 Found 309 hits with Last Name = 'zessis' and Initial = 'r'
Found 309 hits with Last Name = 'zessis' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50378535
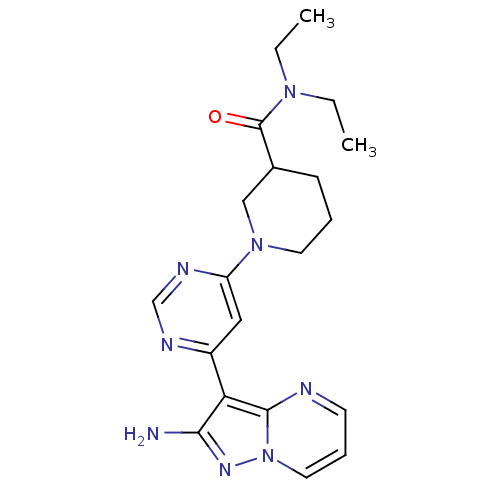
(CHEMBL1204012)Show SMILES CCN(CC)C(=O)C1CCCN(C1)c1cc(ncn1)-c1c(N)nn2cccnc12 Show InChI InChI=1S/C20H26N8O/c1-3-26(4-2)20(29)14-7-5-9-27(12-14)16-11-15(23-13-24-16)17-18(21)25-28-10-6-8-22-19(17)28/h6,8,10-11,13-14H,3-5,7,9,12H2,1-2H3,(H2,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50303076
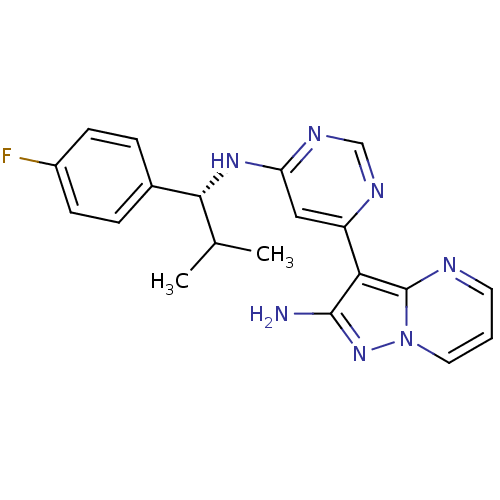
((R)-3-(6-(1-(4-fluorophenyl)-2-methylpropylamino)p...)Show SMILES CC(C)[C@@H](Nc1cc(ncn1)-c1c(N)nn2cccnc12)c1ccc(F)cc1 |r| Show InChI InChI=1S/C20H20FN7/c1-12(2)18(13-4-6-14(21)7-5-13)26-16-10-15(24-11-25-16)17-19(22)27-28-9-3-8-23-20(17)28/h3-12,18H,1-2H3,(H2,22,27)(H,24,25,26)/t18-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50378535

(CHEMBL1204012)Show SMILES CCN(CC)C(=O)C1CCCN(C1)c1cc(ncn1)-c1c(N)nn2cccnc12 Show InChI InChI=1S/C20H26N8O/c1-3-26(4-2)20(29)14-7-5-9-27(12-14)16-11-15(23-13-24-16)17-18(21)25-28-10-6-8-22-19(17)28/h6,8,10-11,13-14H,3-5,7,9,12H2,1-2H3,(H2,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50303070

((R)-3-(6-(1-(4-fluorophenyl)ethylamino)pyrimidin-4...)Show SMILES C[C@@H](Nc1cc(ncn1)-c1c(N)nn2cccnc12)c1ccc(F)cc1 |r| Show InChI InChI=1S/C18H16FN7/c1-11(12-3-5-13(19)6-4-12)24-15-9-14(22-10-23-15)16-17(20)25-26-8-2-7-21-18(16)26/h2-11H,1H3,(H2,20,25)(H,22,23,24)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542738
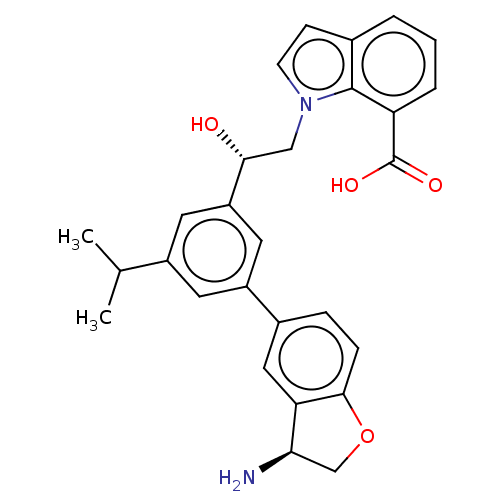
(CHEMBL4637027)Show SMILES CC(C)c1cc(cc(c1)-c1ccc2OC[C@@H](N)c2c1)[C@H](O)Cn1ccc2cccc(C(O)=O)c12 |r| Show InChI InChI=1S/C28H28N2O4/c1-16(2)19-10-20(18-6-7-26-23(13-18)24(29)15-34-26)12-21(11-19)25(31)14-30-9-8-17-4-3-5-22(27(17)30)28(32)33/h3-13,16,24-25,31H,14-15,29H2,1-2H3,(H,32,33)/t24-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50303075
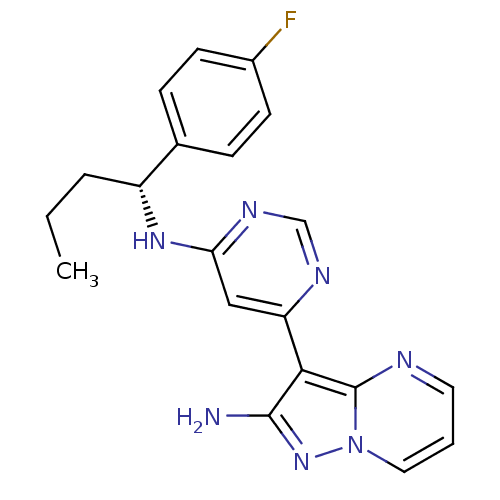
((R)-3-(6-(1-(4-fluorophenyl)butylamino)pyrimidin-4...)Show SMILES CCC[C@@H](Nc1cc(ncn1)-c1c(N)nn2cccnc12)c1ccc(F)cc1 |r| Show InChI InChI=1S/C20H20FN7/c1-2-4-15(13-5-7-14(21)8-6-13)26-17-11-16(24-12-25-17)18-19(22)27-28-10-3-9-23-20(18)28/h3,5-12,15H,2,4H2,1H3,(H2,22,27)(H,24,25,26)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50303074
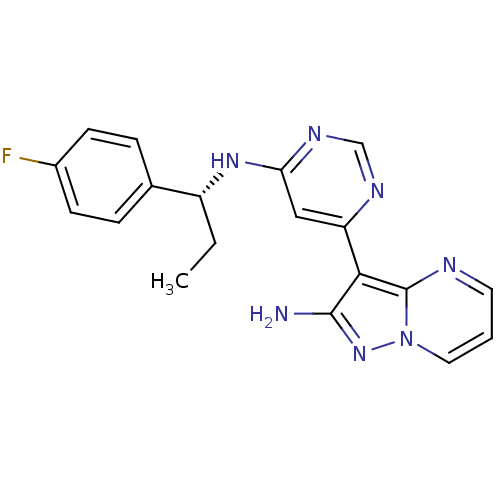
((R)-3-(6-(1-(4-fluorophenyl)propylamino)pyrimidin-...)Show SMILES CC[C@@H](Nc1cc(ncn1)-c1c(N)nn2cccnc12)c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H18FN7/c1-2-14(12-4-6-13(20)7-5-12)25-16-10-15(23-11-24-16)17-18(21)26-27-9-3-8-22-19(17)27/h3-11,14H,2H2,1H3,(H2,21,26)(H,23,24,25)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50303076

((R)-3-(6-(1-(4-fluorophenyl)-2-methylpropylamino)p...)Show SMILES CC(C)[C@@H](Nc1cc(ncn1)-c1c(N)nn2cccnc12)c1ccc(F)cc1 |r| Show InChI InChI=1S/C20H20FN7/c1-12(2)18(13-4-6-14(21)7-5-13)26-16-10-15(24-11-25-16)17-19(22)27-28-9-3-8-23-20(17)28/h3-12,18H,1-2H3,(H2,22,27)(H,24,25,26)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542731

(CHEMBL4642845)Show SMILES CC(C)c1cc(cc(c1)-c1ccc2OC[C@@H](N)c2c1)[C@@H]1CNc2cccc(CC(O)=O)c2O1 |r| Show InChI InChI=1S/C27H28N2O4/c1-15(2)18-8-19(16-6-7-24-21(11-16)22(28)14-32-24)10-20(9-18)25-13-29-23-5-3-4-17(12-26(30)31)27(23)33-25/h3-11,15,22,25,29H,12-14,28H2,1-2H3,(H,30,31)/t22-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542741
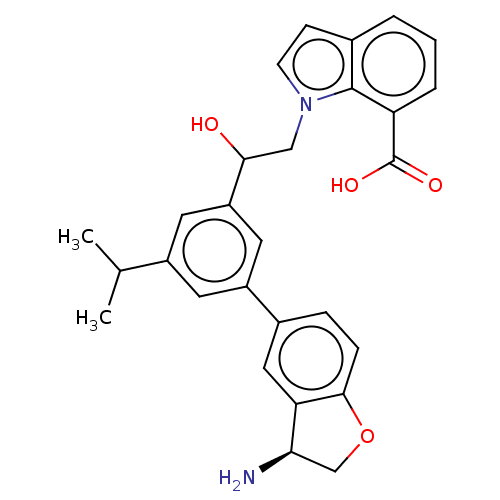
(CHEMBL4647950)Show SMILES CC(C)c1cc(cc(c1)-c1ccc2OC[C@@H](N)c2c1)C(O)Cn1ccc2cccc(C(O)=O)c12 |r| Show InChI InChI=1S/C28H28N2O4/c1-16(2)19-10-20(18-6-7-26-23(13-18)24(29)15-34-26)12-21(11-19)25(31)14-30-9-8-17-4-3-5-22(27(17)30)28(32)33/h3-13,16,24-25,31H,14-15,29H2,1-2H3,(H,32,33)/t24-,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50542731

(CHEMBL4642845)Show SMILES CC(C)c1cc(cc(c1)-c1ccc2OC[C@@H](N)c2c1)[C@@H]1CNc2cccc(CC(O)=O)c2O1 |r| Show InChI InChI=1S/C27H28N2O4/c1-15(2)18-8-19(16-6-7-24-21(11-16)22(28)14-32-24)10-20(9-18)25-13-29-23-5-3-4-17(12-26(30)31)27(23)33-25/h3-11,15,22,25,29H,12-14,28H2,1-2H3,(H,30,31)/t22-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50542738

(CHEMBL4637027)Show SMILES CC(C)c1cc(cc(c1)-c1ccc2OC[C@@H](N)c2c1)[C@H](O)Cn1ccc2cccc(C(O)=O)c12 |r| Show InChI InChI=1S/C28H28N2O4/c1-16(2)19-10-20(18-6-7-26-23(13-18)24(29)15-34-26)12-21(11-19)25(31)14-30-9-8-17-4-3-5-22(27(17)30)28(32)33/h3-13,16,24-25,31H,14-15,29H2,1-2H3,(H,32,33)/t24-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50303065
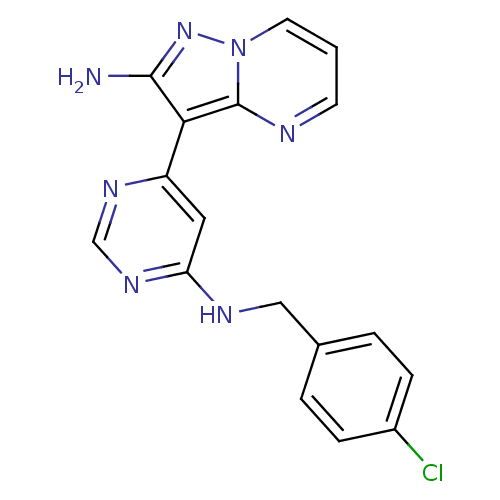
(3-(6-(4-chlorobenzylamino)pyrimidin-4-yl)pyrazolo[...)Show InChI InChI=1S/C17H14ClN7/c18-12-4-2-11(3-5-12)9-21-14-8-13(22-10-23-14)15-16(19)24-25-7-1-6-20-17(15)25/h1-8,10H,9H2,(H2,19,24)(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50303067

((R)-3-(6-(1-phenylethylamino)pyrimidin-4-yl)pyrazo...)Show SMILES C[C@@H](Nc1cc(ncn1)-c1c(N)nn2cccnc12)c1ccccc1 |r| Show InChI InChI=1S/C18H17N7/c1-12(13-6-3-2-4-7-13)23-15-10-14(21-11-22-15)16-17(19)24-25-9-5-8-20-18(16)25/h2-12H,1H3,(H2,19,24)(H,21,22,23)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50303064
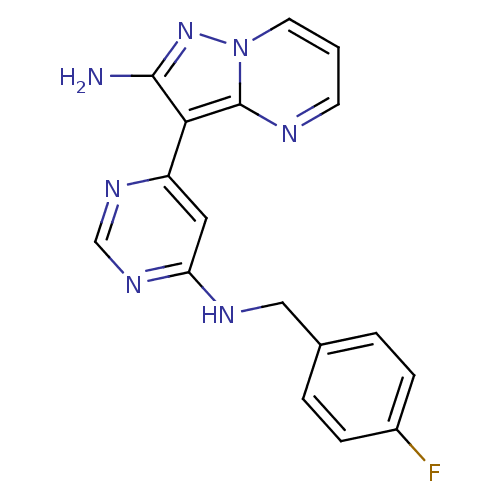
(3-(6-(4-fluorobenzylamino)pyrimidin-4-yl)pyrazolo[...)Show InChI InChI=1S/C17H14FN7/c18-12-4-2-11(3-5-12)9-21-14-8-13(22-10-23-14)15-16(19)24-25-7-1-6-20-17(15)25/h1-8,10H,9H2,(H2,19,24)(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542740
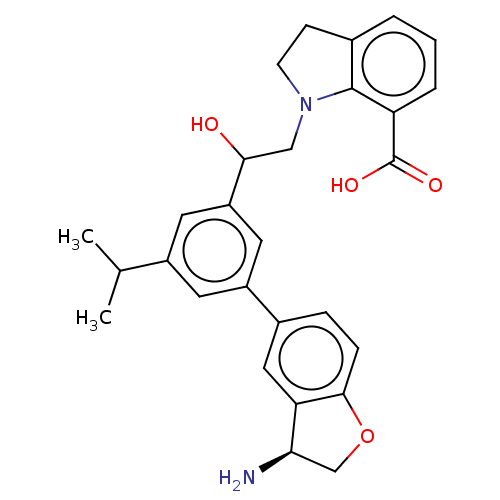
(CHEMBL4646398)Show SMILES CC(C)c1cc(cc(c1)-c1ccc2OC[C@@H](N)c2c1)C(O)CN1CCc2cccc(C(O)=O)c12 |r| Show InChI InChI=1S/C28H30N2O4/c1-16(2)19-10-20(18-6-7-26-23(13-18)24(29)15-34-26)12-21(11-19)25(31)14-30-9-8-17-4-3-5-22(27(17)30)28(32)33/h3-7,10-13,16,24-25,31H,8-9,14-15,29H2,1-2H3,(H,32,33)/t24-,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50542724
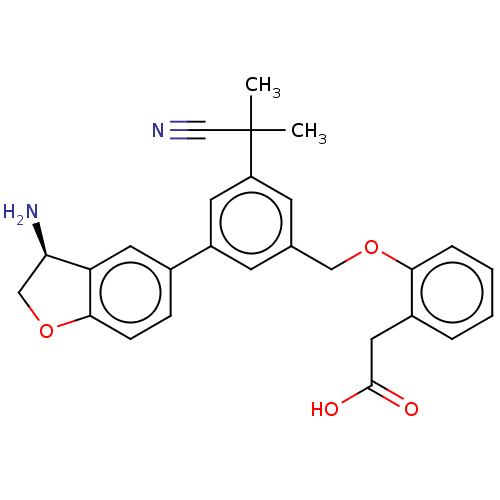
(CHEMBL4636415)Show SMILES CC(C)(C#N)c1cc(COc2ccccc2CC(O)=O)cc(c1)-c1ccc2OC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C27H26N2O4/c1-27(2,16-28)21-10-17(14-32-24-6-4-3-5-19(24)13-26(30)31)9-20(11-21)18-7-8-25-22(12-18)23(29)15-33-25/h3-12,23H,13-15,29H2,1-2H3,(H,30,31)/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50542740

(CHEMBL4646398)Show SMILES CC(C)c1cc(cc(c1)-c1ccc2OC[C@@H](N)c2c1)C(O)CN1CCc2cccc(C(O)=O)c12 |r| Show InChI InChI=1S/C28H30N2O4/c1-16(2)19-10-20(18-6-7-26-23(13-18)24(29)15-34-26)12-21(11-19)25(31)14-30-9-8-17-4-3-5-22(27(17)30)28(32)33/h3-7,10-13,16,24-25,31H,8-9,14-15,29H2,1-2H3,(H,32,33)/t24-,25?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50542723
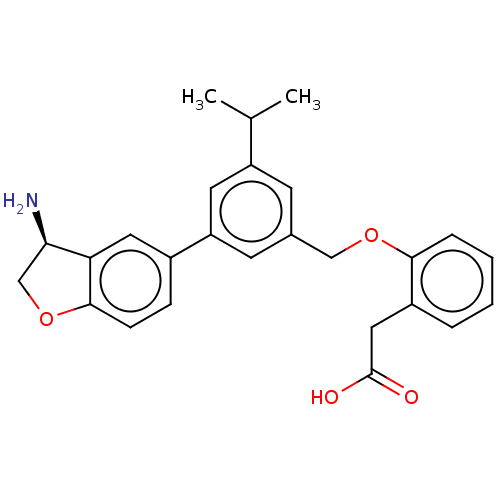
(CHEMBL4643449)Show SMILES CC(C)c1cc(COc2ccccc2CC(O)=O)cc(c1)-c1ccc2OC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C26H27NO4/c1-16(2)20-9-17(14-30-24-6-4-3-5-19(24)13-26(28)29)10-21(11-20)18-7-8-25-22(12-18)23(27)15-31-25/h3-12,16,23H,13-15,27H2,1-2H3,(H,28,29)/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542723

(CHEMBL4643449)Show SMILES CC(C)c1cc(COc2ccccc2CC(O)=O)cc(c1)-c1ccc2OC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C26H27NO4/c1-16(2)20-9-17(14-30-24-6-4-3-5-19(24)13-26(28)29)10-21(11-20)18-7-8-25-22(12-18)23(27)15-31-25/h3-12,16,23H,13-15,27H2,1-2H3,(H,28,29)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542733
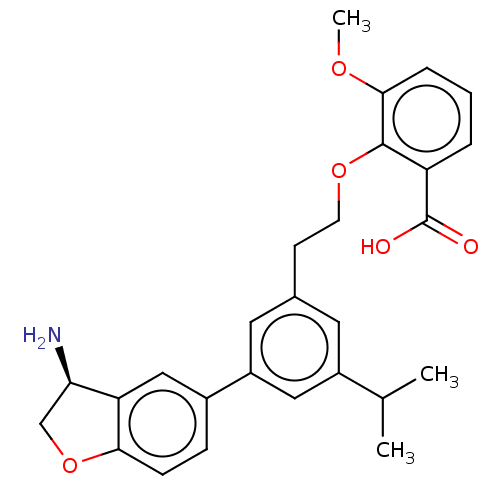
(CHEMBL4646141)Show SMILES COc1cccc(C(O)=O)c1OCCc1cc(cc(c1)-c1ccc2OC[C@@H](N)c2c1)C(C)C |r| Show InChI InChI=1S/C27H29NO5/c1-16(2)19-11-17(9-10-32-26-21(27(29)30)5-4-6-25(26)31-3)12-20(13-19)18-7-8-24-22(14-18)23(28)15-33-24/h4-8,11-14,16,23H,9-10,15,28H2,1-3H3,(H,29,30)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542728
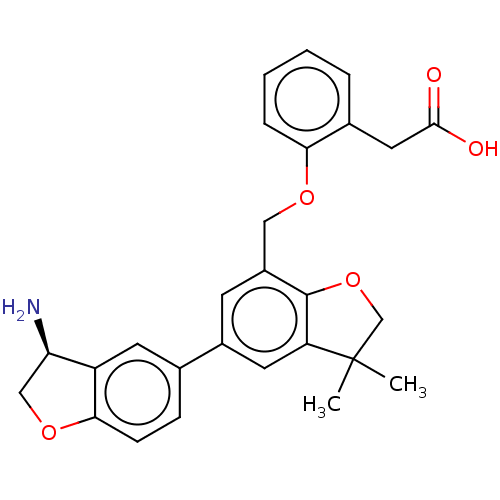
(CHEMBL4635912)Show SMILES CC1(C)COc2c1cc(cc2COc1ccccc1CC(O)=O)-c1ccc2OC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C27H27NO5/c1-27(2)15-33-26-19(13-31-23-6-4-3-5-17(23)12-25(29)30)9-18(11-21(26)27)16-7-8-24-20(10-16)22(28)14-32-24/h3-11,22H,12-15,28H2,1-2H3,(H,29,30)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50303057

(1-(6-(2-aminopyrazolo[1,5-a]pyrimidin-3-yl)pyrimid...)Show SMILES CCCN(CC(F)(F)F)C(=O)C1CCCN(C1)c1cc(ncn1)-c1c(N)nn2cccnc12 Show InChI InChI=1S/C21H25F3N8O/c1-2-7-31(12-21(22,23)24)20(33)14-5-3-8-30(11-14)16-10-15(27-13-28-16)17-18(25)29-32-9-4-6-26-19(17)32/h4,6,9-10,13-14H,2-3,5,7-8,11-12H2,1H3,(H2,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50303073
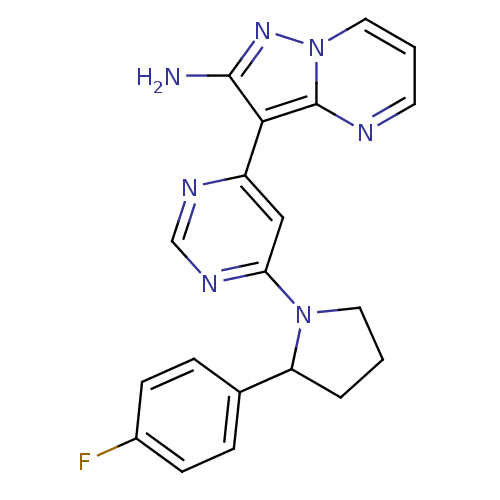
(3-(6-(2-(4-fluorophenyl)pyrrolidin-1-yl)pyrimidin-...)Show SMILES Nc1nn2cccnc2c1-c1cc(ncn1)N1CCCC1c1ccc(F)cc1 Show InChI InChI=1S/C20H18FN7/c21-14-6-4-13(5-7-14)16-3-1-9-27(16)17-11-15(24-12-25-17)18-19(22)26-28-10-2-8-23-20(18)28/h2,4-8,10-12,16H,1,3,9H2,(H2,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50303078
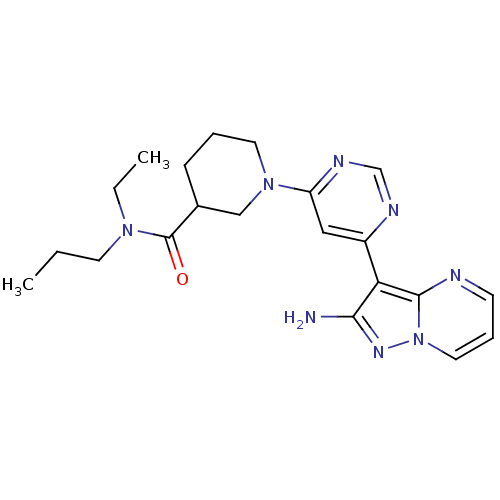
(1-(6-(2-aminopyrazolo[1,5-a]pyrimidin-3-yl)pyrimid...)Show SMILES CCCN(CC)C(=O)C1CCCN(C1)c1cc(ncn1)-c1c(N)nn2cccnc12 Show InChI InChI=1S/C21H28N8O/c1-3-9-27(4-2)21(30)15-7-5-10-28(13-15)17-12-16(24-14-25-17)18-19(22)26-29-11-6-8-23-20(18)29/h6,8,11-12,14-15H,3-5,7,9-10,13H2,1-2H3,(H2,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542724

(CHEMBL4636415)Show SMILES CC(C)(C#N)c1cc(COc2ccccc2CC(O)=O)cc(c1)-c1ccc2OC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C27H26N2O4/c1-27(2,16-28)21-10-17(14-32-24-6-4-3-5-19(24)13-26(30)31)9-20(11-21)18-7-8-25-22(12-18)23(29)15-33-25/h3-12,23H,13-15,29H2,1-2H3,(H,30,31)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50303056

(1-(6-(2-aminopyrazolo[1,5-a]pyrimidin-3-yl)pyrimid...)Show SMILES CCN(CC(F)(F)F)C(=O)C1CCCN(C1)c1cc(ncn1)-c1c(N)nn2cccnc12 Show InChI InChI=1S/C20H23F3N8O/c1-2-29(11-20(21,22)23)19(32)13-5-3-7-30(10-13)15-9-14(26-12-27-15)16-17(24)28-31-8-4-6-25-18(16)31/h4,6,8-9,12-13H,2-3,5,7,10-11H2,1H3,(H2,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542725
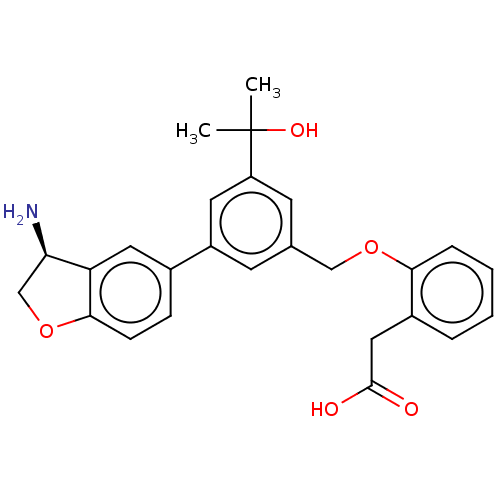
(CHEMBL4637683)Show SMILES CC(C)(O)c1cc(COc2ccccc2CC(O)=O)cc(c1)-c1ccc2OC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C26H27NO5/c1-26(2,30)20-10-16(14-31-23-6-4-3-5-18(23)13-25(28)29)9-19(11-20)17-7-8-24-21(12-17)22(27)15-32-24/h3-12,22,30H,13-15,27H2,1-2H3,(H,28,29)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50542730
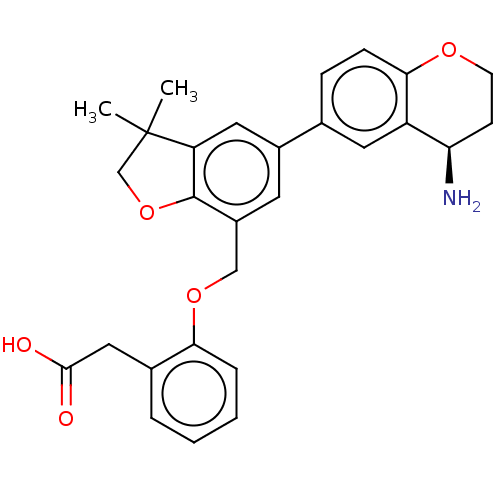
(CHEMBL4647909)Show SMILES CC1(C)COc2c1cc(cc2COc1ccccc1CC(O)=O)-c1ccc2OCC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C28H29NO5/c1-28(2)16-34-27-20(15-33-24-6-4-3-5-18(24)14-26(30)31)11-19(13-22(27)28)17-7-8-25-21(12-17)23(29)9-10-32-25/h3-8,11-13,23H,9-10,14-16,29H2,1-2H3,(H,30,31)/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50303058
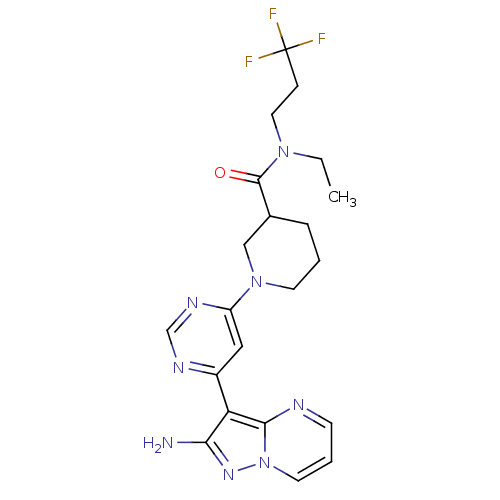
(1-(6-(2-aminopyrazolo[1,5-a]pyrimidin-3-yl)pyrimid...)Show SMILES CCN(CCC(F)(F)F)C(=O)C1CCCN(C1)c1cc(ncn1)-c1c(N)nn2cccnc12 Show InChI InChI=1S/C21H25F3N8O/c1-2-30(10-6-21(22,23)24)20(33)14-5-3-8-31(12-14)16-11-15(27-13-28-16)17-18(25)29-32-9-4-7-26-19(17)32/h4,7,9,11,13-14H,2-3,5-6,8,10,12H2,1H3,(H2,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50303055

(1-(6-(2-aminopyrazolo[1,5-a]pyrimidin-3-yl)pyrimid...)Show SMILES CCCN(CCC)C(=O)C1CCCN(C1)c1cc(ncn1)-c1c(N)nn2cccnc12 Show InChI InChI=1S/C22H30N8O/c1-3-9-28(10-4-2)22(31)16-7-5-11-29(14-16)18-13-17(25-15-26-18)19-20(23)27-30-12-6-8-24-21(19)30/h6,8,12-13,15-16H,3-5,7,9-11,14H2,1-2H3,(H2,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50303059
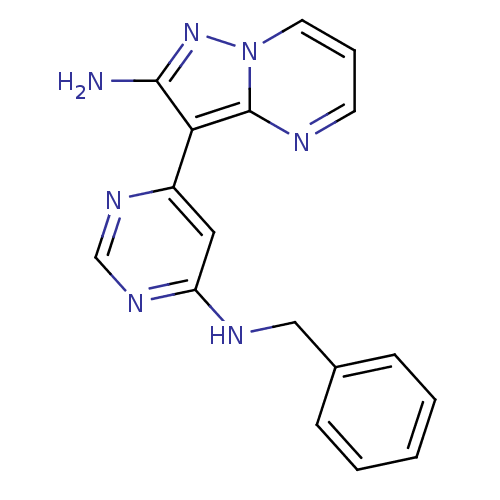
(3-(6-(benzylamino)pyrimidin-4-yl)pyrazolo[1,5-a]py...)Show InChI InChI=1S/C17H15N7/c18-16-15(17-19-7-4-8-24(17)23-16)13-9-14(22-11-21-13)20-10-12-5-2-1-3-6-12/h1-9,11H,10H2,(H2,18,23)(H,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50542725

(CHEMBL4637683)Show SMILES CC(C)(O)c1cc(COc2ccccc2CC(O)=O)cc(c1)-c1ccc2OC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C26H27NO5/c1-26(2,30)20-10-16(14-31-23-6-4-3-5-18(23)13-25(28)29)9-19(11-20)17-7-8-24-21(12-17)22(27)15-32-24/h3-12,22,30H,13-15,27H2,1-2H3,(H,28,29)/t22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542730

(CHEMBL4647909)Show SMILES CC1(C)COc2c1cc(cc2COc1ccccc1CC(O)=O)-c1ccc2OCC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C28H29NO5/c1-28(2)16-34-27-20(15-33-24-6-4-3-5-18(24)14-26(30)31)11-19(13-22(27)28)17-7-8-25-21(12-17)23(29)9-10-32-25/h3-8,11-13,23H,9-10,14-16,29H2,1-2H3,(H,30,31)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542735
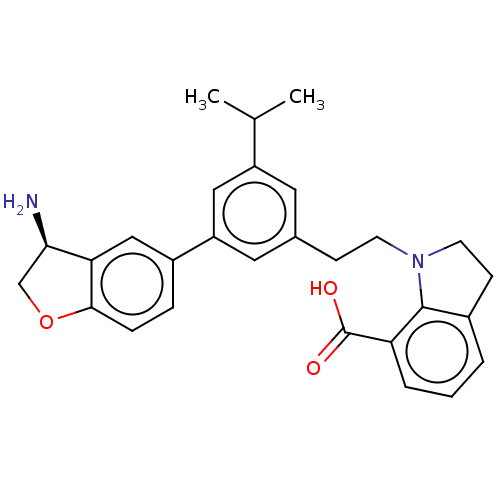
(CHEMBL4635286)Show SMILES CC(C)c1cc(CCN2CCc3cccc(C(O)=O)c23)cc(c1)-c1ccc2OC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C28H30N2O3/c1-17(2)21-12-18(8-10-30-11-9-19-4-3-5-23(27(19)30)28(31)32)13-22(14-21)20-6-7-26-24(15-20)25(29)16-33-26/h3-7,12-15,17,25H,8-11,16,29H2,1-2H3,(H,31,32)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50542728

(CHEMBL4635912)Show SMILES CC1(C)COc2c1cc(cc2COc1ccccc1CC(O)=O)-c1ccc2OC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C27H27NO5/c1-27(2)15-33-26-19(13-31-23-6-4-3-5-17(23)12-25(29)30)9-18(11-21(26)27)16-7-8-24-20(10-16)22(28)14-32-24/h3-11,22H,12-15,28H2,1-2H3,(H,29,30)/t22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50303070

((R)-3-(6-(1-(4-fluorophenyl)ethylamino)pyrimidin-4...)Show SMILES C[C@@H](Nc1cc(ncn1)-c1c(N)nn2cccnc12)c1ccc(F)cc1 |r| Show InChI InChI=1S/C18H16FN7/c1-11(12-3-5-13(19)6-4-12)24-15-9-14(22-10-23-15)16-17(20)25-26-8-2-7-21-18(16)26/h2-11H,1H3,(H2,20,25)(H,22,23,24)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50303066
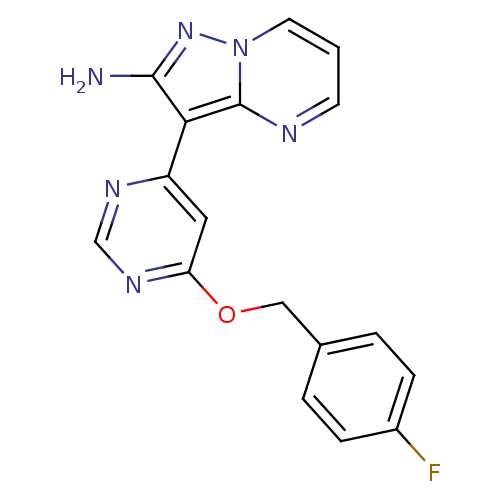
(3-(6-(4-fluorobenzyloxy)pyrimidin-4-yl)pyrazolo[1,...)Show InChI InChI=1S/C17H13FN6O/c18-12-4-2-11(3-5-12)9-25-14-8-13(21-10-22-14)15-16(19)23-24-7-1-6-20-17(15)24/h1-8,10H,9H2,(H2,19,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Complement factor D
(Homo sapiens (Human)) | BDBM50524338
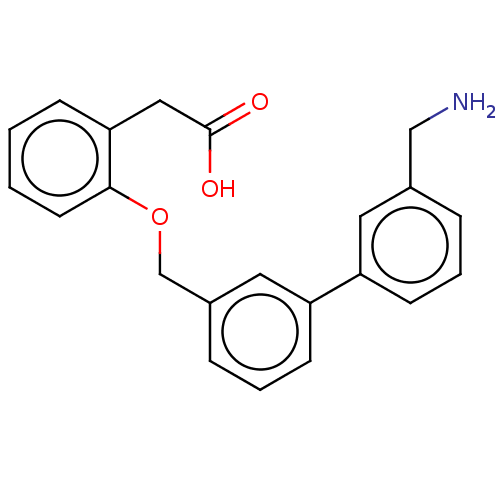
(CHEMBL4468000)Show InChI InChI=1S/C22H21NO3/c23-14-16-5-3-8-18(11-16)19-9-4-6-17(12-19)15-26-21-10-2-1-7-20(21)13-22(24)25/h1-12H,13-15,23H2,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human complement FD by TR-FRET assay |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50303069
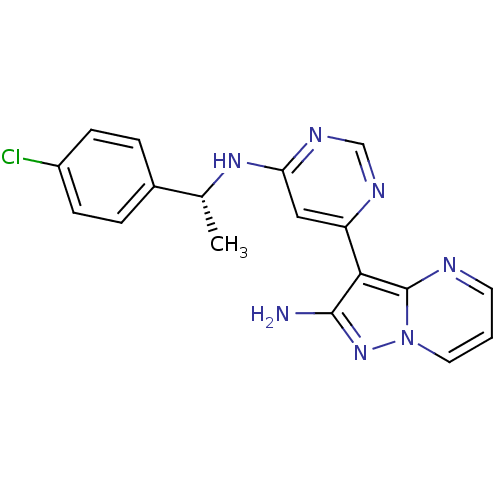
((R)-3-(6-(1-(4-chlorophenyl)ethylamino)pyrimidin-4...)Show SMILES C[C@@H](Nc1cc(ncn1)-c1c(N)nn2cccnc12)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H16ClN7/c1-11(12-3-5-13(19)6-4-12)24-15-9-14(22-10-23-15)16-17(20)25-26-8-2-7-21-18(16)26/h2-11H,1H3,(H2,20,25)(H,22,23,24)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50502595
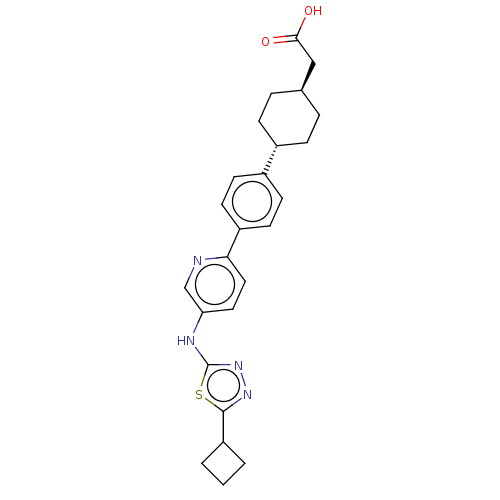
(CHEMBL4554065)Show SMILES OC(=O)C[C@H]1CC[C@@H](CC1)c1ccc(cc1)-c1ccc(Nc2nnc(s2)C2CCC2)cn1 |r,wU:7.10,wD:4.3,(51.91,-2.96,;50.57,-3.73,;50.58,-5.27,;49.24,-2.97,;47.91,-3.74,;46.57,-2.97,;45.23,-3.74,;45.24,-5.28,;46.58,-6.05,;47.91,-5.28,;43.92,-6.05,;43.92,-7.59,;42.59,-8.37,;41.25,-7.6,;41.25,-6.06,;42.57,-5.29,;39.93,-8.37,;38.58,-7.6,;37.26,-8.38,;37.27,-9.92,;35.94,-10.69,;34.6,-9.92,;33.19,-10.55,;32.16,-9.41,;32.93,-8.07,;34.44,-8.39,;32.3,-6.66,;32.85,-5.23,;31.41,-4.68,;30.86,-6.12,;38.6,-10.68,;39.93,-9.91,)| Show InChI InChI=1S/C25H28N4O2S/c30-23(31)14-16-4-6-17(7-5-16)18-8-10-19(11-9-18)22-13-12-21(15-26-22)27-25-29-28-24(32-25)20-2-1-3-20/h8-13,15-17,20H,1-7,14H2,(H,27,29)(H,30,31)/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human his-tagged DGAT1 expressed in Sf9 insect cells using oleoyl-CoA and diolein as substrates incubated for 30 mins by LC... |
ACS Med Chem Lett 10: 1128-1133 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00117
BindingDB Entry DOI: 10.7270/Q28W3HJK |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50542733

(CHEMBL4646141)Show SMILES COc1cccc(C(O)=O)c1OCCc1cc(cc(c1)-c1ccc2OC[C@@H](N)c2c1)C(C)C |r| Show InChI InChI=1S/C27H29NO5/c1-16(2)19-11-17(9-10-32-26-21(27(29)30)5-4-6-25(26)31-3)12-20(13-19)18-7-8-24-22(14-18)23(28)15-33-24/h4-8,11-14,16,23H,9-10,15,28H2,1-3H3,(H,29,30)/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542726

(CHEMBL4642766)Show SMILES CC(C)(C(N)=O)c1cc(COc2ccccc2CC(O)=O)cc(c1)-c1ccc2OC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C27H28N2O5/c1-27(2,26(29)32)20-10-16(14-33-23-6-4-3-5-18(23)13-25(30)31)9-19(11-20)17-7-8-24-21(12-17)22(28)15-34-24/h3-12,22H,13-15,28H2,1-2H3,(H2,29,32)(H,30,31)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542731

(CHEMBL4642845)Show SMILES CC(C)c1cc(cc(c1)-c1ccc2OC[C@@H](N)c2c1)[C@@H]1CNc2cccc(CC(O)=O)c2O1 |r| Show InChI InChI=1S/C27H28N2O4/c1-15(2)18-8-19(16-6-7-24-21(11-16)22(28)14-32-24)10-20(9-18)25-13-29-23-5-3-4-17(12-26(30)31)27(23)33-25/h3-11,15,22,25,29H,12-14,28H2,1-2H3,(H,30,31)/t22-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal human plasma F11a catalytic domain expressed in Escherichia coli strain BL21(DE3) using D-Leu-Pro-Arg*Rh110-D-Pro as substra... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542738

(CHEMBL4637027)Show SMILES CC(C)c1cc(cc(c1)-c1ccc2OC[C@@H](N)c2c1)[C@H](O)Cn1ccc2cccc(C(O)=O)c12 |r| Show InChI InChI=1S/C28H28N2O4/c1-16(2)19-10-20(18-6-7-26-23(13-18)24(29)15-34-26)12-21(11-19)25(31)14-30-9-8-17-4-3-5-22(27(17)30)28(32)33/h3-13,16,24-25,31H,14-15,29H2,1-2H3,(H,32,33)/t24-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal human plasma F11a catalytic domain expressed in Escherichia coli strain BL21(DE3) using D-Leu-Pro-Arg*Rh110-D-Pro as substra... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50303075

((R)-3-(6-(1-(4-fluorophenyl)butylamino)pyrimidin-4...)Show SMILES CCC[C@@H](Nc1cc(ncn1)-c1c(N)nn2cccnc12)c1ccc(F)cc1 |r| Show InChI InChI=1S/C20H20FN7/c1-2-4-15(13-5-7-14(21)8-6-13)26-17-11-16(24-12-25-17)18-19(22)27-28-10-3-9-23-20(18)28/h3,5-12,15H,2,4H2,1H3,(H2,22,27)(H,24,25,26)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50303072
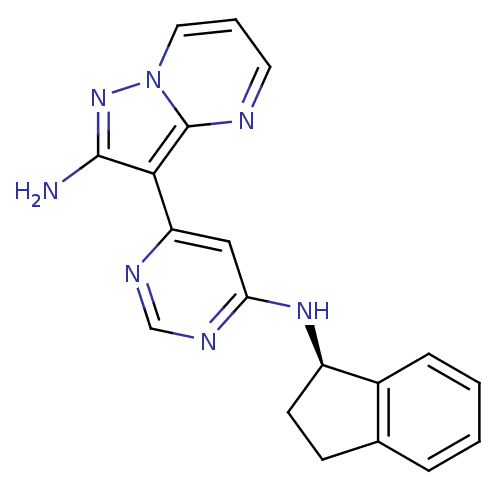
((R)-3-(6-(2,3-dihydro-1H-inden-1-ylamino)pyrimidin...)Show SMILES Nc1nn2cccnc2c1-c1cc(N[C@@H]2CCc3ccccc23)ncn1 |r| Show InChI InChI=1S/C19H17N7/c20-18-17(19-21-8-3-9-26(19)25-18)15-10-16(23-11-22-15)24-14-7-6-12-4-1-2-5-13(12)14/h1-5,8-11,14H,6-7H2,(H2,20,25)(H,22,23,24)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50542726

(CHEMBL4642766)Show SMILES CC(C)(C(N)=O)c1cc(COc2ccccc2CC(O)=O)cc(c1)-c1ccc2OC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C27H28N2O5/c1-27(2,26(29)32)20-10-16(14-33-23-6-4-3-5-18(23)13-25(30)31)9-19(11-20)17-7-8-24-21(12-17)22(28)15-34-24/h3-12,22H,13-15,28H2,1-2H3,(H2,29,32)(H,30,31)/t22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50303076

((R)-3-(6-(1-(4-fluorophenyl)-2-methylpropylamino)p...)Show SMILES CC(C)[C@@H](Nc1cc(ncn1)-c1c(N)nn2cccnc12)c1ccc(F)cc1 |r| Show InChI InChI=1S/C20H20FN7/c1-12(2)18(13-4-6-14(21)7-5-13)26-16-10-15(24-11-25-16)17-19(22)27-28-9-3-8-23-20(17)28/h3-12,18H,1-2H3,(H2,22,27)(H,24,25,26)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50542732
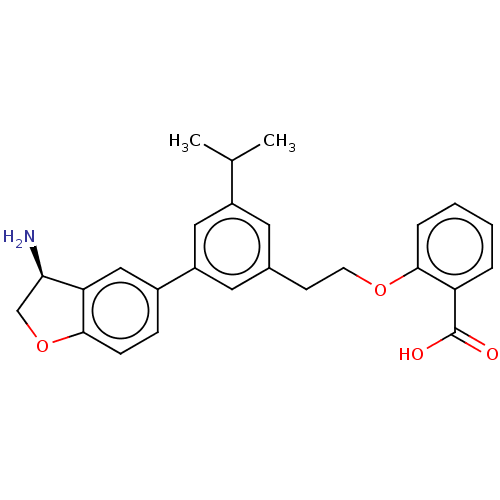
(CHEMBL4647925)Show SMILES CC(C)c1cc(CCOc2ccccc2C(O)=O)cc(c1)-c1ccc2OC[C@@H](N)c2c1 |r| Show InChI InChI=1S/C26H27NO4/c1-16(2)19-11-17(9-10-30-24-6-4-3-5-21(24)26(28)29)12-20(13-19)18-7-8-25-22(14-18)23(27)15-31-25/h3-8,11-14,16,23H,9-10,15,27H2,1-2H3,(H,28,29)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... |
J Med Chem 63: 8088-8113 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00279
BindingDB Entry DOI: 10.7270/Q2KS6W36 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data