

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2X purinoceptor 1 (RAT) | BDBM50240455 (CHEMBL1253351) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at rat P2X1 receptor | J Med Chem 63: 6164-6178 (2020) Article DOI: 10.1021/acs.jmedchem.0c00435 BindingDB Entry DOI: 10.7270/Q24F1V93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50540410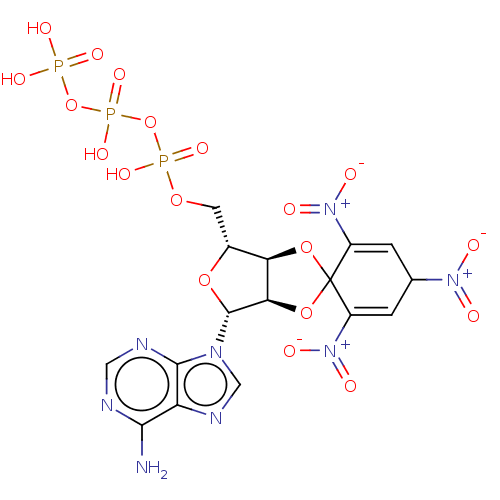 (CHEMBL337395) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at rat P2X1 receptor | J Med Chem 63: 6164-6178 (2020) Article DOI: 10.1021/acs.jmedchem.0c00435 BindingDB Entry DOI: 10.7270/Q24F1V93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50102298 (4-(4-Formyl-5-hydroxy-6-methyl-3-phosphonomethyl-p...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50102295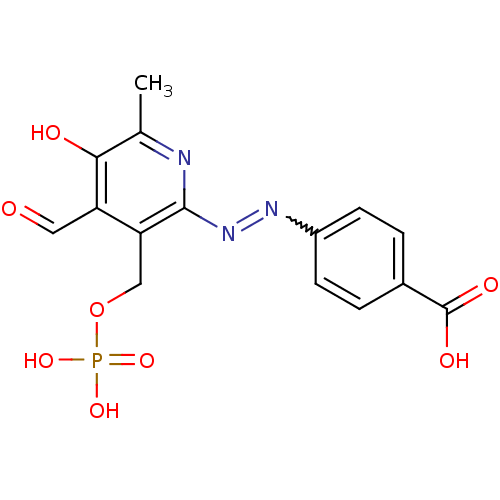 (4-(4-Formyl-5-hydroxy-6-methyl-3-phosphonooxymethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50102299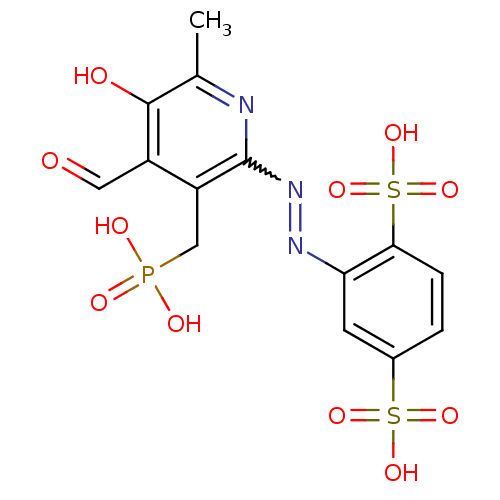 (2-(4-Formyl-5-hydroxy-6-methyl-3-phosphonomethyl-p...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50102300 (CHEMBL331250 | [4-(4-Formyl-5-hydroxy-6-methyl-3-p...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50118221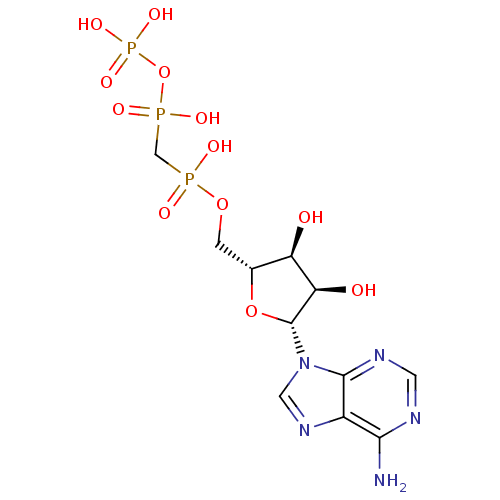 (9H-purine derivative | CHEMBL132722 | DIPHOSPHOMET...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Carlo Bo Curated by ChEMBL | Assay Description Inhibitory concentration against human Adenosine A3 receptor expressed in HEK293 cells using 0.1 nM [3H]AB-MECA | J Med Chem 48: 6887-96 (2005) Article DOI: 10.1021/jm058018d BindingDB Entry DOI: 10.7270/Q28C9X1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50102301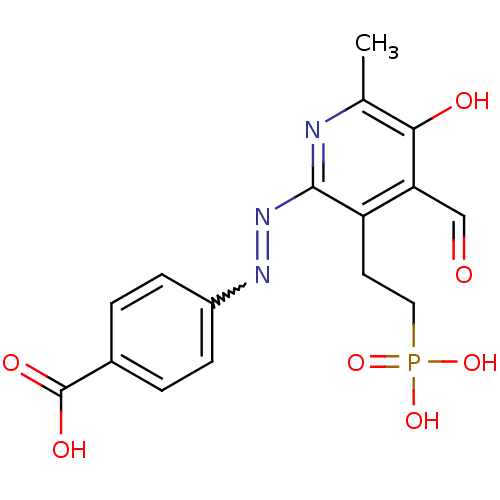 (4-[4-Formyl-5-hydroxy-6-methyl-3-(2-phosphono-ethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50102296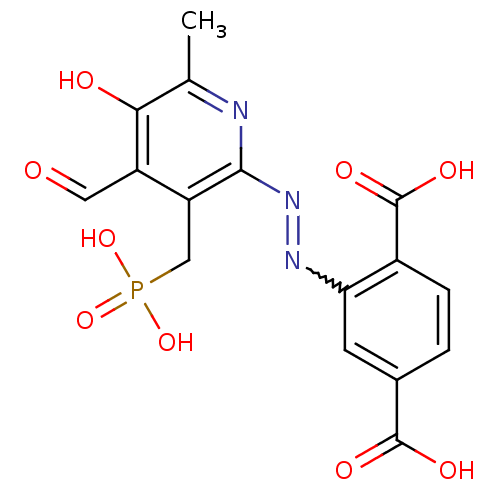 (2-(4-Formyl-5-hydroxy-6-methyl-3-phosphonomethyl-p...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50102304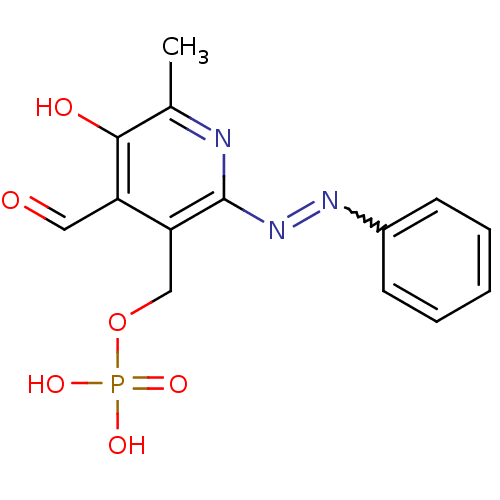 (CHEMBL116926 | Phosphoric acid mono-(4-formyl-5-hy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50102297 (CHEMBL118007 | [2-(3,5-Bis-phosphonomethyl-phenyla...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50102295 (4-(4-Formyl-5-hydroxy-6-methyl-3-phosphonooxymethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at recombinant rat P2X1 receptor expressed in Xenopus oocytes assessed as ion flux stimulation | Eur J Med Chem 70: 811-30 (2013) Article DOI: 10.1016/j.ejmech.2013.10.026 BindingDB Entry DOI: 10.7270/Q2M61MQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50064800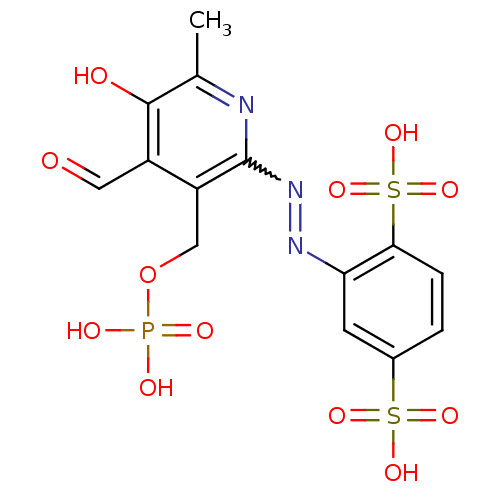 (2-(4-Formyl-5-hydroxy-6-methyl-3-phosphonooxymethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM85043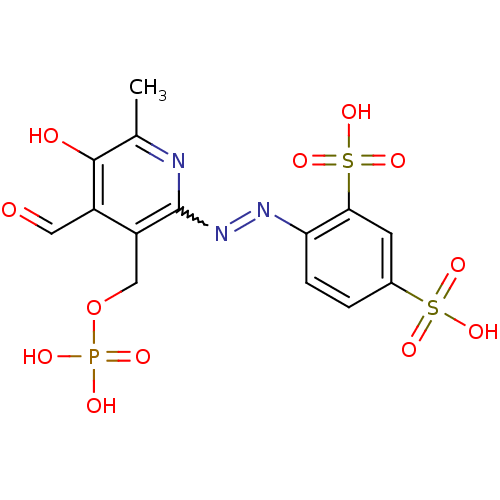 (CAS_149017-66-3 | CHEMBL69234 | NSC_6093163 | PPAD...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50336781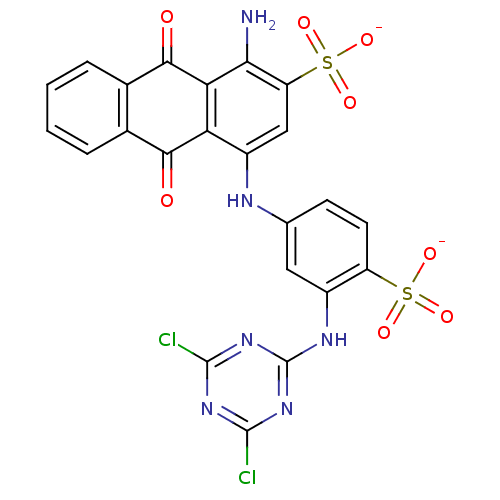 (CHEMBL1672104 | Disodium 1-Amino-4-[3-(4,6-dichlor...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 422 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity against rat P2X1 receptor expressed in Xenopus laevis oocyte assessed as inhibition of alpha, beta-meATP-induced inward current b... | J Med Chem 54: 817-30 (2012) Article DOI: 10.1021/jm1012193 BindingDB Entry DOI: 10.7270/Q2VH5PTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50540409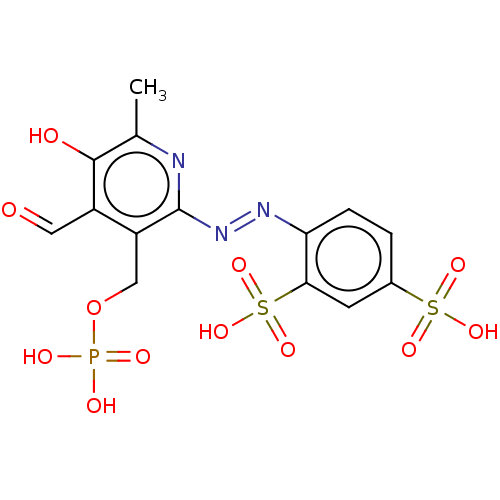 (CHEBI:34941 | CHEMBL69234) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at rat P2X1 receptor | J Med Chem 63: 6164-6178 (2020) Article DOI: 10.1021/acs.jmedchem.0c00435 BindingDB Entry DOI: 10.7270/Q24F1V93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50179360 (CHEMBL3040216) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at rat P2X1 receptor | J Med Chem 63: 6164-6178 (2020) Article DOI: 10.1021/acs.jmedchem.0c00435 BindingDB Entry DOI: 10.7270/Q24F1V93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50118245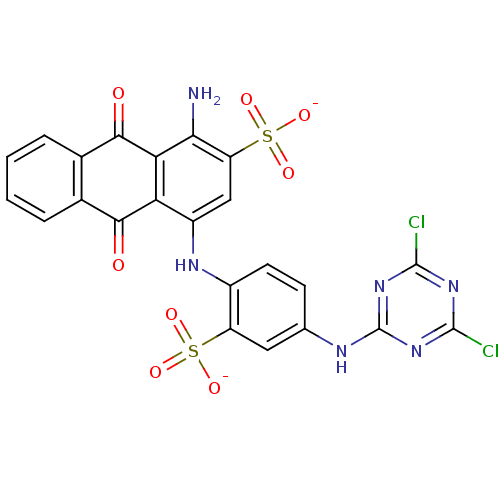 (CHEMBL133572 | MG 40-3) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against P2X purinoceptor 1 (P2X1) like receptor from rat vas deferens | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50168286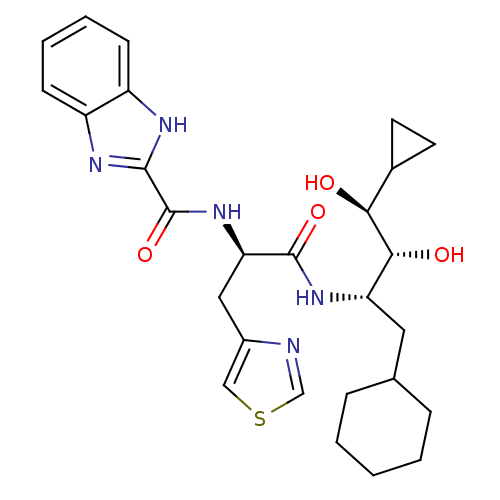 (1H-Benzoimidazole-2-carboxylic acid [(R)-1-((1S,2R...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at rat P2X1 receptor | J Med Chem 63: 6164-6178 (2020) Article DOI: 10.1021/acs.jmedchem.0c00435 BindingDB Entry DOI: 10.7270/Q24F1V93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50179360 (CHEMBL3040216) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at P2X1 receptor in rat vas deferens assessed as inhibition of alpha/beta-meATP-induced calcium influx | J Med Chem 59: 7410-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01690 BindingDB Entry DOI: 10.7270/Q2BC431Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50336781 (CHEMBL1672104 | Disodium 1-Amino-4-[3-(4,6-dichlor...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at P2X1 receptor in rat vas deferens assessed as inhibition of alphabeta me-ATP-induced response | Eur J Med Chem 151: 462-481 (2018) Article DOI: 10.1016/j.ejmech.2018.03.023 BindingDB Entry DOI: 10.7270/Q2S75JWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50118237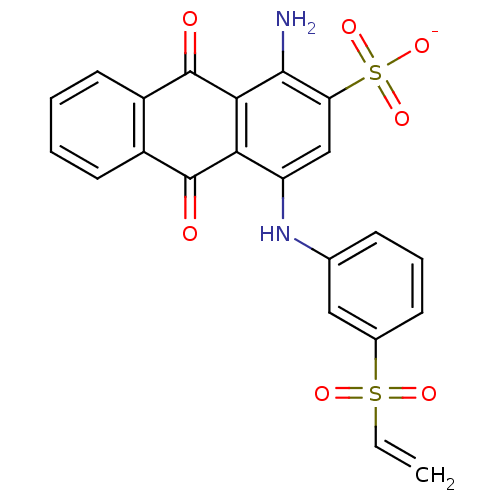 (CHEMBL130059 | Uniblue A) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against P2X purinoceptor 1 (P2X1) from rat vas deferens | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50118218 (CHEMBL131271 | Disodium 1-amino-4-[4-(4,6-dichloro...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against P2X purinoceptor 1 (P2X1) like receptor from rat vas deferens | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50118231 (CHEMBL134193 | Cibachron Blue 3GA) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against P2X purinoceptor 1 (P2X1) from rat vas deferens | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50064801 (2-(4,7-Dihydroxy-3-methyl-7-oxo-5,9-dihydro-6,8-di...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Compound was tested in a functional ion channel assay of ATP-induced current at recombinant rat P2X1 receptor expressed in Xenopus oocytes. | J Med Chem 41: 2201-6 (1998) Article DOI: 10.1021/jm980183o BindingDB Entry DOI: 10.7270/Q2DB80ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50064801 (2-(4,7-Dihydroxy-3-methyl-7-oxo-5,9-dihydro-6,8-di...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50064800 (2-(4-Formyl-5-hydroxy-6-methyl-3-phosphonooxymethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Compound was tested in a functional ion channel assay of ATP-induced current at recombinant rat P2X1 receptor expressed in Xenopus oocytes. | J Med Chem 41: 2201-6 (1998) Article DOI: 10.1021/jm980183o BindingDB Entry DOI: 10.7270/Q2DB80ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM85043 (CAS_149017-66-3 | CHEMBL69234 | NSC_6093163 | PPAD...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 9.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Compound was tested in a functional ion channel assay of ATP-induced current at recombinant rat P2X1 receptor expressed in Xenopus oocytes. | J Med Chem 41: 2201-6 (1998) Article DOI: 10.1021/jm980183o BindingDB Entry DOI: 10.7270/Q2DB80ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||