

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chitinase (Ostrinia furnacalis) | BDBM50514507 (CHEMBL1583158) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of Ostrinia furnacalis chitinase h catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by flu... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Mus musculus) | BDBM50514508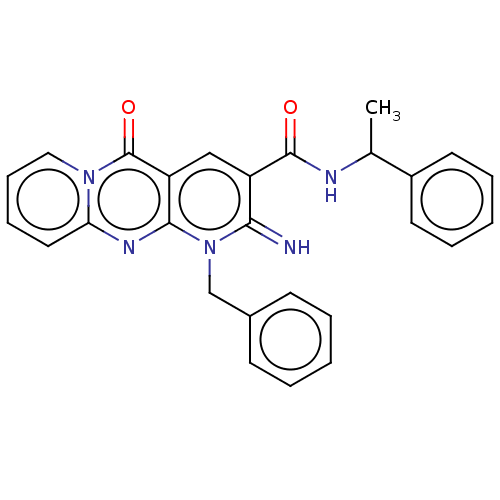 (CHEMBL4549449) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of mouse CHIT1 catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fluorescence based micr... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50514508 (CHEMBL4549449) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of human CHIT1 catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fluorescence based micr... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50514507 (CHEMBL1583158) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase b catalytic domain overexpressed in Escherichia coli BL21(DE3) cells using 4MU-(GlcNAc)2 as substrate aft... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Ostrinia furnacalis) | BDBM50514508 (CHEMBL4549449) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of Ostrinia furnacalis chitinase h catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by flu... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50514508 (CHEMBL4549449) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase b catalytic domain overexpressed in Escherichia coli BL21(DE3) cells using 4MU-(GlcNAc)2 as substrate aft... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50514508 (CHEMBL4549449) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of mouse acidic mammalian chitinase catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fl... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50514507 (CHEMBL1583158) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of human CHIT1 catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fluorescence based micr... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50514507 (CHEMBL1583158) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of mouse acidic mammalian chitinase catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fl... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Mus musculus) | BDBM50514507 (CHEMBL1583158) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of mouse CHIT1 catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fluorescence based micr... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50514508 (CHEMBL4549449) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 3.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of human acidic mammalian chitinase catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fl... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50514507 (CHEMBL1583158) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 9.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of human acidic mammalian chitinase catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fl... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M) (Homo sapiens (Human)) | BDBM14775 (3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Structure 12: 2233-47 (2004) Article DOI: 10.1016/j.str.2004.10.004 BindingDB Entry DOI: 10.7270/Q25B00Q1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M] (Homo sapiens (Human)) | BDBM14775 (3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Structure 12: 2233-47 (2004) Article DOI: 10.1016/j.str.2004.10.004 BindingDB Entry DOI: 10.7270/Q25B00Q1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M) (Homo sapiens (Human)) | BDBM14774 (3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Structure 12: 2233-47 (2004) Article DOI: 10.1016/j.str.2004.10.004 BindingDB Entry DOI: 10.7270/Q25B00Q1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M] (Homo sapiens (Human)) | BDBM14774 (3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Structure 12: 2233-47 (2004) Article DOI: 10.1016/j.str.2004.10.004 BindingDB Entry DOI: 10.7270/Q25B00Q1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase [531-875] (Homo sapiens (Human)) | BDBM14776 (2-{2-ethoxy-5-[(4-ethylpiperazine-1-)sulfonyl]phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Structure 12: 2233-47 (2004) Article DOI: 10.1016/j.str.2004.10.004 BindingDB Entry DOI: 10.7270/Q25B00Q1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase [531-875] (Homo sapiens (Human)) | BDBM14777 ((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Structure 12: 2233-47 (2004) Article DOI: 10.1016/j.str.2004.10.004 BindingDB Entry DOI: 10.7270/Q25B00Q1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase [531-875] (Homo sapiens (Human)) | BDBM14390 (5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Structure 12: 2233-47 (2004) Article DOI: 10.1016/j.str.2004.10.004 BindingDB Entry DOI: 10.7270/Q25B00Q1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isoform 4 of Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (PDE11A1) 35-489] (Homo sapiens (Human)) | BDBM14777 ((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Structure 12: 2233-47 (2004) Article DOI: 10.1016/j.str.2004.10.004 BindingDB Entry DOI: 10.7270/Q25B00Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M) (Homo sapiens (Human)) | BDBM14773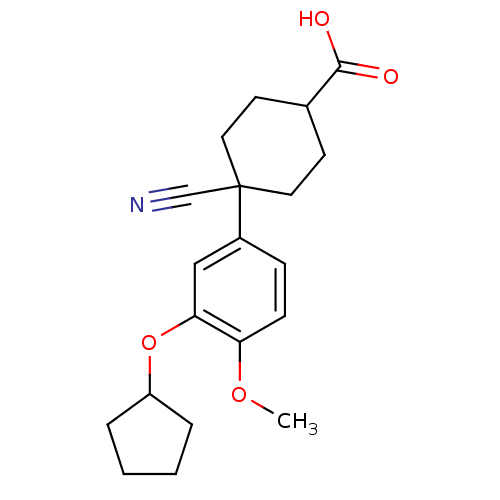 (4-cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)cyclo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Structure 12: 2233-47 (2004) Article DOI: 10.1016/j.str.2004.10.004 BindingDB Entry DOI: 10.7270/Q25B00Q1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM25617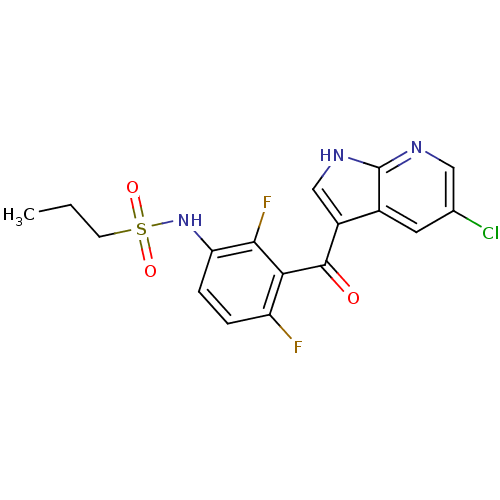 (N-[3-({5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl}carb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Plexxikon | Assay Description The in vitro kinase activities of wild type or mutants were determined by measuring phosphorylation of biotinylated-MEK protein using Perkin-Elmer s ... | Proc Natl Acad Sci U S A 105: 3041-6 (2008) Article DOI: 10.1073/pnas.0711741105 BindingDB Entry DOI: 10.7270/Q2SB441T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M) (Homo sapiens (Human)) | BDBM14797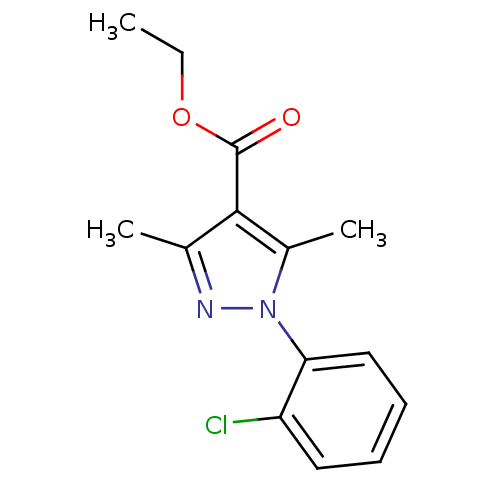 (Pyrazole carboxylic ester 20 | ethyl 1-(2-chloroph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Nat Biotechnol 23: 201-7 (2005) Article DOI: 10.1038/nbt1059 BindingDB Entry DOI: 10.7270/Q21N7ZCN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M) (Homo sapiens (Human)) | BDBM14798 (Pyrazole carboxylic ester 21 | ethyl 3,5-dimethyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Nat Biotechnol 23: 201-7 (2005) Article DOI: 10.1038/nbt1059 BindingDB Entry DOI: 10.7270/Q21N7ZCN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M] (Homo sapiens (Human)) | BDBM14773 (4-cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)cyclo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Structure 12: 2233-47 (2004) Article DOI: 10.1016/j.str.2004.10.004 BindingDB Entry DOI: 10.7270/Q25B00Q1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University | Assay Description Newly synthesized coumarin thioureas were tested against electric eel AChE and horse serum BChE. The cholinesterase inhibitory activity was measured ... | Bioorg Chem 63: 58-63 (2015) Article DOI: 10.1016/j.bioorg.2015.09.009 BindingDB Entry DOI: 10.7270/Q2W957Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M] (Homo sapiens (Human)) | BDBM14798 (Pyrazole carboxylic ester 21 | ethyl 3,5-dimethyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Nat Biotechnol 23: 201-7 (2005) Article DOI: 10.1038/nbt1059 BindingDB Entry DOI: 10.7270/Q21N7ZCN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM173566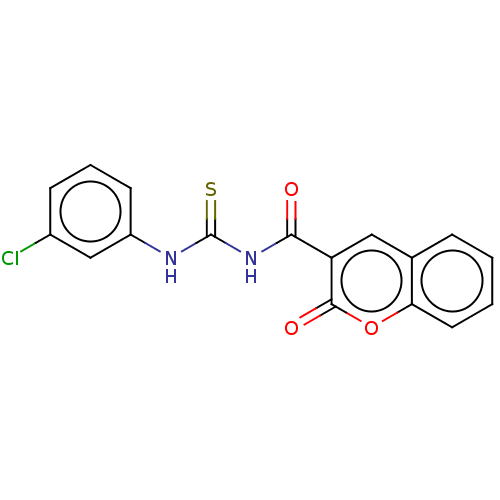 (1-(2-Oxo-2H-chromene-3-carbonyl)-3-(3-chlorophenyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University | Assay Description Newly synthesized coumarin thioureas were tested against electric eel AChE and horse serum BChE. The cholinesterase inhibitory activity was measured ... | Bioorg Chem 63: 58-63 (2015) Article DOI: 10.1016/j.bioorg.2015.09.009 BindingDB Entry DOI: 10.7270/Q2W957Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M] (Homo sapiens (Human)) | BDBM14797 (Pyrazole carboxylic ester 20 | ethyl 1-(2-chloroph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Nat Biotechnol 23: 201-7 (2005) Article DOI: 10.1038/nbt1059 BindingDB Entry DOI: 10.7270/Q21N7ZCN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM173563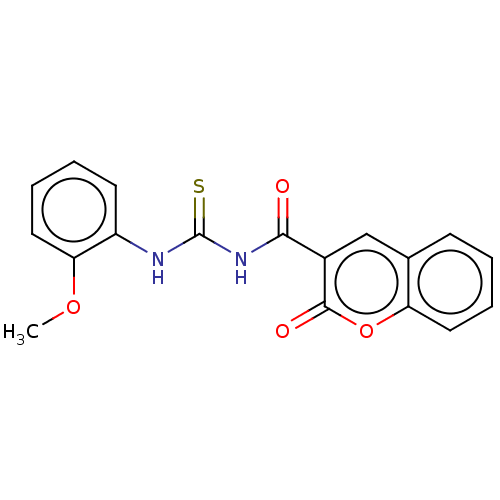 (1-(2-Oxo-2H-chromene-3-carbonyl)-3-(2-methoxypheny...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University | Assay Description Newly synthesized coumarin thioureas were tested against electric eel AChE and horse serum BChE. The cholinesterase inhibitory activity was measured ... | Bioorg Chem 63: 58-63 (2015) Article DOI: 10.1016/j.bioorg.2015.09.009 BindingDB Entry DOI: 10.7270/Q2W957Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM25617 (N-[3-({5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl}carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Plexxikon | Assay Description Enzyme activity was assayed using Z-LYTE Enzymatic Kinase Assay format (Invitrogen Corp., Carlsbad, CA) according to the manufacturer instructions. | Proc Natl Acad Sci U S A 105: 3041-6 (2008) Article DOI: 10.1073/pnas.0711741105 BindingDB Entry DOI: 10.7270/Q2SB441T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M) (Homo sapiens (Human)) | BDBM14796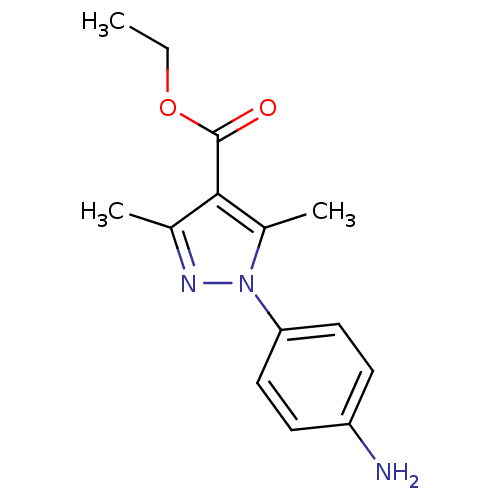 (Pyrazole carboxylic ester 19 | ethyl 1-(4-aminophe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Nat Biotechnol 23: 201-7 (2005) Article DOI: 10.1038/nbt1059 BindingDB Entry DOI: 10.7270/Q21N7ZCN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM25617 (N-[3-({5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl}carb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Plexxikon | Assay Description The in vitro kinase activities of wild type or mutants were determined by measuring phosphorylation of biotinylated-MEK protein using Perkin-Elmer s ... | Proc Natl Acad Sci U S A 105: 3041-6 (2008) Article DOI: 10.1073/pnas.0711741105 BindingDB Entry DOI: 10.7270/Q2SB441T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227634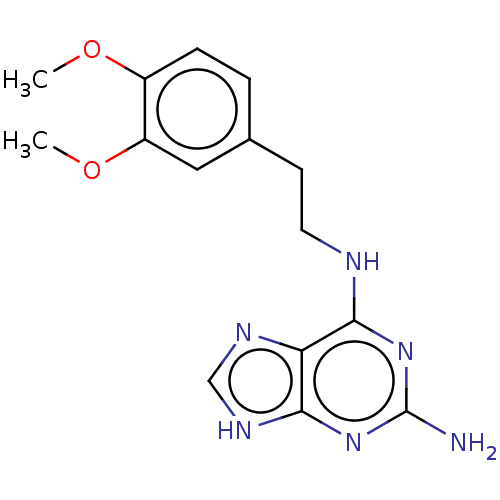 (MTH1 inhibitor, 121) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM173571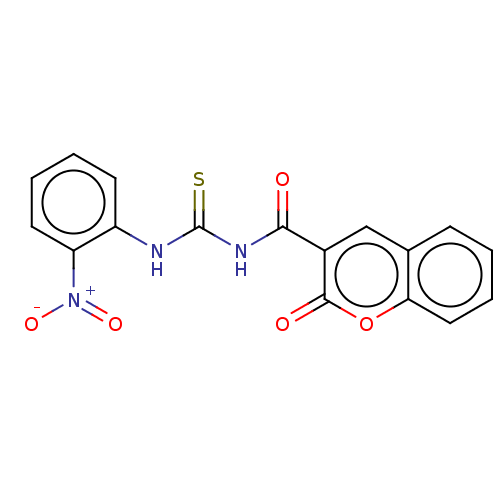 (1-(2-Oxo-2H-chromene-3-carbonyl)-3-(2-nitrophenyl)...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University | Assay Description Newly synthesized coumarin thioureas were tested against electric eel AChE and horse serum BChE. The cholinesterase inhibitory activity was measured ... | Bioorg Chem 63: 58-63 (2015) Article DOI: 10.1016/j.bioorg.2015.09.009 BindingDB Entry DOI: 10.7270/Q2W957Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227638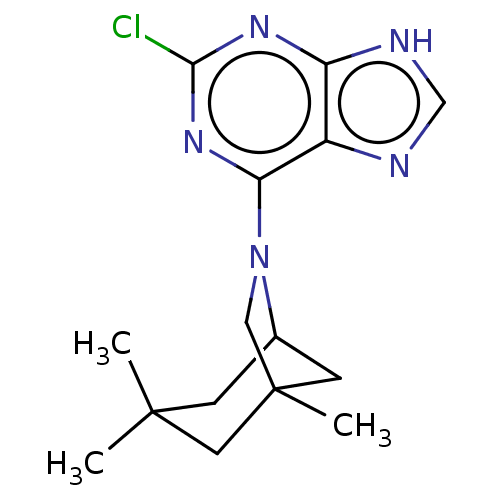 (MTH1 inhibitor, 132) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 4 of Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (PDE11A1) 35-489] (Homo sapiens (Human)) | BDBM14776 (2-{2-ethoxy-5-[(4-ethylpiperazine-1-)sulfonyl]phen...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Structure 12: 2233-47 (2004) Article DOI: 10.1016/j.str.2004.10.004 BindingDB Entry DOI: 10.7270/Q25B00Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M) (Homo sapiens (Human)) | BDBM14785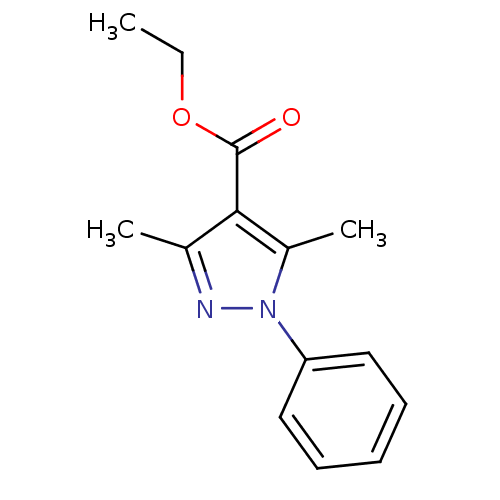 (Pyrazole carboxylic ester 8 | ethyl 3,5-dimethyl-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Nat Biotechnol 23: 201-7 (2005) Article DOI: 10.1038/nbt1059 BindingDB Entry DOI: 10.7270/Q21N7ZCN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B (Homo sapiens (Human)) | BDBM14776 (2-{2-ethoxy-5-[(4-ethylpiperazine-1-)sulfonyl]phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Structure 12: 2233-47 (2004) Article DOI: 10.1016/j.str.2004.10.004 BindingDB Entry DOI: 10.7270/Q25B00Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M] (Homo sapiens (Human)) | BDBM14785 (Pyrazole carboxylic ester 8 | ethyl 3,5-dimethyl-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Nat Biotechnol 23: 201-7 (2005) Article DOI: 10.1038/nbt1059 BindingDB Entry DOI: 10.7270/Q21N7ZCN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227638 (MTH1 inhibitor, 132) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M] (Homo sapiens (Human)) | BDBM14796 (Pyrazole carboxylic ester 19 | ethyl 1-(4-aminophe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Nat Biotechnol 23: 201-7 (2005) Article DOI: 10.1038/nbt1059 BindingDB Entry DOI: 10.7270/Q21N7ZCN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M) (Homo sapiens (Human)) | BDBM14769 (6-(3,4-Dimethoxy-phenyl)-4,5-dimethyl-4,5-dihydro-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Structure 12: 2233-47 (2004) Article DOI: 10.1016/j.str.2004.10.004 BindingDB Entry DOI: 10.7270/Q25B00Q1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227634 (MTH1 inhibitor, 121) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M] (Homo sapiens (Human)) | BDBM14772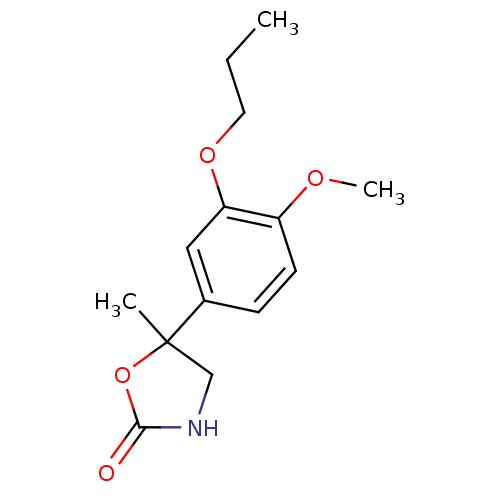 ((R,S)-Mesopram | 5-(4-methoxy-3-propoxy-phenyl)-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Structure 12: 2233-47 (2004) Article DOI: 10.1016/j.str.2004.10.004 BindingDB Entry DOI: 10.7270/Q25B00Q1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M] (Homo sapiens (Human)) | BDBM14361 ((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Structure 12: 2233-47 (2004) Article DOI: 10.1016/j.str.2004.10.004 BindingDB Entry DOI: 10.7270/Q25B00Q1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [650-1084] (Homo sapiens (Human)) | BDBM14776 (2-{2-ethoxy-5-[(4-ethylpiperazine-1-)sulfonyl]phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Structure 12: 2233-47 (2004) Article DOI: 10.1016/j.str.2004.10.004 BindingDB Entry DOI: 10.7270/Q25B00Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227632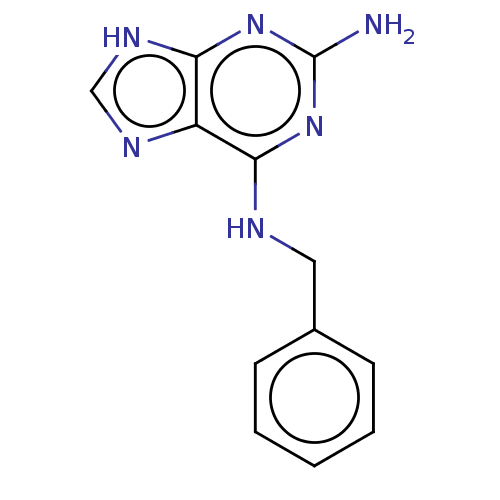 (MTH1 inhibitor, 53) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A [226-593] (Homo sapiens (Human)) | BDBM14776 (2-{2-ethoxy-5-[(4-ethylpiperazine-1-)sulfonyl]phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Plexxikon | Assay Description Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-... | Structure 12: 2233-47 (2004) Article DOI: 10.1016/j.str.2004.10.004 BindingDB Entry DOI: 10.7270/Q25B00Q1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM173567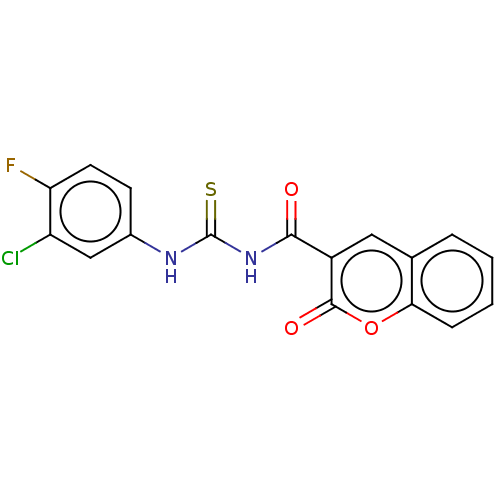 (1-(2-Oxo-2H-chromene-3-carbonyl)-3-(3-chloro-4-flu...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University | Assay Description Newly synthesized coumarin thioureas were tested against electric eel AChE and horse serum BChE. The cholinesterase inhibitory activity was measured ... | Bioorg Chem 63: 58-63 (2015) Article DOI: 10.1016/j.bioorg.2015.09.009 BindingDB Entry DOI: 10.7270/Q2W957Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 241 total ) | Next | Last >> |