

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50188594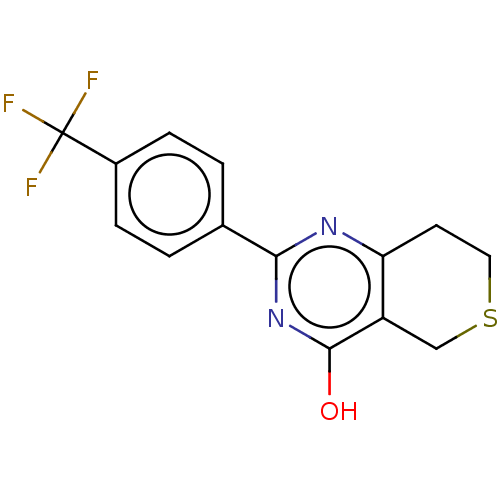 (CHEBI:62878 | CHEMBL1086580) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic activity against histamine H3 receptor in guinea pig ileum | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50407813 (CHEMBL5194149) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 2.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist potency at cloned recombinant human adenosine A2B receptor transfected in CHO cells by cAMP assay | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259594 (US9505749, 107) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0882 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259593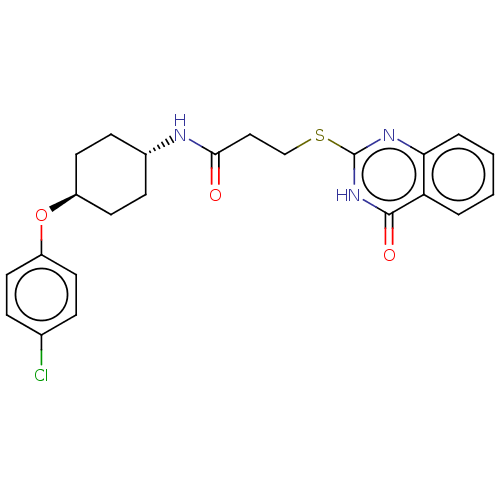 (US9505749, 105) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0982 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50234915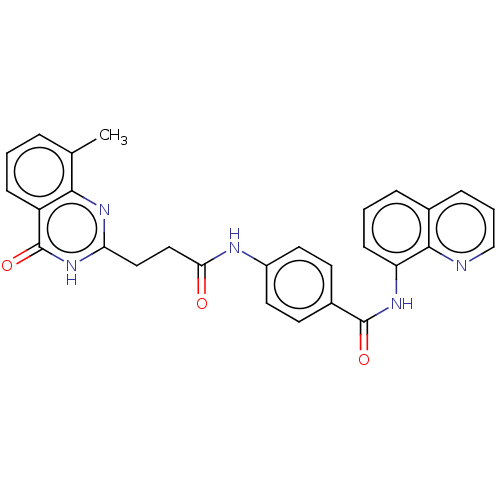 (CHEMBL4069447) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-GST-tagged TNKS2 (849 to 1166 residues) expressed in baculovirus infected Sf9 cells using biotinylated... | J Med Chem 60: 814-820 (2017) Article DOI: 10.1021/acs.jmedchem.6b01574 BindingDB Entry DOI: 10.7270/Q20R9RPP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50234915 (CHEMBL4069447) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of TNKS2 (unknown origin) | J Med Chem 60: 814-820 (2017) Article DOI: 10.1021/acs.jmedchem.6b01574 BindingDB Entry DOI: 10.7270/Q20R9RPP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50234915 (CHEMBL4069447) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-GST-tagged TNKS2 (849 to 1166 residues) expressed in baculovirus infected Sf9 cells using biotinylated... | J Med Chem 60: 814-820 (2017) Article DOI: 10.1021/acs.jmedchem.6b01574 BindingDB Entry DOI: 10.7270/Q20R9RPP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50234915 (CHEMBL4069447) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50169061 (CHEMBL3805393) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of GST-tagged TNKS2 (873 to 1166 residues) (unknown origin) autoparsylation using biotinylated NAD incubated for 1 hr by ELISA | ACS Med Chem Lett 7: 209-10 (2016) Article DOI: 10.1021/acsmedchemlett.6b00017 BindingDB Entry DOI: 10.7270/Q2F76FFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259584 (US9505749, 95) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50169056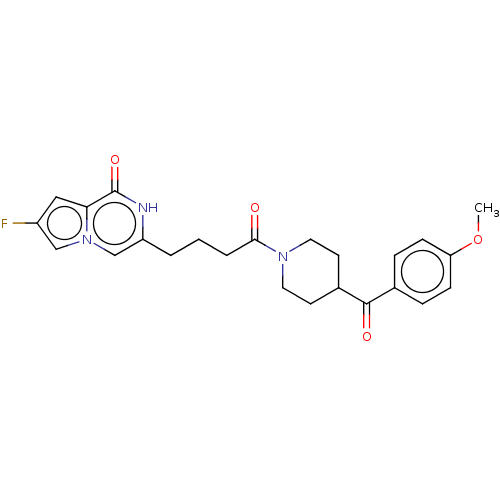 (CHEMBL3806163) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of GST-tagged TNKS2 (873 to 1166 residues) (unknown origin) autoparsylation using biotinylated NAD incubated for 1 hr by ELISA | ACS Med Chem Lett 7: 209-10 (2016) Article DOI: 10.1021/acsmedchemlett.6b00017 BindingDB Entry DOI: 10.7270/Q2F76FFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259612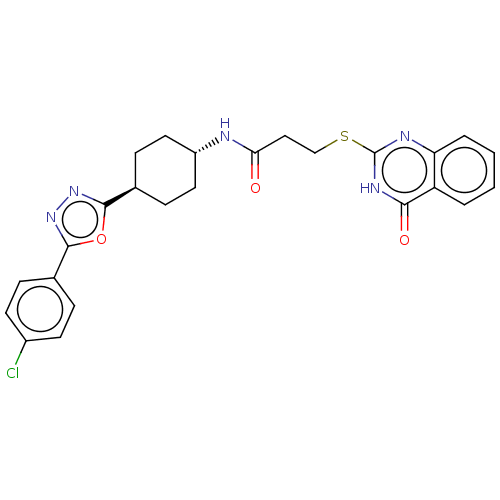 (US9505749, 125) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.179 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50169057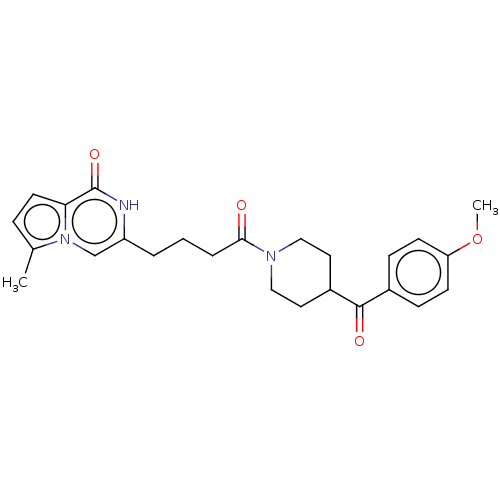 (CHEMBL3805896) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of GST-tagged TNKS2 (873 to 1166 residues) (unknown origin) autoparsylation using biotinylated NAD incubated for 1 hr by ELISA | ACS Med Chem Lett 7: 209-10 (2016) Article DOI: 10.1021/acsmedchemlett.6b00017 BindingDB Entry DOI: 10.7270/Q2F76FFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM435386 (US10570116, Compound C2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description TNKS 1 and 2: The activity ELISA is performed in 384 well Glutathione coated microtiter plates (Express capture Glutathione coated plate, Biocat, Hei... | US Patent US10570116 (2020) BindingDB Entry DOI: 10.7270/Q2J968R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50169060 (CHEMBL3804939) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of GST-tagged TNKS2 (873 to 1166 residues) (unknown origin) autoparsylation using biotinylated NAD incubated for 1 hr by ELISA | ACS Med Chem Lett 7: 209-10 (2016) Article DOI: 10.1021/acsmedchemlett.6b00017 BindingDB Entry DOI: 10.7270/Q2F76FFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50544643 (CHEMBL4634919) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Lauren Mccallister Curated by ChEMBL | Assay Description Inhibition of human GST-tagged tankyrase 2 autophosphorylation | ACS Med Chem Lett 11: 1676-1677 (2020) Article DOI: 10.1021/acsmedchemlett.0c00390 BindingDB Entry DOI: 10.7270/Q2FF3WZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259519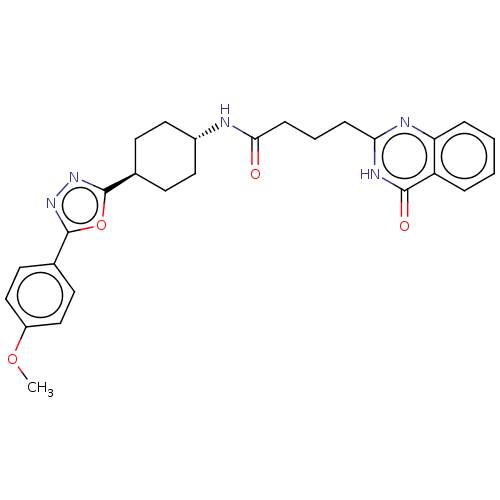 (US9505749, 30) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.191 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259541 (US9505749, 52) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.193 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259524 (US9505749, 35) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.196 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50234916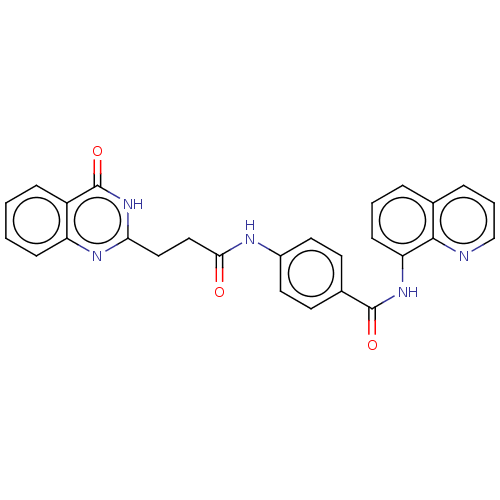 (CHEMBL4098188) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-GST-tagged TNKS2 (849 to 1166 residues) expressed in baculovirus infected Sf9 cells using biotinylated... | J Med Chem 60: 814-820 (2017) Article DOI: 10.1021/acs.jmedchem.6b01574 BindingDB Entry DOI: 10.7270/Q20R9RPP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50234916 (CHEMBL4098188) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM435395 (US10570116, Compound C6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description TNKS 1 and 2: The activity ELISA is performed in 384 well Glutathione coated microtiter plates (Express capture Glutathione coated plate, Biocat, Hei... | US Patent US10570116 (2020) BindingDB Entry DOI: 10.7270/Q2J968R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50234916 (CHEMBL4098188) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.204 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-GST-tagged TNKS2 (849 to 1166 residues) expressed in baculovirus infected Sf9 cells using biotinylated... | J Med Chem 60: 814-820 (2017) Article DOI: 10.1021/acs.jmedchem.6b01574 BindingDB Entry DOI: 10.7270/Q20R9RPP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259550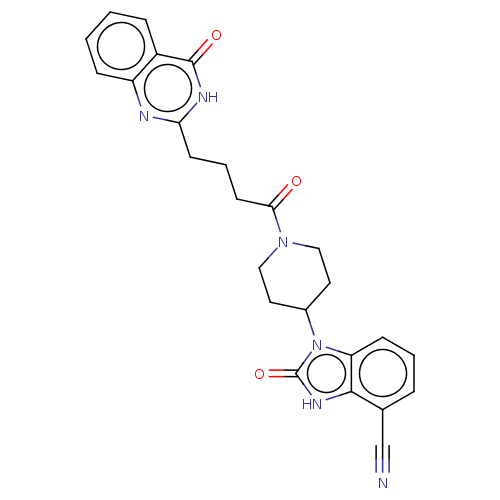 (US9505749, 61) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.206 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM435441 (US10570116, Compound C49) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description TNKS 1 and 2: The activity ELISA is performed in 384 well Glutathione coated microtiter plates (Express capture Glutathione coated plate, Biocat, Hei... | US Patent US10570116 (2020) BindingDB Entry DOI: 10.7270/Q2J968R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM435439 (US10570116, Compound C47) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description TNKS 1 and 2: The activity ELISA is performed in 384 well Glutathione coated microtiter plates (Express capture Glutathione coated plate, Biocat, Hei... | US Patent US10570116 (2020) BindingDB Entry DOI: 10.7270/Q2J968R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259574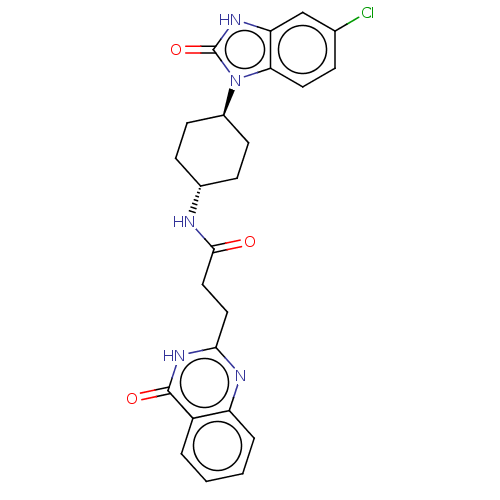 (US9505749, 85) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.227 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259520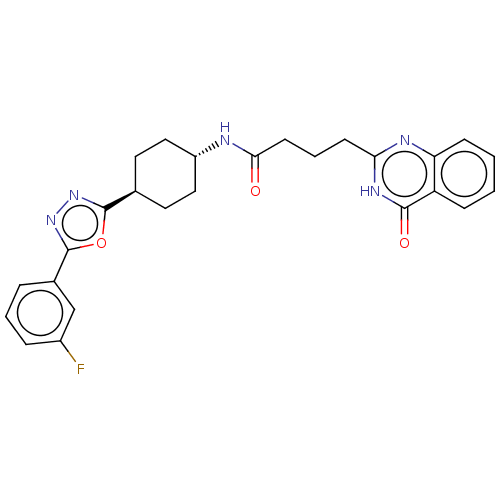 (US9505749, 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.237 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM435442 (US10570116, Compound C50) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description TNKS 1 and 2: The activity ELISA is performed in 384 well Glutathione coated microtiter plates (Express capture Glutathione coated plate, Biocat, Hei... | US Patent US10570116 (2020) BindingDB Entry DOI: 10.7270/Q2J968R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM435444 (US10570116, Compound C52) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description TNKS 1 and 2: The activity ELISA is performed in 384 well Glutathione coated microtiter plates (Express capture Glutathione coated plate, Biocat, Hei... | US Patent US10570116 (2020) BindingDB Entry DOI: 10.7270/Q2J968R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50544642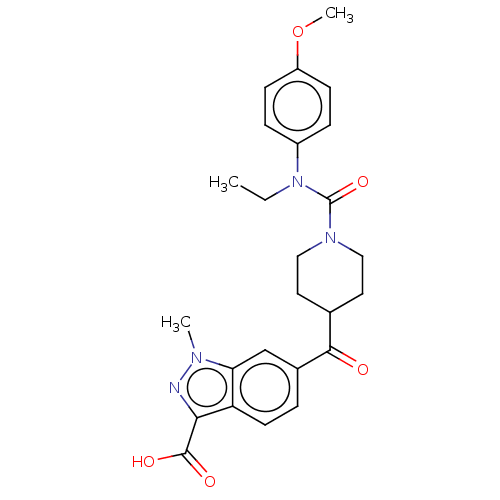 (CHEMBL4632401) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Lauren Mccallister Curated by ChEMBL | Assay Description Inhibition of human GST-tagged tankyrase 2 autophosphorylation | ACS Med Chem Lett 11: 1676-1677 (2020) Article DOI: 10.1021/acsmedchemlett.0c00390 BindingDB Entry DOI: 10.7270/Q2FF3WZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50544640 (CHEMBL4640008) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Lauren Mccallister Curated by ChEMBL | Assay Description Inhibition of human GST-tagged tankyrase 2 autophosphorylation | ACS Med Chem Lett 11: 1676-1677 (2020) Article DOI: 10.1021/acsmedchemlett.0c00390 BindingDB Entry DOI: 10.7270/Q2FF3WZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259599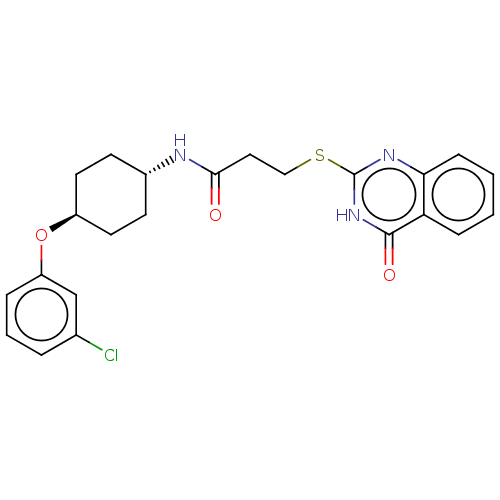 (US9505749, 112) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.264 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50544645 (CHEMBL4643690) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Lauren Mccallister Curated by ChEMBL | Assay Description Inhibition of human GST-tagged tankyrase 2 autophosphorylation | ACS Med Chem Lett 11: 1676-1677 (2020) Article DOI: 10.1021/acsmedchemlett.0c00390 BindingDB Entry DOI: 10.7270/Q2FF3WZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259525 (US9505749, 36) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50169058 (CHEMBL3806049) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of GST-tagged TNKS2 (873 to 1166 residues) (unknown origin) autoparsylation using biotinylated NAD incubated for 1 hr by ELISA | ACS Med Chem Lett 7: 209-10 (2016) Article DOI: 10.1021/acsmedchemlett.6b00017 BindingDB Entry DOI: 10.7270/Q2F76FFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50544644 (CHEMBL4639702) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Lauren Mccallister Curated by ChEMBL | Assay Description Inhibition of human GST-tagged tankyrase 2 autophosphorylation | ACS Med Chem Lett 11: 1676-1677 (2020) Article DOI: 10.1021/acsmedchemlett.0c00390 BindingDB Entry DOI: 10.7270/Q2FF3WZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259557 (US9505749, 68) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.292 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259600 (US9505749, 113) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.294 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50540762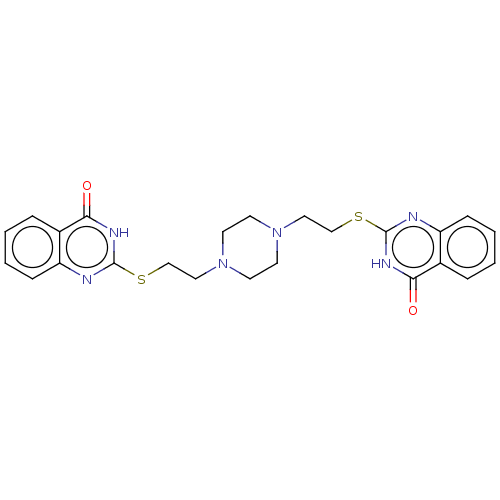 (CHEMBL4638701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal GST-tagged TNKS2 ADP-ART catalytic domain (1001 to 1327 residues) using histone H2A as substrate incubated... | ACS Med Chem Lett 11: 862-868 (2020) Article DOI: 10.1021/acsmedchemlett.9b00654 BindingDB Entry DOI: 10.7270/Q21J9FBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259521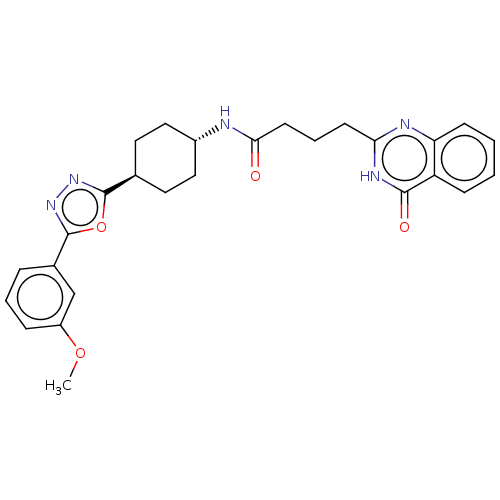 (US9505749, 32) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.301 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259596 (US9505749, 109) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259517 (US9505749, 28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.303 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM435430 (US10570116, Compound C38) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description TNKS 1 and 2: The activity ELISA is performed in 384 well Glutathione coated microtiter plates (Express capture Glutathione coated plate, Biocat, Hei... | US Patent US10570116 (2020) BindingDB Entry DOI: 10.7270/Q2J968R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM435409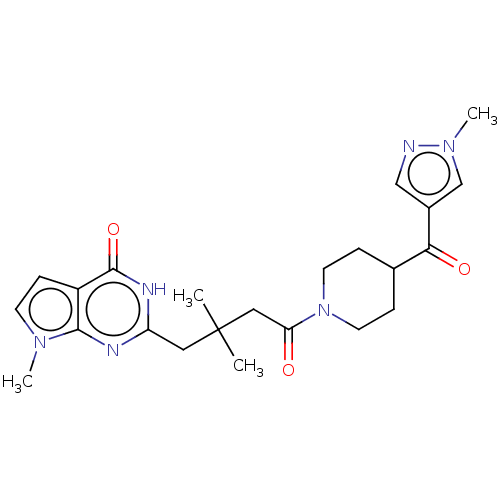 (US10570116, Compound C17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description TNKS 1 and 2: The activity ELISA is performed in 384 well Glutathione coated microtiter plates (Express capture Glutathione coated plate, Biocat, Hei... | US Patent US10570116 (2020) BindingDB Entry DOI: 10.7270/Q2J968R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259522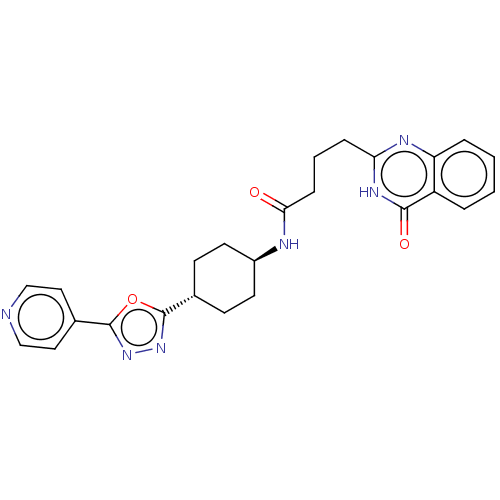 (US9505749, 33) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.341 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259575 (US9505749, 86) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.347 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM435410 (US10570116, Compound C18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description TNKS 1 and 2: The activity ELISA is performed in 384 well Glutathione coated microtiter plates (Express capture Glutathione coated plate, Biocat, Hei... | US Patent US10570116 (2020) BindingDB Entry DOI: 10.7270/Q2J968R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 [946-1162] (Homo sapiens (Human)) | BDBM259588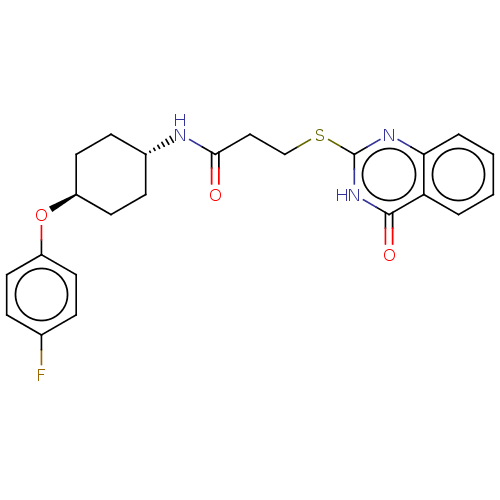 (US9505749, 100) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.362 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9505749 (2016) BindingDB Entry DOI: 10.7270/Q2FN154F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM435384 (US10570116, Compound C1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description TNKS 1 and 2: The activity ELISA is performed in 384 well Glutathione coated microtiter plates (Express capture Glutathione coated plate, Biocat, Hei... | US Patent US10570116 (2020) BindingDB Entry DOI: 10.7270/Q2J968R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1210 total ) | Next | Last >> |