

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Wt: 581.0 BDBM5445  Purchase Purchase | Wt: 264.3 BDBM19149  Purchase Purchase | Wt: 376.4 BDBM19410 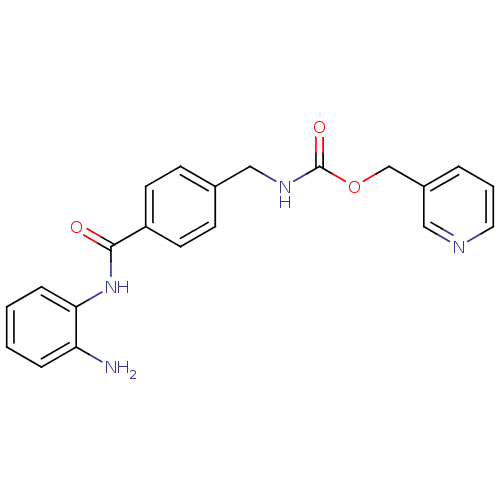 Purchase Purchase | Wt: 351.4 BDBM19423  Purchase Purchase | Wt: 269.2 BDBM19422  Purchase Purchase |
| Wt: 242.2 BDBM19426 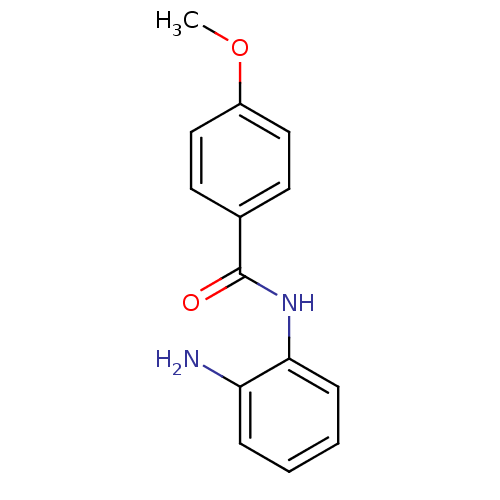 Purchase Purchase | Wt: 379.4 BDBM19428 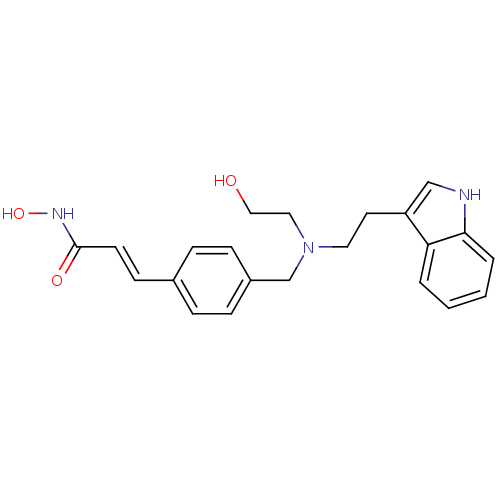 Purchase Purchase | Wt: 302.3 BDBM19130 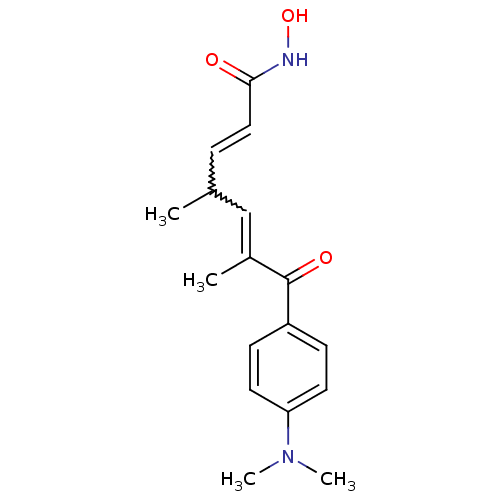 | Wt: 721.8 BDBM22449 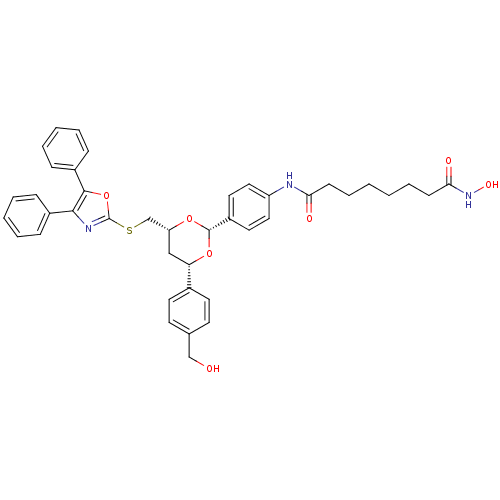 Purchase Purchase | Wt: 397.4 BDBM24622  Purchase Purchase |
| Wt: 396.4 BDBM24624 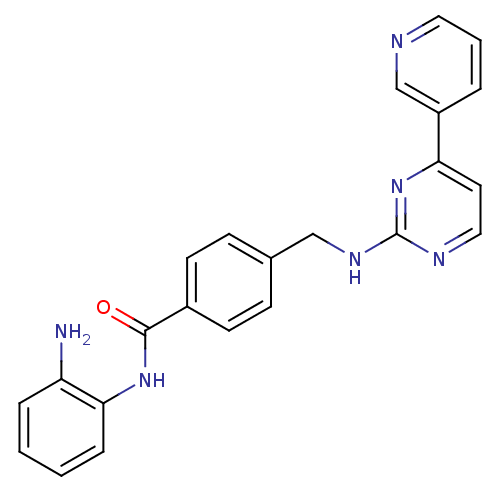 Purchase Purchase | Wt: 318.3 BDBM25150  Purchase Purchase | Wt: 88.1 BDBM26109 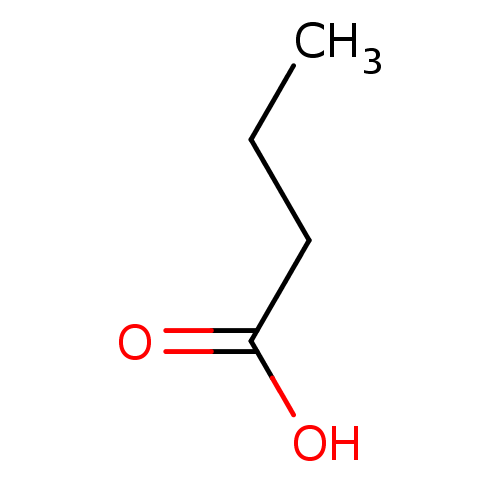 Purchase Purchase | Wt: 349.4 BDBM29589  Purchase Purchase | Wt: 163.1 BDBM36184 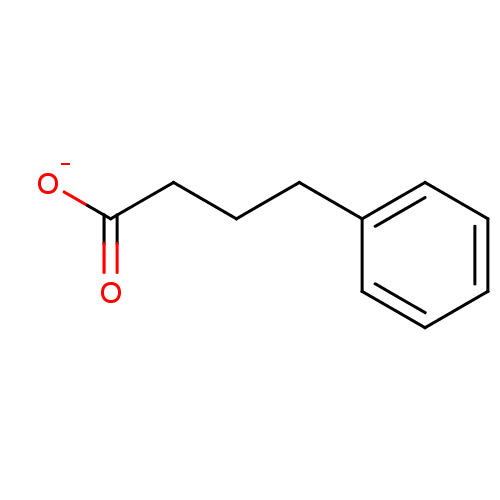 Purchase Purchase |
| Displayed 1 to 15 (of 63 total ) | Next | Last >> |
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19410 (CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | 8.0 | 37 |
MethylGene Inc | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5543-6 (2007) Article DOI: 10.1021/jm701079h BindingDB Entry DOI: 10.7270/Q2445JS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19410 (CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 8.0 | 37 |
MethylGene Inc | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5543-6 (2007) Article DOI: 10.1021/jm701079h BindingDB Entry DOI: 10.7270/Q2445JS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19422 (4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5543-6 (2007) Article DOI: 10.1021/jm701079h BindingDB Entry DOI: 10.7270/Q2445JS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19422 (4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5543-6 (2007) Article DOI: 10.1021/jm701079h BindingDB Entry DOI: 10.7270/Q2445JS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19422 (4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5543-6 (2007) Article DOI: 10.1021/jm701079h BindingDB Entry DOI: 10.7270/Q2445JS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM19422 (4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5543-6 (2007) Article DOI: 10.1021/jm701079h BindingDB Entry DOI: 10.7270/Q2445JS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5543-6 (2007) Article DOI: 10.1021/jm701079h BindingDB Entry DOI: 10.7270/Q2445JS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5543-6 (2007) Article DOI: 10.1021/jm701079h BindingDB Entry DOI: 10.7270/Q2445JS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5543-6 (2007) Article DOI: 10.1021/jm701079h BindingDB Entry DOI: 10.7270/Q2445JS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5543-6 (2007) Article DOI: 10.1021/jm701079h BindingDB Entry DOI: 10.7270/Q2445JS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19426 (N-(2-aminophenyl)-4-methoxybenzamide | benzamide-t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5543-6 (2007) Article DOI: 10.1021/jm701079h BindingDB Entry DOI: 10.7270/Q2445JS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19426 (N-(2-aminophenyl)-4-methoxybenzamide | benzamide-t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5543-6 (2007) Article DOI: 10.1021/jm701079h BindingDB Entry DOI: 10.7270/Q2445JS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19426 (N-(2-aminophenyl)-4-methoxybenzamide | benzamide-t...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5543-6 (2007) Article DOI: 10.1021/jm701079h BindingDB Entry DOI: 10.7270/Q2445JS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM19426 (N-(2-aminophenyl)-4-methoxybenzamide | benzamide-t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5543-6 (2007) Article DOI: 10.1021/jm701079h BindingDB Entry DOI: 10.7270/Q2445JS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19428 ((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5543-6 (2007) Article DOI: 10.1021/jm701079h BindingDB Entry DOI: 10.7270/Q2445JS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19428 ((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5543-6 (2007) Article DOI: 10.1021/jm701079h BindingDB Entry DOI: 10.7270/Q2445JS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19428 ((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5543-6 (2007) Article DOI: 10.1021/jm701079h BindingDB Entry DOI: 10.7270/Q2445JS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM19428 ((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5543-6 (2007) Article DOI: 10.1021/jm701079h BindingDB Entry DOI: 10.7270/Q2445JS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Mus musculus) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Nagoya City University | Assay Description The enzyme activity was assayed using recombinant HDAC and [3H] acetyl-labeled histones as substrate. The released [3H]acetic acid was extracted and ... | J Med Chem 50: 5425-38 (2007) Article DOI: 10.1021/jm7009217 BindingDB Entry DOI: 10.7270/Q2XK8CT6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Nagoya City University | Assay Description The enzyme activity was assayed using recombinant HDAC and [3H] acetyl-labeled histones as substrate. The released [3H]acetic acid was extracted and ... | J Med Chem 50: 5425-38 (2007) Article DOI: 10.1021/jm7009217 BindingDB Entry DOI: 10.7270/Q2XK8CT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University | Assay Description The enzyme activity was assayed using recombinant HDAC and [3H] acetyl-labeled histones as substrate. The released [3H]acetic acid was extracted and ... | J Med Chem 50: 5425-38 (2007) Article DOI: 10.1021/jm7009217 BindingDB Entry DOI: 10.7270/Q2XK8CT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Mus musculus) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 196 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Southampton | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5720-5726 (2007) Article DOI: 10.1021/jm0703800 BindingDB Entry DOI: 10.7270/Q2ST7N4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 775 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Southampton | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | J Med Chem 50: 5720-5726 (2007) Article DOI: 10.1021/jm0703800 BindingDB Entry DOI: 10.7270/Q2ST7N4B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Kyushu Institute of Technology | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | Bioorg Med Chem 14: 3438-46 (2006) Article DOI: 10.1016/j.bmc.2005.12.063 BindingDB Entry DOI: 10.7270/Q2P55KRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Kyushu Institute of Technology | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | Bioorg Med Chem 14: 3438-46 (2006) Article DOI: 10.1016/j.bmc.2005.12.063 BindingDB Entry DOI: 10.7270/Q2P55KRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Mus musculus) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Kyushu Institute of Technology | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | Bioorg Med Chem 14: 3438-46 (2006) Article DOI: 10.1016/j.bmc.2005.12.063 BindingDB Entry DOI: 10.7270/Q2P55KRB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 164 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24624 (CHEMBL272980 | MGCD-0103 | MGCD0103 | N-(2-aminoph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 8.0 | 37 |
MethylGene Inc. | Assay Description The HDAC enzyme in vitro assay was based on a homogeneous fluorescence release assay. Purified recombinant HDAC enzymes were incubated with compounds... | J Med Chem 51: 4072-5 (2008) Article DOI: 10.1021/jm800251w BindingDB Entry DOI: 10.7270/Q28P5XTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM24624 (CHEMBL272980 | MGCD-0103 | MGCD0103 | N-(2-aminoph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | 8.0 | 37 |
MethylGene Inc. | Assay Description The HDAC enzyme in vitro assay was based on a homogeneous fluorescence release assay. Purified recombinant HDAC enzymes were incubated with compounds... | J Med Chem 51: 4072-5 (2008) Article DOI: 10.1021/jm800251w BindingDB Entry DOI: 10.7270/Q28P5XTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24624 (CHEMBL272980 | MGCD-0103 | MGCD0103 | N-(2-aminoph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
MethylGene Inc. | Assay Description The HDAC enzyme in vitro assay was based on a homogeneous fluorescence release assay. Purified recombinant HDAC enzymes were incubated with compounds... | J Med Chem 51: 4072-5 (2008) Article DOI: 10.1021/jm800251w BindingDB Entry DOI: 10.7270/Q28P5XTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM24624 (CHEMBL272980 | MGCD-0103 | MGCD0103 | N-(2-aminoph...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | 8.0 | 37 |
MethylGene Inc. | Assay Description The HDAC enzyme in vitro assay was based on a homogeneous fluorescence release assay. Purified recombinant HDAC enzymes were incubated with compounds... | J Med Chem 51: 4072-5 (2008) Article DOI: 10.1021/jm800251w BindingDB Entry DOI: 10.7270/Q28P5XTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM24624 (CHEMBL272980 | MGCD-0103 | MGCD0103 | N-(2-aminoph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
MethylGene Inc. | Assay Description The HDAC enzyme in vitro assay was based on a homogeneous fluorescence release assay. Purified recombinant HDAC enzymes were incubated with compounds... | J Med Chem 51: 4072-5 (2008) Article DOI: 10.1021/jm800251w BindingDB Entry DOI: 10.7270/Q28P5XTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24624 (CHEMBL272980 | MGCD-0103 | MGCD0103 | N-(2-aminoph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
MethylGene Inc. | Assay Description The HDAC enzyme in vitro assay was based on a homogeneous fluorescence release assay. Purified recombinant HDAC enzymes were incubated with compounds... | J Med Chem 51: 4072-5 (2008) Article DOI: 10.1021/jm800251w BindingDB Entry DOI: 10.7270/Q28P5XTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM24624 (CHEMBL272980 | MGCD-0103 | MGCD0103 | N-(2-aminoph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
MethylGene Inc. | Assay Description The HDAC enzyme in vitro assay was based on a homogeneous fluorescence release assay. Purified recombinant HDAC enzymes were incubated with compounds... | J Med Chem 51: 4072-5 (2008) Article DOI: 10.1021/jm800251w BindingDB Entry DOI: 10.7270/Q28P5XTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM24624 (CHEMBL272980 | MGCD-0103 | MGCD0103 | N-(2-aminoph...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
MethylGene Inc. | Assay Description The HDAC enzyme in vitro assay was based on a homogeneous fluorescence release assay. Purified recombinant HDAC enzymes were incubated with compounds... | J Med Chem 51: 4072-5 (2008) Article DOI: 10.1021/jm800251w BindingDB Entry DOI: 10.7270/Q28P5XTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM24624 (CHEMBL272980 | MGCD-0103 | MGCD0103 | N-(2-aminoph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
MethylGene Inc. | Assay Description The HDAC enzyme in vitro assay was based on a homogeneous fluorescence release assay. Purified recombinant HDAC enzymes were incubated with compounds... | J Med Chem 51: 4072-5 (2008) Article DOI: 10.1021/jm800251w BindingDB Entry DOI: 10.7270/Q28P5XTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5445 (CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GSK | Assay Description Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP/[gamma-33P]ATP, and purified kinase in t... | Proc Natl Acad Sci U S A 105: 2773-8 (2008) Article DOI: 10.1073/pnas.0708281105 BindingDB Entry DOI: 10.7270/Q27S7M35 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5445 (CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GSK | Assay Description Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP/[gamma-33P]ATP, and purified kinase in t... | Proc Natl Acad Sci U S A 105: 2773-8 (2008) Article DOI: 10.1073/pnas.0708281105 BindingDB Entry DOI: 10.7270/Q27S7M35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM5445 (CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 367 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GSK | Assay Description Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP/[gamma-33P]ATP, and purified kinase in t... | Proc Natl Acad Sci U S A 105: 2773-8 (2008) Article DOI: 10.1073/pnas.0708281105 BindingDB Entry DOI: 10.7270/Q27S7M35 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Georgia Institute of Technology | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 52: 456-68 (2009) Article DOI: 10.1021/jm801128g BindingDB Entry DOI: 10.7270/Q2542KXF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| << First | Previous | Displayed 201 to 250 (of 3861 total ) | Next | Last >> |
| Cell (A) | Syringe (B) | Cell Links | Syringe Links | Cell + Syr Links | ΔG° kcal/mole | -TΔS° kcal/mole | ΔH° kcal/mole | log K | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|
| Methyl-accepting chemotaxis protein (McpS) (Pseudomonas putida (Arthrobacter siderocapsulatus)) | BDBM26109 (Butyrate | butanoic acid | butanoic acid, 4) | GoogleScholar | CHEBI DrugBank KEGG MMDB PC cid PC sid PDB | -5.41 | 2.80 | -7.88 | 4.04 | n/a | 20 | |
CSIC | J Biol Chem 285: 23126-36 (2010) | |||||||||
BDBM11 | BDBM36184 | CHEBI KEGG MMDB PC cid PC sid PDB | CHEBI DrugBank PC cid PC sid | -3.60 | -0.783 | -2.81 | 2.64 | 6.90 | 25 | |
NIST | J Phys Chem B 101: 87-100 (1997) | |||||||||
| Histone deacetylase 1/3/5/8 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | DrugBank GoogleScholar KEGG PDB | CHEBI DrugBank MMDB PC cid PC sid PDB | -8.36 | 2.42 | -10.9 | 6.13 | n/a | 25 | |
North Dakota State University | Biochemistry 52: 8139-49 (2013) | |||||||||
| Histone deacetylase 1/3/5/8 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | DrugBank GoogleScholar KEGG PDB | MMDB PC cid PC sid PDB | -8.60 | 0.214 | -8.84 | 6.31 | n/a | 25 | |
North Dakota State University | Biochemistry 52: 8139-49 (2013) | |||||||||
| Histone deacetylase 8 (HDAC8) (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | DrugBank GoogleScholar KEGG PDB | CHEBI DrugBank MMDB PC cid PC sid PDB | -8.36 | 0.0519 | -8.96 | 6.34 | 7.5 | -248 | |
North Dakota State University | Biochemistry 53: 7445-58 (2014) | |||||||||