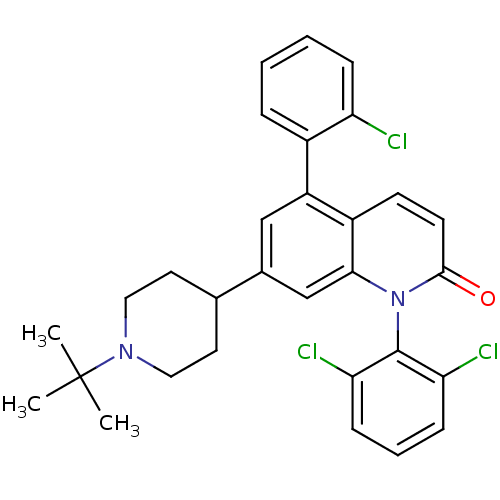
(7-(1-tert-butylpiperidin-4-yl)-5-(2-chlorophenyl)-...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccccc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(19.17,-37.34,;17.84,-38.12,;18.62,-39.45,;17.07,-36.79,;16.51,-38.89,;16.51,-40.43,;15.18,-41.2,;13.84,-40.43,;13.83,-38.89,;15.17,-38.12,;12.51,-41.21,;12.51,-42.76,;11.17,-43.53,;11.18,-45.07,;9.84,-45.84,;9.84,-47.37,;11.17,-48.15,;12.51,-47.37,;12.51,-45.83,;13.84,-45.06,;9.84,-42.76,;8.51,-43.54,;7.17,-42.76,;7.17,-41.22,;5.84,-40.45,;8.51,-40.45,;8.51,-38.92,;7.18,-38.15,;5.85,-38.92,;7.18,-36.61,;8.52,-35.84,;9.85,-36.61,;9.85,-38.15,;11.18,-38.92,;9.84,-41.22,;11.17,-40.45,)| Show InChI InChI=1S/C30H29Cl3N2O/c1-30(2,3)34-15-13-19(14-16-34)20-17-23(21-7-4-5-8-24(21)31)22-11-12-28(36)35(27(22)18-20)29-25(32)9-6-10-26(29)33/h4-12,17-19H,13-16H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122385
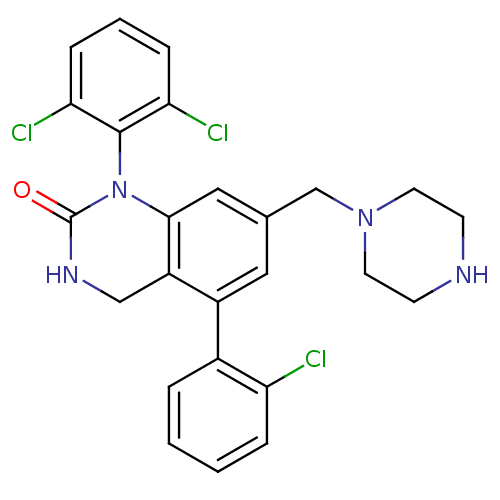
(5-(2-Chloro-phenyl)-1-(2,6-dichloro-phenyl)-7-pipe...)Show SMILES Clc1ccccc1-c1cc(CN2CCNCC2)cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl Show InChI InChI=1S/C25H23Cl3N4O/c26-20-5-2-1-4-17(20)18-12-16(15-31-10-8-29-9-11-31)13-23-19(18)14-30-25(33)32(23)24-21(27)6-3-7-22(24)28/h1-7,12-13,29H,8-11,14-15H2,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM15459

(4-[1-methyl-2-(piperidin-4-yl)-4-[3-(trifluorometh...)Show SMILES C[C@H](Nc1cc(ccn1)-c1c(nc(C2CCNCC2)n1C)-c1cccc(c1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C29H30F3N5/c1-19(20-7-4-3-5-8-20)35-25-18-23(13-16-34-25)27-26(22-9-6-10-24(17-22)29(30,31)32)36-28(37(27)2)21-11-14-33-15-12-21/h3-10,13,16-19,21,33H,11-12,14-15H2,1-2H3,(H,34,35)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of wild type p38beta (unknown origin) |
J Biol Chem 282: 34663-71 (2007)
Article DOI: 10.1074/jbc.M704236200
BindingDB Entry DOI: 10.7270/Q2G44Q2F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301624
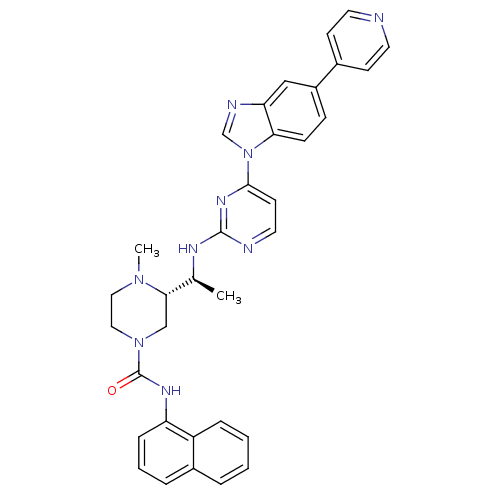
((S)-4-methyl-N(S)-4-methyl-N-(naphthalen-1-yl)-3-(...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2cc(ccc12)-c1ccncc1)[C@@H]1CN(CCN1C)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C34H33N9O/c1-23(31-21-42(19-18-41(31)2)34(44)39-28-9-5-7-25-6-3-4-8-27(25)28)38-33-36-17-14-32(40-33)43-22-37-29-20-26(10-11-30(29)43)24-12-15-35-16-13-24/h3-17,20,22-23,31H,18-19,21H2,1-2H3,(H,39,44)(H,36,38,40)/t23-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50375790
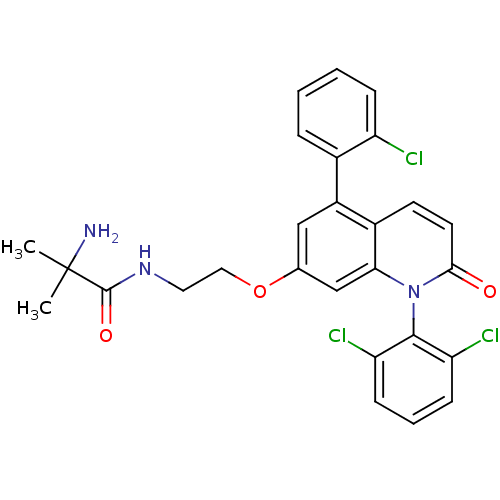
(CHEMBL270004)Show SMILES CC(C)(N)C(=O)NCCOc1cc(-c2ccccc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(6.02,.44,;5.14,-.82,;4.29,-2.11,;6.4,-1.7,;3.86,.05,;2.61,-.87,;3.82,1.59,;2.48,2.33,;1.15,1.54,;-.19,2.29,;-1.51,1.49,;-1.49,-.05,;-2.81,-.83,;-2.79,-2.37,;-4.12,-3.16,;-4.1,-4.7,;-2.75,-5.45,;-1.42,-4.66,;-1.45,-3.12,;-.13,-2.33,;-4.15,-.08,;-5.46,-.87,;-6.8,-.12,;-6.82,1.42,;-8.17,2.17,;-5.5,2.21,;-5.52,3.75,;-6.86,4.5,;-8.18,3.71,;-6.88,6.04,;-5.56,6.83,;-4.21,6.08,;-4.19,4.54,;-2.85,3.79,;-4.16,1.45,;-2.85,2.24,)| Show InChI InChI=1S/C27H24Cl3N3O3/c1-27(2,31)26(35)32-12-13-36-16-14-19(17-6-3-4-7-20(17)28)18-10-11-24(34)33(23(18)15-16)25-21(29)8-5-9-22(25)30/h3-11,14-15H,12-13,31H2,1-2H3,(H,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2222-6 (2008)
Article DOI: 10.1016/j.bmcl.2006.10.097
BindingDB Entry DOI: 10.7270/Q23T9J36 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM15240

(4-[1-methyl-2-(piperidin-4-yl)-4-[3-(trifluorometh...)Show SMILES C[C@H](Nc1nccc(n1)-c1c(nc(C2CCNCC2)n1C)-c1cccc(c1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C28H29F3N6/c1-18(19-7-4-3-5-8-19)34-27-33-16-13-23(35-27)25-24(21-9-6-10-22(17-21)28(29,30)31)36-26(37(25)2)20-11-14-32-15-12-20/h3-10,13,16-18,20,32H,11-12,14-15H2,1-2H3,(H,33,34,35)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with wild-type or mutant p38alpha enzymes, and substrates in the presence ATP/[gam... |
Nat Struct Biol 10: 764-9 (2003)
Article DOI: 10.1038/nsb949
BindingDB Entry DOI: 10.7270/Q2SX6BF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122390
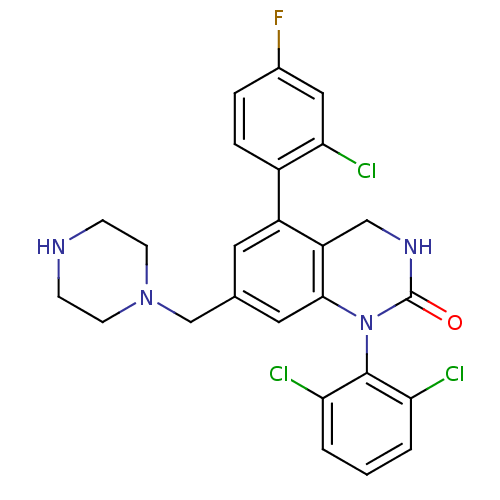
(5-(2-Chloro-4-fluoro-phenyl)-1-(2,6-dichloro-pheny...)Show SMILES Fc1ccc(c(Cl)c1)-c1cc(CN2CCNCC2)cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl Show InChI InChI=1S/C25H22Cl3FN4O/c26-20-2-1-3-21(27)24(20)33-23-11-15(14-32-8-6-30-7-9-32)10-18(19(23)13-31-25(33)34)17-5-4-16(29)12-22(17)28/h1-5,10-12,30H,6-9,13-14H2,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175745

(7-(1-tert-butylpiperidin-4-yl)-1-(2,6-dichlorophen...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccc(F)cc2F)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(5.3,.54,;3.97,-.23,;4.75,-1.56,;3.2,1.1,;2.64,-1,;2.64,-2.54,;1.31,-3.31,;-.03,-2.54,;-.04,-1.01,;1.3,-.23,;-1.36,-3.31,;-1.35,-4.86,;-2.69,-5.64,;-2.69,-7.17,;-4.03,-7.94,;-4.03,-9.48,;-2.69,-10.25,;-2.69,-11.79,;-1.35,-9.47,;-1.36,-7.94,;-.03,-7.16,;-4.03,-4.86,;-5.36,-5.63,;-6.69,-4.85,;-6.69,-3.31,;-8.02,-2.53,;-5.34,-2.55,;-5.33,-1.01,;-6.66,-.24,;-8,-1,;-6.66,1.3,;-5.31,2.07,;-3.98,1.29,;-3.99,-.25,;-2.67,-1.03,;-4.02,-3.32,;-2.69,-2.55,)| Show InChI InChI=1S/C30H28Cl2F2N2O/c1-30(2,3)35-13-11-18(12-14-35)19-15-23(21-8-7-20(33)17-26(21)34)22-9-10-28(37)36(27(22)16-19)29-24(31)5-4-6-25(29)32/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14 [G110D]
(Mus musculus (mouse)) | BDBM15240

(4-[1-methyl-2-(piperidin-4-yl)-4-[3-(trifluorometh...)Show SMILES C[C@H](Nc1nccc(n1)-c1c(nc(C2CCNCC2)n1C)-c1cccc(c1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C28H29F3N6/c1-18(19-7-4-3-5-8-19)34-27-33-16-13-23(35-27)25-24(21-9-6-10-22(17-21)28(29,30)31)36-26(37(25)2)20-11-14-32-15-12-20/h3-10,13,16-18,20,32H,11-12,14-15H2,1-2H3,(H,33,34,35)/t18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with wild-type or mutant p38alpha enzymes, and substrates in the presence ATP/[gam... |
Nat Struct Biol 10: 764-9 (2003)
Article DOI: 10.1038/nsb949
BindingDB Entry DOI: 10.7270/Q2SX6BF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50375797
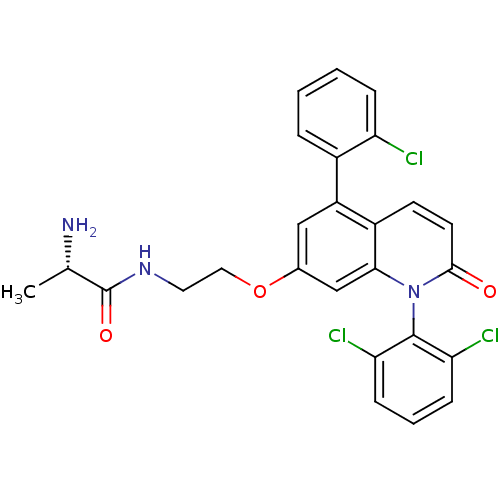
(CHEMBL269792)Show SMILES C[C@H](N)C(=O)NCCOc1cc(-c2ccccc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |wU:1.1,(30.05,-2.81,;29.98,-1.26,;31.21,-.38,;28.7,-.38,;27.45,-1.31,;28.66,1.16,;27.32,1.9,;25.99,1.11,;24.65,1.86,;23.33,1.07,;23.35,-.48,;22.03,-1.26,;22.05,-2.8,;20.72,-3.59,;20.74,-5.13,;22.09,-5.88,;23.42,-5.09,;23.39,-3.55,;24.71,-2.76,;20.69,-.51,;19.37,-1.3,;18.03,-.55,;18.01,.99,;16.67,1.74,;19.33,1.79,;19.31,3.33,;17.98,4.08,;16.65,3.29,;17.95,5.61,;19.28,6.41,;20.63,5.66,;20.65,4.12,;21.99,3.36,;20.67,1.03,;21.99,1.81,)| Show InChI InChI=1S/C26H22Cl3N3O3/c1-15(30)26(34)31-11-12-35-16-13-19(17-5-2-3-6-20(17)27)18-9-10-24(33)32(23(18)14-16)25-21(28)7-4-8-22(25)29/h2-10,13-15H,11-12,30H2,1H3,(H,31,34)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2222-6 (2008)
Article DOI: 10.1016/j.bmcl.2006.10.097
BindingDB Entry DOI: 10.7270/Q23T9J36 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM17060
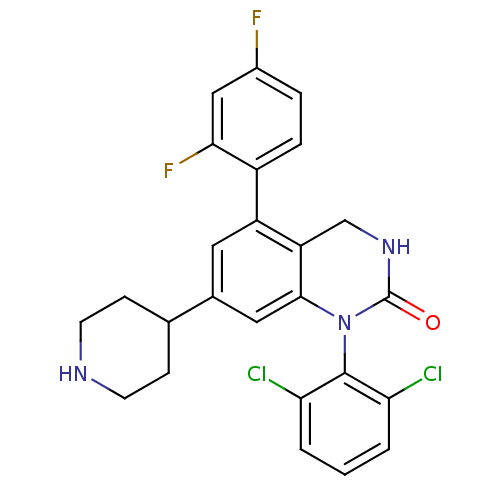
(1-(2,6-dichlorophenyl)-5-(2,4-difluorophenyl)-7-(p...)Show SMILES Fc1ccc(c(F)c1)-c1cc(cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl)C1CCNCC1 Show InChI InChI=1S/C25H21Cl2F2N3O/c26-20-2-1-3-21(27)24(20)32-23-11-15(14-6-8-30-9-7-14)10-18(19(23)13-31-25(32)33)17-5-4-16(28)12-22(17)29/h1-5,10-12,14,30H,6-9,13H2,(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50222624
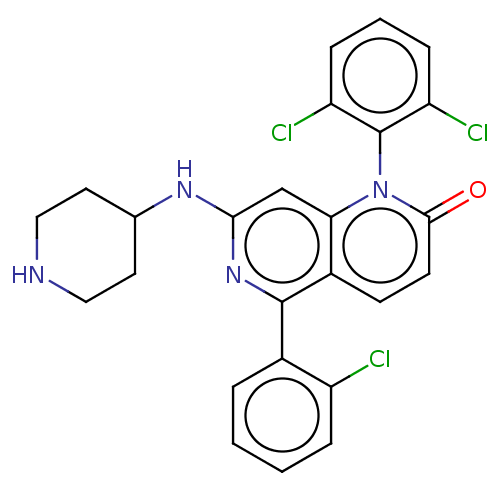
(CHEMBL357598)Show SMILES Clc1ccccc1-c1nc(NC2CCNCC2)cc2n(-c3c(Cl)cccc3Cl)c(=O)ccc12 |(10.11,-3.62,;8.78,-4.39,;8.78,-5.93,;7.43,-6.7,;6.1,-5.93,;6.11,-4.39,;7.45,-3.62,;7.47,-2.1,;8.8,-1.33,;8.8,.21,;10.13,.97,;11.46,.2,;12.79,.97,;14.12,.19,;14.12,-1.36,;12.79,-2.12,;11.46,-1.34,;7.47,.98,;6.14,.23,;4.81,.98,;4.81,2.52,;6.14,3.29,;7.47,2.52,;6.14,4.85,;4.81,5.62,;3.48,4.83,;3.48,3.29,;2.15,2.54,;3.48,.23,;2.13,1,;3.46,-1.31,;4.79,-2.08,;6.14,-1.31,)| Show InChI InChI=1S/C25H21Cl3N4O/c26-18-5-2-1-4-16(18)24-17-8-9-23(33)32(25-19(27)6-3-7-20(25)28)21(17)14-22(31-24)30-15-10-12-29-13-11-15/h1-9,14-15,29H,10-13H2,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of p38 MAP kinase |
Bioorg Med Chem Lett 13: 467-70 (2003)
BindingDB Entry DOI: 10.7270/Q22F7QM4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122384
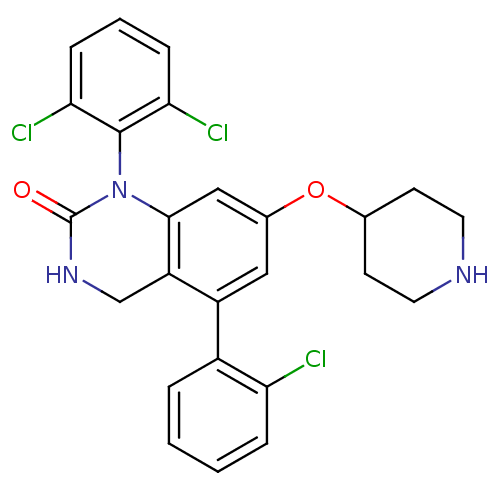
(5-(2-Chloro-phenyl)-1-(2,6-dichloro-phenyl)-7-(pip...)Show SMILES Clc1ccccc1-c1cc(OC2CCNCC2)cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl Show InChI InChI=1S/C25H22Cl3N3O2/c26-20-5-2-1-4-17(20)18-12-16(33-15-8-10-29-11-9-15)13-23-19(18)14-30-25(32)31(23)24-21(27)6-3-7-22(24)28/h1-7,12-13,15,29H,8-11,14H2,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122379

(7-(1-tert-Butyl-piperidin-4-yl)-5-(2-chloro-4-fluo...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc2N(C(=O)NCc2c(c1)-c1ccc(F)cc1Cl)c1c(Cl)cccc1Cl Show InChI InChI=1S/C29H29Cl3FN3O/c1-29(2,3)35-11-9-17(10-12-35)18-13-21(20-8-7-19(33)15-25(20)32)22-16-34-28(37)36(26(22)14-18)27-23(30)5-4-6-24(27)31/h4-8,13-15,17H,9-12,16H2,1-3H3,(H,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50222627
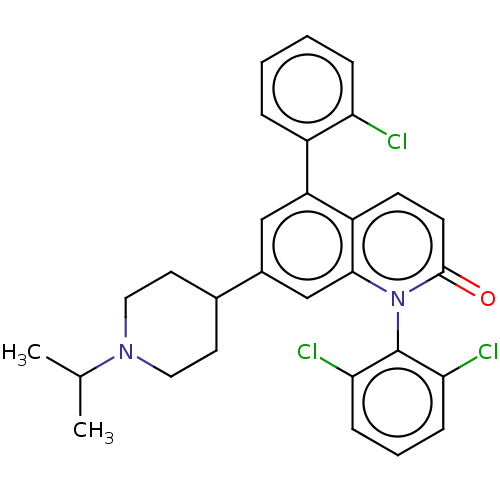
(CHEMBL356125)Show SMILES CC(C)N1CCC(CC1)c1cc(-c2ccccc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(15.97,6.5,;15.96,4.96,;17.29,4.19,;14.61,4.2,;14.61,2.66,;13.28,1.91,;11.95,2.68,;11.95,4.22,;13.28,4.97,;10.62,1.91,;10.62,.37,;9.27,-.4,;9.27,-1.94,;7.92,-2.69,;7.92,-4.23,;9.25,-5.02,;10.59,-4.25,;10.59,-2.71,;11.92,-1.92,;7.94,.37,;6.61,-.4,;5.28,.39,;5.28,1.91,;3.95,2.68,;6.63,2.68,;6.63,4.22,;7.96,4.99,;9.29,4.22,;7.96,6.53,;6.63,7.32,;5.3,6.53,;5.3,4.99,;3.95,4.24,;7.96,1.91,;9.29,2.68,)| Show InChI InChI=1S/C29H27Cl3N2O/c1-18(2)33-14-12-19(13-15-33)20-16-23(21-6-3-4-7-24(21)30)22-10-11-28(35)34(27(22)17-20)29-25(31)8-5-9-26(29)32/h3-11,16-19H,12-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of p38 MAP kinase |
Bioorg Med Chem Lett 13: 467-70 (2003)
BindingDB Entry DOI: 10.7270/Q22F7QM4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301605
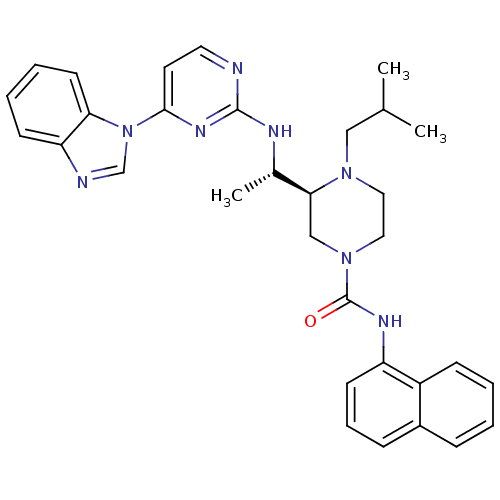
((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...)Show SMILES CC(C)CN1CCN(C[C@H]1[C@H](C)Nc1nccc(n1)-n1cnc2ccccc12)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C32H36N8O/c1-22(2)19-38-17-18-39(32(41)36-26-13-8-10-24-9-4-5-11-25(24)26)20-29(38)23(3)35-31-33-16-15-30(37-31)40-21-34-27-12-6-7-14-28(27)40/h4-16,21-23,29H,17-20H2,1-3H3,(H,36,41)(H,33,35,37)/t23-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50375796
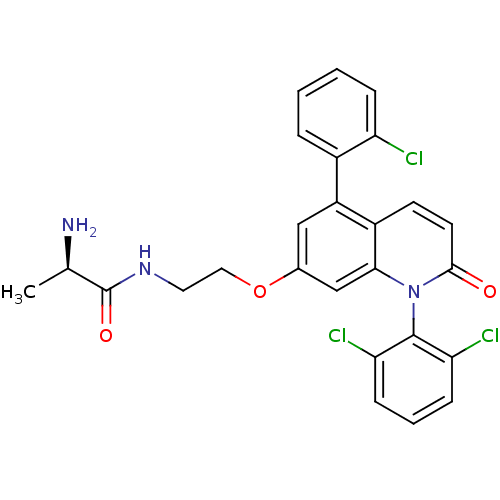
(CHEMBL255403)Show SMILES C[C@@H](N)C(=O)NCCOc1cc(-c2ccccc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |wD:1.1,(6.2,-17.24,;6.13,-15.68,;7.35,-14.8,;4.85,-14.8,;3.6,-15.73,;4.81,-13.26,;3.47,-12.52,;2.14,-13.31,;.8,-12.56,;-.53,-13.36,;-.5,-14.9,;-1.82,-15.69,;-1.8,-17.22,;-3.13,-18.01,;-3.11,-19.55,;-1.76,-20.31,;-.44,-19.51,;-.46,-17.97,;.86,-17.18,;-3.16,-14.94,;-4.48,-15.72,;-5.82,-14.98,;-5.84,-13.43,;-7.19,-12.69,;-4.52,-12.64,;-4.54,-11.1,;-5.87,-10.35,;-7.2,-11.14,;-5.9,-8.81,;-4.57,-8.02,;-3.23,-8.77,;-3.2,-10.31,;-1.86,-11.06,;-3.18,-13.4,;-1.86,-12.61,)| Show InChI InChI=1S/C26H22Cl3N3O3/c1-15(30)26(34)31-11-12-35-16-13-19(17-5-2-3-6-20(17)27)18-9-10-24(33)32(23(18)14-16)25-21(28)7-4-8-22(25)29/h2-10,13-15H,11-12,30H2,1H3,(H,31,34)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2222-6 (2008)
Article DOI: 10.1016/j.bmcl.2006.10.097
BindingDB Entry DOI: 10.7270/Q23T9J36 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301607
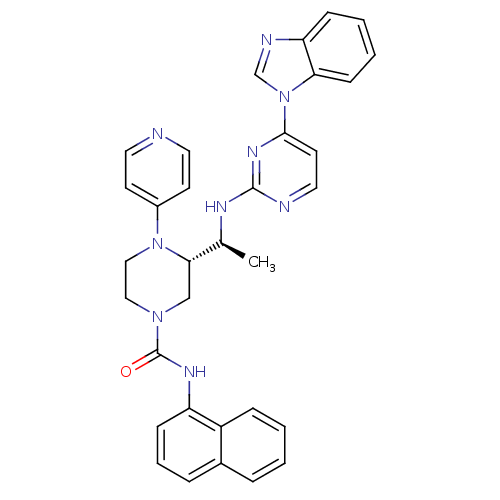
((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2ccccc12)[C@@H]1CN(CCN1c1ccncc1)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C33H31N9O/c1-23(37-32-35-18-15-31(39-32)42-22-36-28-10-4-5-12-29(28)42)30-21-40(19-20-41(30)25-13-16-34-17-14-25)33(43)38-27-11-6-8-24-7-2-3-9-26(24)27/h2-18,22-23,30H,19-21H2,1H3,(H,38,43)(H,35,37,39)/t23-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50175745

(7-(1-tert-butylpiperidin-4-yl)-1-(2,6-dichlorophen...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccc(F)cc2F)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(5.3,.54,;3.97,-.23,;4.75,-1.56,;3.2,1.1,;2.64,-1,;2.64,-2.54,;1.31,-3.31,;-.03,-2.54,;-.04,-1.01,;1.3,-.23,;-1.36,-3.31,;-1.35,-4.86,;-2.69,-5.64,;-2.69,-7.17,;-4.03,-7.94,;-4.03,-9.48,;-2.69,-10.25,;-2.69,-11.79,;-1.35,-9.47,;-1.36,-7.94,;-.03,-7.16,;-4.03,-4.86,;-5.36,-5.63,;-6.69,-4.85,;-6.69,-3.31,;-8.02,-2.53,;-5.34,-2.55,;-5.33,-1.01,;-6.66,-.24,;-8,-1,;-6.66,1.3,;-5.31,2.07,;-3.98,1.29,;-3.99,-.25,;-2.67,-1.03,;-4.02,-3.32,;-2.69,-2.55,)| Show InChI InChI=1S/C30H28Cl2F2N2O/c1-30(2,3)35-13-11-18(12-14-35)19-15-23(21-8-7-20(33)17-26(21)34)22-9-10-28(37)36(27(22)16-19)29-24(31)5-4-6-25(29)32/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of wild type p38beta (unknown origin) |
J Biol Chem 282: 34663-71 (2007)
Article DOI: 10.1074/jbc.M704236200
BindingDB Entry DOI: 10.7270/Q2G44Q2F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301594
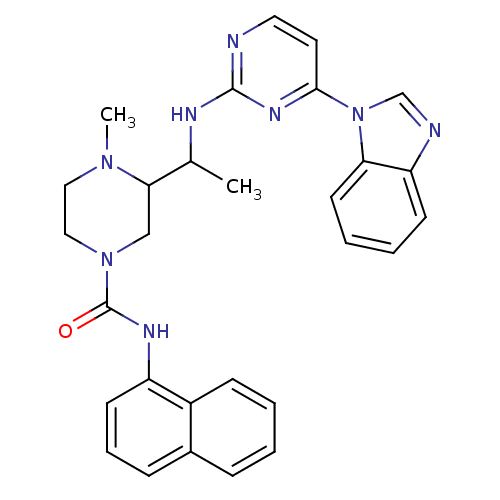
(3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...)Show SMILES CC(Nc1nccc(n1)-n1cnc2ccccc12)C1CN(CCN1C)C(=O)Nc1cccc2ccccc12 Show InChI InChI=1S/C29H30N8O/c1-20(32-28-30-15-14-27(34-28)37-19-31-24-11-5-6-13-25(24)37)26-18-36(17-16-35(26)2)29(38)33-23-12-7-9-21-8-3-4-10-22(21)23/h3-15,19-20,26H,16-18H2,1-2H3,(H,33,38)(H,30,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301594

(3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...)Show SMILES CC(Nc1nccc(n1)-n1cnc2ccccc12)C1CN(CCN1C)C(=O)Nc1cccc2ccccc12 Show InChI InChI=1S/C29H30N8O/c1-20(32-28-30-15-14-27(34-28)37-19-31-24-11-5-6-13-25(24)37)26-18-36(17-16-35(26)2)29(38)33-23-12-7-9-21-8-3-4-10-22(21)23/h3-15,19-20,26H,16-18H2,1-2H3,(H,33,38)(H,30,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175762
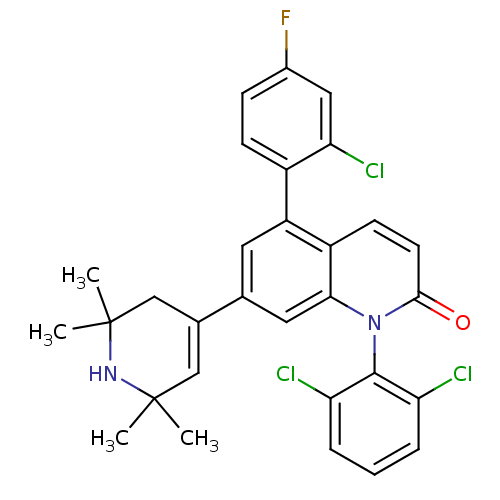
(5-(2-chloro-4-fluorophenyl)-1-(2,6-dichlorophenyl)...)Show SMILES CC1(C)CC(=CC(C)(C)N1)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |c:4,(2.04,.58,;2.06,2.12,;3.39,2.89,;.73,1.35,;-.61,2.12,;-.61,3.66,;.72,4.43,;2.05,5.21,;-.61,5.2,;2.06,3.66,;-1.94,1.34,;-1.94,-.21,;-3.27,-.98,;-3.27,-2.51,;-4.61,-3.28,;-4.61,-4.82,;-3.27,-5.59,;-3.27,-7.13,;-1.94,-4.81,;-1.94,-3.28,;-.61,-2.5,;-4.61,-.21,;-5.94,-.98,;-7.28,-.21,;-7.28,1.34,;-8.61,2.11,;-5.94,2.1,;-5.94,3.64,;-7.27,4.4,;-8.6,3.63,;-7.27,5.94,;-5.93,6.72,;-4.6,5.94,;-4.6,4.41,;-3.27,3.63,;-4.61,1.34,;-3.28,2.11,)| Show InChI InChI=1S/C30H26Cl3FN2O/c1-29(2)15-18(16-30(3,4)35-29)17-12-22(20-9-8-19(34)14-25(20)33)21-10-11-27(37)36(26(21)13-17)28-23(31)6-5-7-24(28)32/h5-15,35H,16H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175758
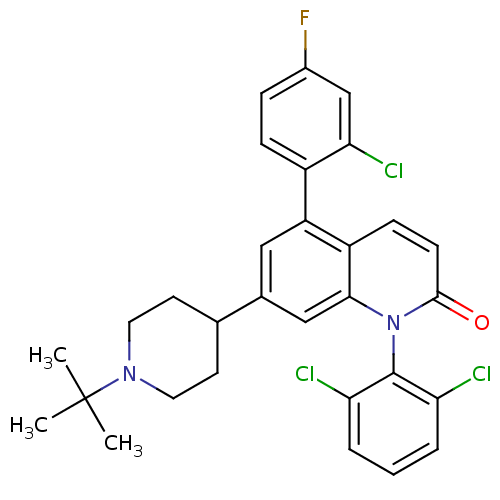
(7-(1-tert-butylpiperidin-4-yl)-5-(2-chloro-4-fluor...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(36.13,-13.37,;34.8,-14.15,;35.58,-15.48,;34.03,-12.81,;33.47,-14.92,;33.47,-16.46,;32.14,-17.22,;30.8,-16.46,;30.79,-14.92,;32.13,-14.15,;29.47,-17.23,;29.47,-18.78,;28.13,-19.55,;28.14,-21.09,;26.8,-21.86,;26.8,-23.4,;28.13,-24.17,;28.14,-25.71,;29.47,-23.39,;29.47,-21.85,;30.8,-21.08,;26.8,-18.78,;25.47,-19.56,;24.13,-18.79,;24.13,-17.24,;22.8,-16.47,;25.47,-16.47,;25.47,-14.94,;24.14,-14.17,;22.81,-14.95,;24.14,-12.64,;25.48,-11.86,;26.81,-12.63,;26.81,-14.17,;28.14,-14.94,;26.8,-17.24,;28.13,-16.47,)| Show InChI InChI=1S/C30H28Cl3FN2O/c1-30(2,3)35-13-11-18(12-14-35)19-15-23(21-8-7-20(34)17-26(21)33)22-9-10-28(37)36(27(22)16-19)29-24(31)5-4-6-25(29)32/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of TNF alpha release in THP1 cells |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50375798
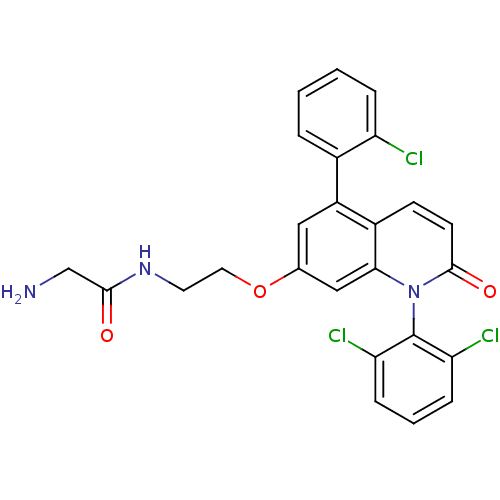
(CHEMBL437024)Show SMILES NCC(=O)NCCOc1cc(-c2ccccc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(7.26,-41.02,;5.95,-41.95,;4.71,-41.09,;3.48,-41.96,;4.7,-39.54,;3.36,-38.78,;2.03,-39.56,;.69,-38.79,;-.64,-39.57,;-.64,-41.11,;-1.97,-41.88,;-1.97,-43.42,;-3.31,-44.19,;-3.31,-45.73,;-1.98,-46.5,;-.64,-45.72,;-.64,-44.18,;.69,-43.41,;-3.3,-41.11,;-4.63,-41.88,;-5.96,-41.11,;-5.96,-39.57,;-7.29,-38.81,;-4.63,-38.79,;-4.63,-37.25,;-5.95,-36.48,;-7.28,-37.26,;-5.95,-34.95,;-4.62,-34.17,;-3.28,-34.94,;-3.28,-36.48,;-1.95,-37.25,;-3.3,-39.57,;-1.97,-38.81,)| Show InChI InChI=1S/C25H20Cl3N3O3/c26-19-5-2-1-4-16(19)18-12-15(34-11-10-30-23(32)14-29)13-22-17(18)8-9-24(33)31(22)25-20(27)6-3-7-21(25)28/h1-9,12-13H,10-11,14,29H2,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2222-6 (2008)
Article DOI: 10.1016/j.bmcl.2006.10.097
BindingDB Entry DOI: 10.7270/Q23T9J36 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122382

(5-(2-Chloro-4-fluoro-phenyl)-7-[1-(1-cyclopropyl-e...)Show SMILES CC(C1CC1)N1CCC(CC1)c1cc2N(C(=O)NCc2c(c1)-c1ccc(F)cc1Cl)c1c(Cl)cccc1Cl Show InChI InChI=1S/C30H29Cl3FN3O/c1-17(18-5-6-18)36-11-9-19(10-12-36)20-13-23(22-8-7-21(34)15-27(22)33)24-16-35-30(38)37(28(24)14-20)29-25(31)3-2-4-26(29)32/h2-4,7-8,13-15,17-19H,5-6,9-12,16H2,1H3,(H,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175760
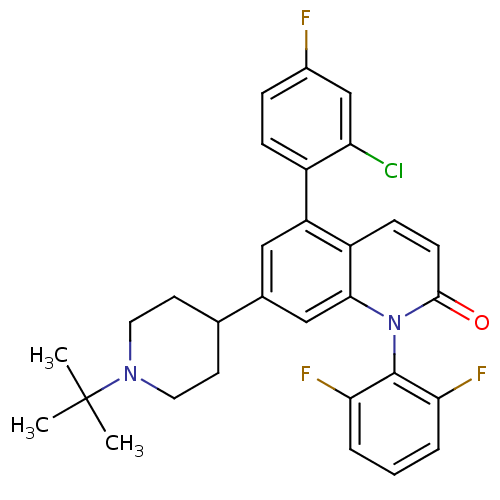
(7-(1-tert-butylpiperidin-4-yl)-5-(2-chloro-4-fluor...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(F)cccc3F)c2c1 |(36.23,-35.35,;34.9,-36.13,;35.68,-37.46,;34.13,-34.8,;33.57,-36.9,;33.57,-38.44,;32.24,-39.21,;30.9,-38.44,;30.9,-36.9,;32.23,-36.13,;29.57,-39.22,;29.57,-40.77,;28.24,-41.54,;28.24,-43.08,;26.9,-43.84,;26.9,-45.38,;28.24,-46.15,;28.24,-47.69,;29.57,-45.38,;29.57,-43.84,;30.9,-43.07,;26.9,-40.77,;25.57,-41.55,;24.23,-40.77,;24.23,-39.23,;22.9,-38.46,;25.57,-38.46,;25.57,-36.92,;24.24,-36.16,;22.91,-36.93,;24.24,-34.62,;25.58,-33.85,;26.91,-34.62,;26.91,-36.16,;28.24,-36.93,;26.9,-39.23,;28.23,-38.46,)| Show InChI InChI=1S/C30H28ClF3N2O/c1-30(2,3)35-13-11-18(12-14-35)19-15-23(21-8-7-20(32)17-24(21)31)22-9-10-28(37)36(27(22)16-19)29-25(33)5-4-6-26(29)34/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175758

(7-(1-tert-butylpiperidin-4-yl)-5-(2-chloro-4-fluor...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(36.13,-13.37,;34.8,-14.15,;35.58,-15.48,;34.03,-12.81,;33.47,-14.92,;33.47,-16.46,;32.14,-17.22,;30.8,-16.46,;30.79,-14.92,;32.13,-14.15,;29.47,-17.23,;29.47,-18.78,;28.13,-19.55,;28.14,-21.09,;26.8,-21.86,;26.8,-23.4,;28.13,-24.17,;28.14,-25.71,;29.47,-23.39,;29.47,-21.85,;30.8,-21.08,;26.8,-18.78,;25.47,-19.56,;24.13,-18.79,;24.13,-17.24,;22.8,-16.47,;25.47,-16.47,;25.47,-14.94,;24.14,-14.17,;22.81,-14.95,;24.14,-12.64,;25.48,-11.86,;26.81,-12.63,;26.81,-14.17,;28.14,-14.94,;26.8,-17.24,;28.13,-16.47,)| Show InChI InChI=1S/C30H28Cl3FN2O/c1-30(2,3)35-13-11-18(12-14-35)19-15-23(21-8-7-20(34)17-26(21)33)22-9-10-28(37)36(27(22)16-19)29-24(31)5-4-6-25(29)32/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175761
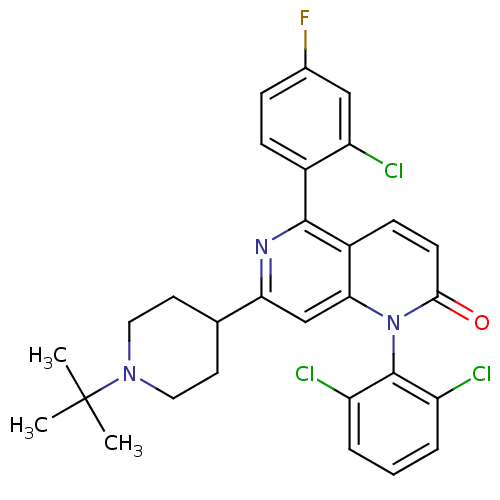
(7-(1-tert-butylpiperidin-4-yl)-5-(2-chloro-4-fluor...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc2n(-c3c(Cl)cccc3Cl)c(=O)ccc2c(n1)-c1ccc(F)cc1Cl |(3.3,5.4,;1.97,4.62,;2.74,3.29,;1.2,5.95,;.64,3.85,;.63,2.31,;-.69,1.54,;-2.04,2.31,;-2.04,3.85,;-.7,4.62,;-3.37,1.53,;-4.7,2.3,;-6.03,1.52,;-7.36,2.29,;-7.36,3.83,;-8.69,4.59,;-10.02,3.82,;-8.7,6.13,;-7.36,6.9,;-6.02,6.13,;-6.02,4.59,;-4.69,3.82,;-8.7,1.52,;-10.04,2.29,;-8.7,-.02,;-7.36,-.8,;-6.03,-.02,;-4.7,-.79,;-3.36,-.02,;-4.7,-2.33,;-6.03,-3.1,;-6.03,-4.63,;-4.7,-5.41,;-4.7,-6.95,;-3.36,-4.63,;-3.37,-3.09,;-2.03,-2.32,)| Show InChI InChI=1S/C29H27Cl3FN3O/c1-29(2,3)35-13-11-17(12-14-35)24-16-25-20(27(34-24)19-8-7-18(33)15-23(19)32)9-10-26(37)36(25)28-21(30)5-4-6-22(28)31/h4-10,15-17H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50222628

(CHEMBL142697)Show SMILES CN1C2CCC1CC(C2)Nc1cc2n(-c3c(Cl)cccc3Cl)c(=O)ccc2c(n1)-c1ccccc1Cl |THB:9:7:1:3.4,(5,-1.84,;4,-.68,;2.5,-.29,;2.5,1.25,;3.83,2.03,;3.79,.89,;2.31,.48,;.98,1.25,;.96,-.29,;-.35,2.02,;-1.7,1.26,;-3.03,2.03,;-4.36,1.28,;-5.69,2.03,;-5.69,3.57,;-4.34,4.36,;-3.01,3.57,;-4.34,5.9,;-5.69,6.67,;-7.02,5.88,;-7.02,4.36,;-8.35,3.59,;-7.02,1.28,;-8.35,2.05,;-7.02,-.26,;-5.69,-1.03,;-4.36,-.26,;-3.03,-1.05,;-1.7,-.28,;-3.03,-2.57,;-4.38,-3.34,;-4.39,-4.88,;-3.06,-5.65,;-1.72,-4.88,;-1.72,-3.34,;-.39,-2.57,)| Show InChI InChI=1S/C28H25Cl3N4O/c1-34-17-9-10-18(34)14-16(13-17)32-25-15-24-20(27(33-25)19-5-2-3-6-21(19)29)11-12-26(36)35(24)28-22(30)7-4-8-23(28)31/h2-8,11-12,15-18H,9-10,13-14H2,1H3,(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of p38 MAP kinase |
Bioorg Med Chem Lett 13: 467-70 (2003)
BindingDB Entry DOI: 10.7270/Q22F7QM4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301588
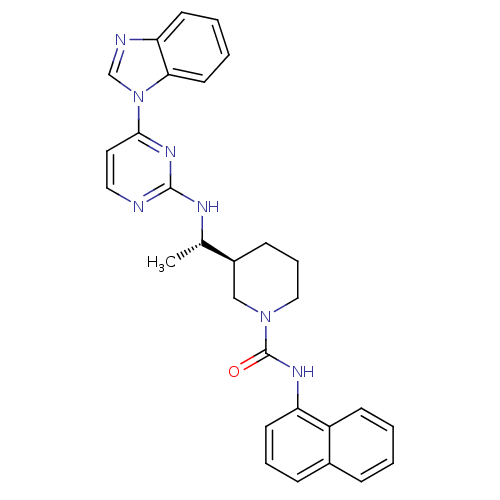
((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...)Show SMILES C[C@H](Nc1nccc(n1)-n1cnc2ccccc12)[C@H]1CCCN(C1)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C29H29N7O/c1-20(32-28-30-16-15-27(34-28)36-19-31-25-12-4-5-14-26(25)36)22-10-7-17-35(18-22)29(37)33-24-13-6-9-21-8-2-3-11-23(21)24/h2-6,8-9,11-16,19-20,22H,7,10,17-18H2,1H3,(H,33,37)(H,30,32,34)/t20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301618
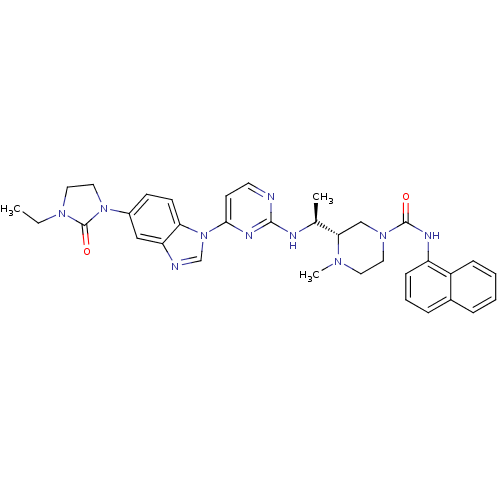
((S)-3-((S)-1-(4-(5-(3-ethyl-2-oxoimidazolidin-1-yl...)Show SMILES CCN1CCN(C1=O)c1ccc2n(cnc2c1)-c1ccnc(N[C@@H](C)[C@@H]2CN(CCN2C)C(=O)Nc2cccc3ccccc23)n1 |r| Show InChI InChI=1S/C34H38N10O2/c1-4-41-18-19-43(34(41)46)25-12-13-29-28(20-25)36-22-44(29)31-14-15-35-32(39-31)37-23(2)30-21-42(17-16-40(30)3)33(45)38-27-11-7-9-24-8-5-6-10-26(24)27/h5-15,20,22-23,30H,4,16-19,21H2,1-3H3,(H,38,45)(H,35,37,39)/t23-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122377

(5-(2-Chloro-4-fluoro-phenyl)-7-(1-cyclobutyl-piper...)Show SMILES Fc1ccc(c(Cl)c1)-c1cc(cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl)C1CCN(CC1)C1CCC1 Show InChI InChI=1S/C29H27Cl3FN3O/c30-24-5-2-6-25(31)28(24)36-27-14-18(17-9-11-35(12-10-17)20-3-1-4-20)13-22(23(27)16-34-29(36)37)21-8-7-19(33)15-26(21)32/h2,5-8,13-15,17,20H,1,3-4,9-12,16H2,(H,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50222629

(CHEMBL142187)Show SMILES CN1C2CCC1CC(C2)Nc1cc2N(C(=O)NCc2c(c1)-c1ccccc1Cl)c1c(Cl)cccc1Cl |THB:9:7:1:3.4| Show InChI InChI=1S/C28H27Cl3N4O/c1-34-18-9-10-19(34)12-16(11-18)33-17-13-21(20-5-2-3-6-23(20)29)22-15-32-28(36)35(26(22)14-17)27-24(30)7-4-8-25(27)31/h2-8,13-14,16,18-19,33H,9-12,15H2,1H3,(H,32,36) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of p38 MAP kinase |
Bioorg Med Chem Lett 13: 467-70 (2003)
BindingDB Entry DOI: 10.7270/Q22F7QM4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175760

(7-(1-tert-butylpiperidin-4-yl)-5-(2-chloro-4-fluor...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(F)cccc3F)c2c1 |(36.23,-35.35,;34.9,-36.13,;35.68,-37.46,;34.13,-34.8,;33.57,-36.9,;33.57,-38.44,;32.24,-39.21,;30.9,-38.44,;30.9,-36.9,;32.23,-36.13,;29.57,-39.22,;29.57,-40.77,;28.24,-41.54,;28.24,-43.08,;26.9,-43.84,;26.9,-45.38,;28.24,-46.15,;28.24,-47.69,;29.57,-45.38,;29.57,-43.84,;30.9,-43.07,;26.9,-40.77,;25.57,-41.55,;24.23,-40.77,;24.23,-39.23,;22.9,-38.46,;25.57,-38.46,;25.57,-36.92,;24.24,-36.16,;22.91,-36.93,;24.24,-34.62,;25.58,-33.85,;26.91,-34.62,;26.91,-36.16,;28.24,-36.93,;26.9,-39.23,;28.23,-38.46,)| Show InChI InChI=1S/C30H28ClF3N2O/c1-30(2,3)35-13-11-18(12-14-35)19-15-23(21-8-7-20(32)17-24(21)31)22-9-10-28(37)36(27(22)16-19)29-25(33)5-4-6-26(29)34/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of TNF alpha release in THP1 cells |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175742

(7-(8-aza-bicyclo[3.2.1]octan-3-yl)-5-(2-chloro-4-f...)Show SMILES Fc1ccc(c(Cl)c1)-c1cc(cc2n(-c3c(Cl)cccc3Cl)c(=O)ccc12)C1CC2CCC(C1)N2 |TLB:10:27:34:30.31,(12.26,-6.73,;12.26,-5.19,;10.92,-4.42,;10.93,-2.88,;12.26,-2.12,;13.59,-2.88,;14.92,-2.11,;13.6,-4.42,;12.26,-.58,;13.59,.19,;13.59,1.74,;12.25,2.5,;10.92,1.73,;9.59,2.5,;9.6,4.03,;8.27,4.8,;6.94,4.03,;8.26,6.33,;9.6,7.11,;10.93,6.34,;10.93,4.8,;12.26,4.03,;8.26,1.73,;6.92,2.5,;8.26,.19,;9.6,-.59,;10.92,.19,;14.92,2.51,;15.86,3.37,;16.12,5.19,;14.93,6.36,;15.46,5.1,;16.7,4.31,;16.49,2.56,;17.68,5.23,)| Show InChI InChI=1S/C28H22Cl3FN2O/c29-23-2-1-3-24(30)28(23)34-26-13-16(15-10-18-5-6-19(11-15)33-18)12-22(21(26)8-9-27(34)35)20-7-4-17(32)14-25(20)31/h1-4,7-9,12-15,18-19,33H,5-6,10-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50301608
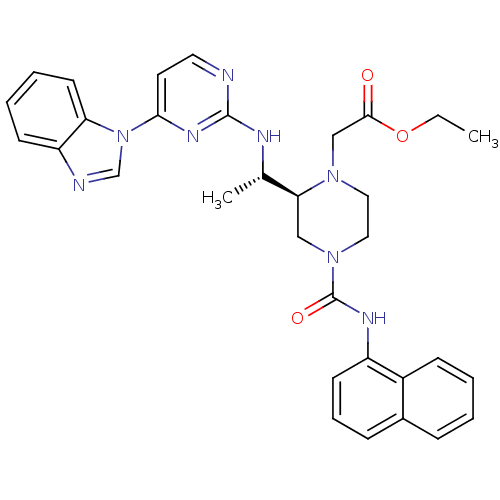
(CHEMBL566507 | ethyl 2-((S)-2-((S)-1-(4-(1H-benzo[...)Show SMILES CCOC(=O)CN1CCN(C[C@H]1[C@H](C)Nc1nccc(n1)-n1cnc2ccccc12)C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C32H34N8O3/c1-3-43-30(41)20-38-17-18-39(32(42)36-25-13-8-10-23-9-4-5-11-24(23)25)19-28(38)22(2)35-31-33-16-15-29(37-31)40-21-34-26-12-6-7-14-27(26)40/h4-16,21-22,28H,3,17-20H2,1-2H3,(H,36,42)(H,33,35,37)/t22-,28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 19: 5440-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.102
BindingDB Entry DOI: 10.7270/Q2GX4BM0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50375788

(CHEMBL257697)Show SMILES NC(CO)C(=O)NCCOc1cc(-c2ccccc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |w:1.0,(30.03,-41.17,;28.7,-41.95,;28.71,-43.49,;30.05,-44.25,;27.36,-41.18,;26.03,-41.96,;27.35,-39.63,;26.01,-38.88,;24.67,-39.65,;23.34,-38.88,;22,-39.66,;22.01,-41.2,;20.68,-41.97,;20.68,-43.51,;19.34,-44.28,;19.34,-45.82,;20.67,-46.59,;22.01,-45.81,;22.01,-44.28,;23.34,-43.5,;19.35,-41.2,;18.02,-41.97,;16.69,-41.21,;16.69,-39.66,;15.35,-38.9,;18.02,-38.89,;18.02,-37.34,;16.7,-36.58,;15.36,-37.35,;16.69,-35.04,;18.03,-34.26,;19.37,-35.03,;19.37,-36.57,;20.7,-37.34,;19.35,-39.66,;20.68,-38.9,)| Show InChI InChI=1S/C26H22Cl3N3O4/c27-19-5-2-1-4-16(19)18-12-15(36-11-10-31-26(35)22(30)14-33)13-23-17(18)8-9-24(34)32(23)25-20(28)6-3-7-21(25)29/h1-9,12-13,22,33H,10-11,14,30H2,(H,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2222-6 (2008)
Article DOI: 10.1016/j.bmcl.2006.10.097
BindingDB Entry DOI: 10.7270/Q23T9J36 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50122391

(5-(2-Chloro-phenyl)-1-(2,6-dichloro-phenyl)-7-(pip...)Show SMILES Clc1ccccc1-c1cc(NC2CCNCC2)cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl Show InChI InChI=1S/C25H23Cl3N4O/c26-20-5-2-1-4-17(20)18-12-16(31-15-8-10-29-11-9-15)13-23-19(18)14-30-25(33)32(23)24-21(27)6-3-7-22(24)28/h1-7,12-13,15,29,31H,8-11,14H2,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of p38 MAP kinase |
Bioorg Med Chem Lett 13: 467-70 (2003)
BindingDB Entry DOI: 10.7270/Q22F7QM4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122395

(5-(2-Chloro-4-fluoro-phenyl)-7-(1-cyclopropylmethy...)Show SMILES Fc1ccc(c(Cl)c1)-c1cc(cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl)C1CCN(CC2CC2)CC1 Show InChI InChI=1S/C29H27Cl3FN3O/c30-24-2-1-3-25(31)28(24)36-27-13-19(18-8-10-35(11-9-18)16-17-4-5-17)12-22(23(27)15-34-29(36)37)21-7-6-20(33)14-26(21)32/h1-3,6-7,12-14,17-18H,4-5,8-11,15-16H2,(H,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122391

(5-(2-Chloro-phenyl)-1-(2,6-dichloro-phenyl)-7-(pip...)Show SMILES Clc1ccccc1-c1cc(NC2CCNCC2)cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl Show InChI InChI=1S/C25H23Cl3N4O/c26-20-5-2-1-4-17(20)18-12-16(31-15-8-10-29-11-9-15)13-23-19(18)14-30-25(33)32(23)24-21(27)6-3-7-22(24)28/h1-7,12-13,15,29,31H,8-11,14H2,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122405

(5-(2-Chloro-4-fluoro-phenyl)-1-(2,6-dichloro-pheny...)Show SMILES CN1CCC(CC1)c1cc2N(C(=O)NCc2c(c1)-c1ccc(F)cc1Cl)c1c(Cl)cccc1Cl Show InChI InChI=1S/C26H23Cl3FN3O/c1-32-9-7-15(8-10-32)16-11-19(18-6-5-17(30)13-23(18)29)20-14-31-26(34)33(24(20)12-16)25-21(27)3-2-4-22(25)28/h2-6,11-13,15H,7-10,14H2,1H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175751

(7-(1-tert-butylpiperidin-4-yl)-1-(2,6-dichlorophen...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc2n(-c3c(Cl)cccc3Cl)c(=O)ccc2c(n1)-c1ccc(F)cc1F |(18.46,6,;17.14,5.23,;17.91,3.89,;16.36,6.56,;15.8,4.46,;15.8,2.92,;14.47,2.15,;13.13,2.91,;13.13,4.45,;14.47,5.22,;11.8,2.14,;10.47,2.9,;9.13,2.13,;7.8,2.9,;7.8,4.43,;6.48,5.2,;5.14,4.43,;6.47,6.73,;7.81,7.52,;9.14,6.74,;9.14,5.2,;10.47,4.43,;6.46,2.13,;5.13,2.9,;6.47,.59,;7.8,-.19,;9.13,.59,;10.47,-.18,;11.81,.59,;10.47,-1.72,;9.13,-2.49,;9.13,-4.03,;10.47,-4.8,;10.47,-6.34,;11.81,-4.02,;11.8,-2.48,;13.13,-1.71,)| Show InChI InChI=1S/C29H27Cl2F2N3O/c1-29(2,3)35-13-11-17(12-14-35)24-16-25-20(27(34-24)19-8-7-18(32)15-23(19)33)9-10-26(37)36(25)28-21(30)5-4-6-22(28)31/h4-10,15-17H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data