 Found 234 hits of ic50 for UniProtKB: P48449
Found 234 hits of ic50 for UniProtKB: P48449 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50055641
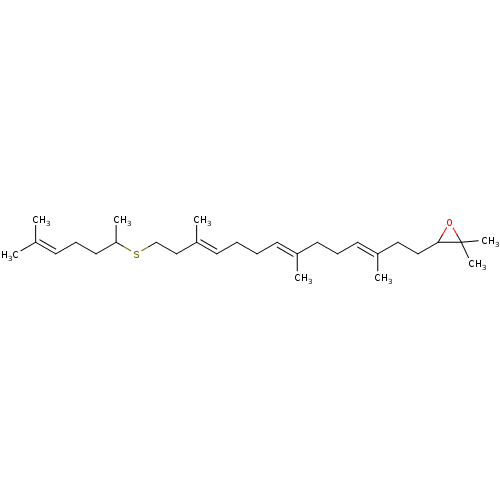
(2,2-dimethyl-3-((3E,7E,11E)-3,7,12-trimethyl-14-(6...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])-[#16]-[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]-[#6]1-[#8]C1([#6])[#6] Show InChI InChI=1S/C29H50OS/c1-23(2)13-11-18-27(6)31-22-21-26(5)15-10-9-14-24(3)16-12-17-25(4)19-20-28-29(7,8)30-28/h13-15,17,27-28H,9-12,16,18-22H2,1-8H3/b24-14+,25-17+,26-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of purified Oxidosqualene-lanosterol cyclase from Candida albicans |
J Med Chem 40: 201-9 (1997)
Article DOI: 10.1021/jm960483a
BindingDB Entry DOI: 10.7270/Q2P26X73 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130785
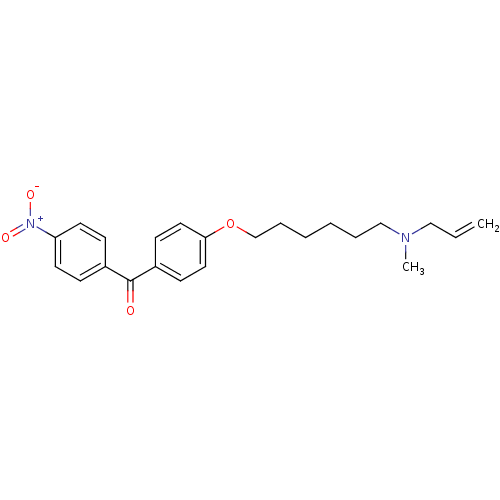
(CHEMBL542453 | CHEMBL609978 | {4-[6-(Allyl-methyl-...)Show SMILES CN(CCCCCCOc1ccc(cc1)C(=O)c1ccc(cc1)[N+]([O-])=O)CC=C Show InChI InChI=1S/C23H28N2O4/c1-3-16-24(2)17-6-4-5-7-18-29-22-14-10-20(11-15-22)23(26)19-8-12-21(13-9-19)25(27)28/h3,8-15H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Torino
Curated by ChEMBL
| Assay Description
Inhibition of oxidosqualene cyclase |
J Med Chem 50: 5039-42 (2007)
Article DOI: 10.1021/jm0704651
BindingDB Entry DOI: 10.7270/Q2ZP47FF |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130785

(CHEMBL542453 | CHEMBL609978 | {4-[6-(Allyl-methyl-...)Show SMILES CN(CCCCCCOc1ccc(cc1)C(=O)c1ccc(cc1)[N+]([O-])=O)CC=C Show InChI InChI=1S/C23H28N2O4/c1-3-16-24(2)17-6-4-5-7-18-29-22-14-10-20(11-15-22)23(26)19-8-12-21(13-9-19)25(27)28/h3,8-15H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50055642
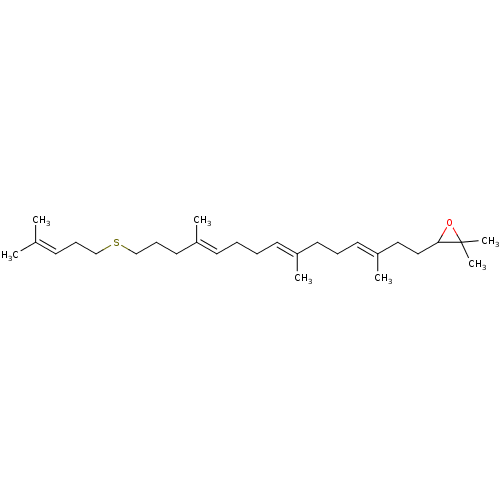
(2,2-Dimethyl-3-[(3E,7E,11E)-3,7,12-trimethyl-15-(4...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]-[#16]-[#6]-[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]-[#6]1-[#8]C1([#6])[#6] Show InChI InChI=1S/C28H48OS/c1-23(2)13-11-21-30-22-12-18-25(4)15-9-8-14-24(3)16-10-17-26(5)19-20-27-28(6,7)29-27/h13-15,17,27H,8-12,16,18-22H2,1-7H3/b24-14+,25-15+,26-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of purified Oxidosqualene-lanosterol cyclase from Candida albicans |
J Med Chem 40: 201-9 (1997)
Article DOI: 10.1021/jm960483a
BindingDB Entry DOI: 10.7270/Q2P26X73 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128063

(Allyl-{6-[3-(4-bromo-phenyl)-benzo[d]isothiazol-6-...)Show SMILES CN(CCCCCCOc1ccc2c(nsc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C23H27BrN2OS/c1-3-14-26(2)15-6-4-5-7-16-27-20-12-13-21-22(17-20)28-25-23(21)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.88 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of oxidosqualene cyclase |
Eur J Med Chem 43: 1462-8 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.019
BindingDB Entry DOI: 10.7270/Q2CR5VKG |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128063

(Allyl-{6-[3-(4-bromo-phenyl)-benzo[d]isothiazol-6-...)Show SMILES CN(CCCCCCOc1ccc2c(nsc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C23H27BrN2OS/c1-3-14-26(2)15-6-4-5-7-16-27-20-12-13-21-22(17-20)28-25-23(21)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Torino
Curated by ChEMBL
| Assay Description
Inhibition of oxidosqualene cyclase |
J Med Chem 50: 5039-42 (2007)
Article DOI: 10.1021/jm0704651
BindingDB Entry DOI: 10.7270/Q2ZP47FF |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128063

(Allyl-{6-[3-(4-bromo-phenyl)-benzo[d]isothiazol-6-...)Show SMILES CN(CCCCCCOc1ccc2c(nsc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C23H27BrN2OS/c1-3-14-26(2)15-6-4-5-7-16-27-20-12-13-21-22(17-20)28-25-23(21)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-Universität
Curated by ChEMBL
| Assay Description
Inhibitory activity against Oxidosqualene-lanosterol cyclase from human liver microsomes |
J Med Chem 46: 2083-92 (2003)
Article DOI: 10.1021/jm0211218
BindingDB Entry DOI: 10.7270/Q2VM4BM9 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128063

(Allyl-{6-[3-(4-bromo-phenyl)-benzo[d]isothiazol-6-...)Show SMILES CN(CCCCCCOc1ccc2c(nsc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C23H27BrN2OS/c1-3-14-26(2)15-6-4-5-7-16-27-20-12-13-21-22(17-20)28-25-23(21)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128063

(Allyl-{6-[3-(4-bromo-phenyl)-benzo[d]isothiazol-6-...)Show SMILES CN(CCCCCCOc1ccc2c(nsc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C23H27BrN2OS/c1-3-14-26(2)15-6-4-5-7-16-27-20-12-13-21-22(17-20)28-25-23(21)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL)
Curated by ChEMBL
| Assay Description
Inhibition of human liver microsome 2,3-OSC after 1 hr by Silica gel plate phosphor imaging |
J Med Chem 55: 4990-5002 (2012)
Article DOI: 10.1021/jm300256z
BindingDB Entry DOI: 10.7270/Q2BZ674B |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128060
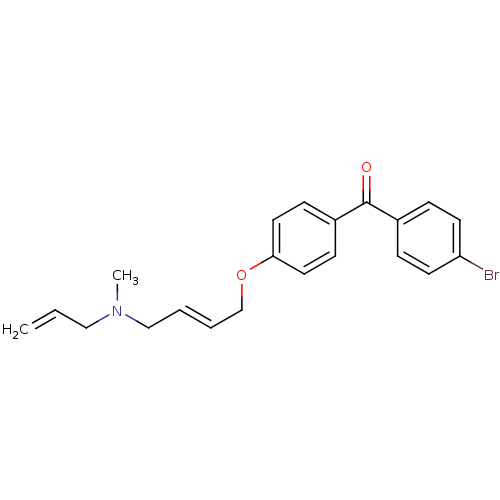
(CHEMBL63524 | N-allyl-4-(4-(4-bromobenzoyl)phenoxy...)Show SMILES CN(CC=C)C\C=C\COc1ccc(cc1)C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C21H22BrNO2/c1-3-14-23(2)15-4-5-16-25-20-12-8-18(9-13-20)21(24)17-6-10-19(22)11-7-17/h3-13H,1,14-16H2,2H3/b5-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-Universität
Curated by ChEMBL
| Assay Description
Inhibitory activity against Oxidosqualene-lanosterol cyclase from human liver microsomes |
J Med Chem 46: 2083-92 (2003)
Article DOI: 10.1021/jm0211218
BindingDB Entry DOI: 10.7270/Q2VM4BM9 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50271572
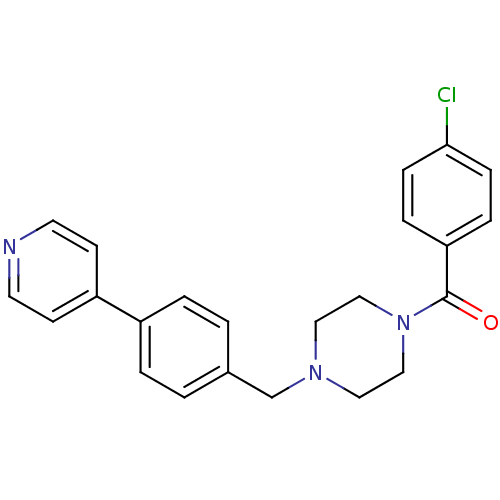
(1-[4-Chlorophenylcarbonyl]-4-[4-(pyridin-4-yl)phen...)Show SMILES Clc1ccc(cc1)C(=O)N1CCN(Cc2ccc(cc2)-c2ccncc2)CC1 Show InChI InChI=1S/C23H22ClN3O/c24-22-7-5-21(6-8-22)23(28)27-15-13-26(14-16-27)17-18-1-3-19(4-2-18)20-9-11-25-12-10-20/h1-12H,13-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of 2,3-oxidosqualene-lanosterol cyclase (unknown origin) |
Bioorg Med Chem 16: 6218-32 (2008)
Article DOI: 10.1016/j.bmc.2008.04.034
BindingDB Entry DOI: 10.7270/Q2M61K14 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128054
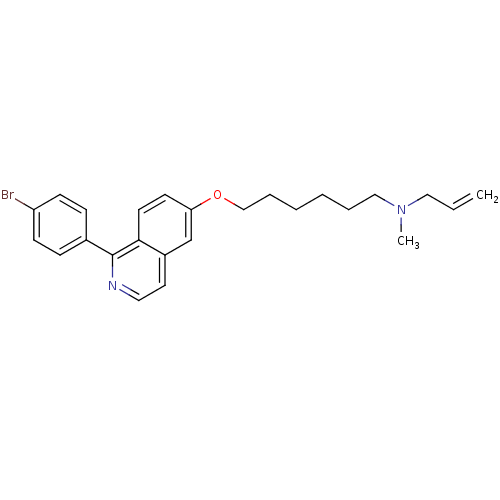
(Allyl-{6-[1-(4-bromo-phenyl)-isoquinolin-6-yloxy]-...)Show SMILES CN(CCCCCCOc1ccc2c(nccc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C25H29BrN2O/c1-3-16-28(2)17-6-4-5-7-18-29-23-12-13-24-21(19-23)14-15-27-25(24)20-8-10-22(26)11-9-20/h3,8-15,19H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.47 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of oxidosqualene cyclase |
Eur J Med Chem 43: 1462-8 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.019
BindingDB Entry DOI: 10.7270/Q2CR5VKG |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128054

(Allyl-{6-[1-(4-bromo-phenyl)-isoquinolin-6-yloxy]-...)Show SMILES CN(CCCCCCOc1ccc2c(nccc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C25H29BrN2O/c1-3-16-28(2)17-6-4-5-7-18-29-23-12-13-24-21(19-23)14-15-27-25(24)20-8-10-22(26)11-9-20/h3,8-15,19H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128054

(Allyl-{6-[1-(4-bromo-phenyl)-isoquinolin-6-yloxy]-...)Show SMILES CN(CCCCCCOc1ccc2c(nccc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C25H29BrN2O/c1-3-16-28(2)17-6-4-5-7-18-29-23-12-13-24-21(19-23)14-15-27-25(24)20-8-10-22(26)11-9-20/h3,8-15,19H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Torino
Curated by ChEMBL
| Assay Description
Inhibition of oxidosqualene cyclase |
J Med Chem 50: 5039-42 (2007)
Article DOI: 10.1021/jm0704651
BindingDB Entry DOI: 10.7270/Q2ZP47FF |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128054

(Allyl-{6-[1-(4-bromo-phenyl)-isoquinolin-6-yloxy]-...)Show SMILES CN(CCCCCCOc1ccc2c(nccc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C25H29BrN2O/c1-3-16-28(2)17-6-4-5-7-18-29-23-12-13-24-21(19-23)14-15-27-25(24)20-8-10-22(26)11-9-20/h3,8-15,19H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-Universität
Curated by ChEMBL
| Assay Description
Inhibitory activity against Oxidosqualene-lanosterol cyclase from human liver microsomes |
J Med Chem 46: 2083-92 (2003)
Article DOI: 10.1021/jm0211218
BindingDB Entry DOI: 10.7270/Q2VM4BM9 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128055
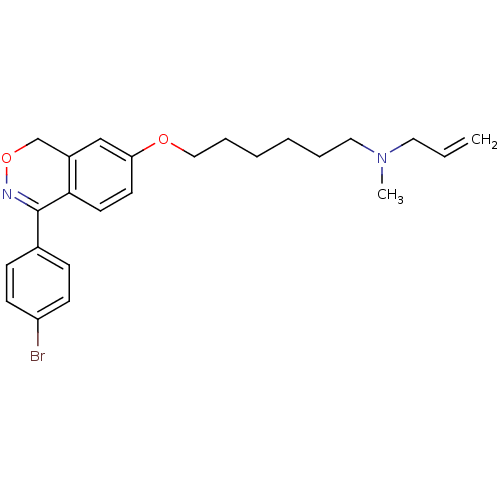
(Allyl-{6-[4-(4-bromo-phenyl)-1H-benzo[d][1,2]oxazi...)Show SMILES CN(CCCCCCOc1ccc2C(=NOCc2c1)c1ccc(Br)cc1)CC=C |c:13| Show InChI InChI=1S/C24H29BrN2O2/c1-3-14-27(2)15-6-4-5-7-16-28-22-12-13-23-20(17-22)18-29-26-24(23)19-8-10-21(25)11-9-19/h3,8-13,17H,1,4-7,14-16,18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.07 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of oxidosqualene cyclase |
Eur J Med Chem 43: 1462-8 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.019
BindingDB Entry DOI: 10.7270/Q2CR5VKG |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130788
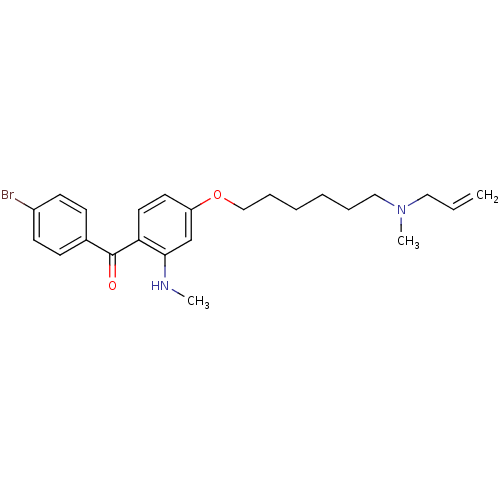
(CHEMBL116705 | CHEMBL611484 | {4-[6-(Allyl-methyl-...)Show SMILES CNc1cc(OCCCCCCN(C)CC=C)ccc1C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C24H31BrN2O2/c1-4-15-27(3)16-7-5-6-8-17-29-21-13-14-22(23(18-21)26-2)24(28)19-9-11-20(25)12-10-19/h4,9-14,18,26H,1,5-8,15-17H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Torino
Curated by ChEMBL
| Assay Description
Inhibition of oxidosqualene cyclase |
J Med Chem 50: 5039-42 (2007)
Article DOI: 10.1021/jm0704651
BindingDB Entry DOI: 10.7270/Q2ZP47FF |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128055

(Allyl-{6-[4-(4-bromo-phenyl)-1H-benzo[d][1,2]oxazi...)Show SMILES CN(CCCCCCOc1ccc2C(=NOCc2c1)c1ccc(Br)cc1)CC=C |c:13| Show InChI InChI=1S/C24H29BrN2O2/c1-3-14-27(2)15-6-4-5-7-16-28-22-12-13-23-20(17-22)18-29-26-24(23)19-8-10-21(25)11-9-19/h3,8-13,17H,1,4-7,14-16,18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130788

(CHEMBL116705 | CHEMBL611484 | {4-[6-(Allyl-methyl-...)Show SMILES CNc1cc(OCCCCCCN(C)CC=C)ccc1C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C24H31BrN2O2/c1-4-15-27(3)16-7-5-6-8-17-29-21-13-14-22(23(18-21)26-2)24(28)19-9-11-20(25)12-10-19/h4,9-14,18,26H,1,5-8,15-17H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128055

(Allyl-{6-[4-(4-bromo-phenyl)-1H-benzo[d][1,2]oxazi...)Show SMILES CN(CCCCCCOc1ccc2C(=NOCc2c1)c1ccc(Br)cc1)CC=C |c:13| Show InChI InChI=1S/C24H29BrN2O2/c1-3-14-27(2)15-6-4-5-7-16-28-22-12-13-23-20(17-22)18-29-26-24(23)19-8-10-21(25)11-9-19/h3,8-13,17H,1,4-7,14-16,18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-Universität
Curated by ChEMBL
| Assay Description
Inhibitory activity against Oxidosqualene-lanosterol cyclase from human liver microsomes |
J Med Chem 46: 2083-92 (2003)
Article DOI: 10.1021/jm0211218
BindingDB Entry DOI: 10.7270/Q2VM4BM9 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128055

(Allyl-{6-[4-(4-bromo-phenyl)-1H-benzo[d][1,2]oxazi...)Show SMILES CN(CCCCCCOc1ccc2C(=NOCc2c1)c1ccc(Br)cc1)CC=C |c:13| Show InChI InChI=1S/C24H29BrN2O2/c1-3-14-27(2)15-6-4-5-7-16-28-22-12-13-23-20(17-22)18-29-26-24(23)19-8-10-21(25)11-9-19/h3,8-13,17H,1,4-7,14-16,18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Torino
Curated by ChEMBL
| Assay Description
Inhibition of oxidosqualene cyclase |
J Med Chem 50: 5039-42 (2007)
Article DOI: 10.1021/jm0704651
BindingDB Entry DOI: 10.7270/Q2ZP47FF |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50133285

((E)-3-Carboxy-acrylateallyl-{6-[4-(4-chloro-benzoy...)Show SMILES C[NH+](CCCCCCOc1ccc(C(=O)c2ccc(Cl)cc2)c(F)c1)CC=C Show InChI InChI=1S/C23H27ClFNO2/c1-3-14-26(2)15-6-4-5-7-16-28-20-12-13-21(22(25)17-20)23(27)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Tested for Oxidosqualene-lanosterol cyclase inhibition in T. brucei |
J Med Chem 46: 4240-3 (2003)
Article DOI: 10.1021/jm034126t
BindingDB Entry DOI: 10.7270/Q26W99GV |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130783

(CHEMBL115020 | CHEMBL611485 | {4-[6-(Allyl-methyl-...)Show SMILES COc1cc(OCCCCCCN(C)CC=C)ccc1C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C24H30BrNO3/c1-4-15-26(2)16-7-5-6-8-17-29-21-13-14-22(23(18-21)28-3)24(27)19-9-11-20(25)12-10-19/h4,9-14,18H,1,5-8,15-17H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Torino
Curated by ChEMBL
| Assay Description
Inhibition of oxidosqualene cyclase |
J Med Chem 50: 5039-42 (2007)
Article DOI: 10.1021/jm0704651
BindingDB Entry DOI: 10.7270/Q2ZP47FF |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50133285

((E)-3-Carboxy-acrylateallyl-{6-[4-(4-chloro-benzoy...)Show SMILES C[NH+](CCCCCCOc1ccc(C(=O)c2ccc(Cl)cc2)c(F)c1)CC=C Show InChI InChI=1S/C23H27ClFNO2/c1-3-14-26(2)15-6-4-5-7-16-28-20-12-13-21(22(25)17-20)23(27)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Tested for Oxidosqualene-lanosterol cyclase inhibition in P. carinii |
J Med Chem 46: 4240-3 (2003)
Article DOI: 10.1021/jm034126t
BindingDB Entry DOI: 10.7270/Q26W99GV |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130783

(CHEMBL115020 | CHEMBL611485 | {4-[6-(Allyl-methyl-...)Show SMILES COc1cc(OCCCCCCN(C)CC=C)ccc1C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C24H30BrNO3/c1-4-15-26(2)16-7-5-6-8-17-29-21-13-14-22(23(18-21)28-3)24(27)19-9-11-20(25)12-10-19/h4,9-14,18H,1,5-8,15-17H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50255431
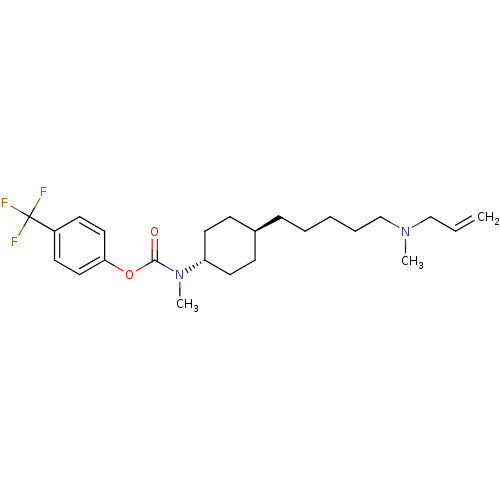
(4-(trifluoromethyl)phenyl 4-(5-(allyl(methyl)amino...)Show SMILES CN(CCCCC[C@H]1CC[C@@H](CC1)N(C)C(=O)Oc1ccc(cc1)C(F)(F)F)CC=C |r,wU:10.13,wD:7.6,(-5.96,-29.96,;-5.96,-31.5,;-4.63,-32.27,;-3.29,-31.5,;-1.96,-32.27,;-.63,-31.5,;.71,-32.27,;2.04,-31.5,;3.36,-32.27,;4.69,-31.51,;4.7,-29.97,;3.37,-29.19,;2.03,-29.96,;6.04,-29.2,;6.04,-27.66,;7.37,-29.98,;7.36,-31.52,;8.71,-29.21,;10.04,-29.99,;10,-31.53,;11.32,-32.32,;12.67,-31.58,;12.7,-30.03,;11.38,-29.24,;13.99,-32.38,;15.32,-33.15,;13.2,-33.7,;14.77,-31.05,;-7.3,-32.27,;-8.63,-31.5,;-9.96,-32.27,)| Show InChI InChI=1S/C24H35F3N2O2/c1-4-17-28(2)18-7-5-6-8-19-9-13-21(14-10-19)29(3)23(30)31-22-15-11-20(12-16-22)24(25,26)27/h4,11-12,15-16,19,21H,1,5-10,13-14,17-18H2,2-3H3/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL)
Curated by ChEMBL
| Assay Description
Inhibition of human liver microsome 2,3-OSC after 1 hr by Silica gel plate phosphor imaging |
J Med Chem 55: 4990-5002 (2012)
Article DOI: 10.1021/jm300256z
BindingDB Entry DOI: 10.7270/Q2BZ674B |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128050
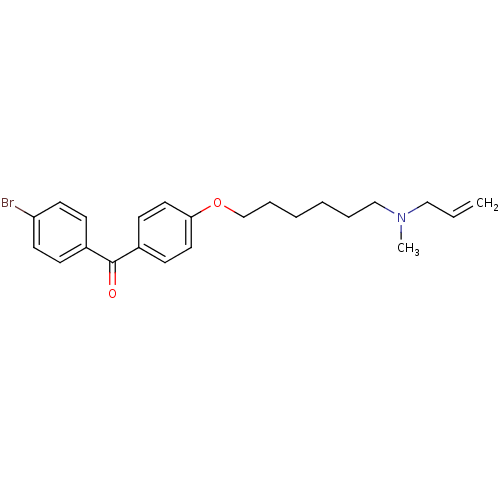
(CHEMBL114259 | CHEMBL416694 | N-allyl-6-(4-(4-brom...)Show InChI InChI=1S/C23H28BrNO2/c1-3-16-25(2)17-6-4-5-7-18-27-22-14-10-20(11-15-22)23(26)19-8-12-21(24)13-9-19/h3,8-15H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.37 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of oxidosqualene cyclase |
Eur J Med Chem 43: 1462-8 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.019
BindingDB Entry DOI: 10.7270/Q2CR5VKG |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128050

(CHEMBL114259 | CHEMBL416694 | N-allyl-6-(4-(4-brom...)Show InChI InChI=1S/C23H28BrNO2/c1-3-16-25(2)17-6-4-5-7-18-27-22-14-10-20(11-15-22)23(26)19-8-12-21(24)13-9-19/h3,8-15H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128050

(CHEMBL114259 | CHEMBL416694 | N-allyl-6-(4-(4-brom...)Show InChI InChI=1S/C23H28BrNO2/c1-3-16-25(2)17-6-4-5-7-18-27-22-14-10-20(11-15-22)23(26)19-8-12-21(24)13-9-19/h3,8-15H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-Universität
Curated by ChEMBL
| Assay Description
Inhibitory activity against Oxidosqualene-lanosterol cyclase from human liver microsomes |
J Med Chem 46: 2083-92 (2003)
Article DOI: 10.1021/jm0211218
BindingDB Entry DOI: 10.7270/Q2VM4BM9 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128050

(CHEMBL114259 | CHEMBL416694 | N-allyl-6-(4-(4-brom...)Show InChI InChI=1S/C23H28BrNO2/c1-3-16-25(2)17-6-4-5-7-18-27-22-14-10-20(11-15-22)23(26)19-8-12-21(24)13-9-19/h3,8-15H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Torino
Curated by ChEMBL
| Assay Description
Inhibition of oxidosqualene cyclase |
J Med Chem 50: 5039-42 (2007)
Article DOI: 10.1021/jm0704651
BindingDB Entry DOI: 10.7270/Q2ZP47FF |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130789

(Allyl-{6-[3-(4-bromo-phenyl)-benzo[b]thiophen-6-yl...)Show SMILES CN(CCCCCCOc1ccc2c(csc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C24H28BrNOS/c1-3-14-26(2)15-6-4-5-7-16-27-21-12-13-22-23(18-28-24(22)17-21)19-8-10-20(25)11-9-19/h3,8-13,17-18H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128065

(CHEMBL304858 | CHEMBL324162 | N-allyl-6-(4-(4-brom...)Show SMILES CN(CCCCCCOc1ccc(C(=O)c2ccc(Br)cc2)c(F)c1)CC=C Show InChI InChI=1S/C23H27BrFNO2/c1-3-14-26(2)15-6-4-5-7-16-28-20-12-13-21(22(25)17-20)23(27)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL)
Curated by ChEMBL
| Assay Description
Inhibition of human liver microsome 2,3-OSC after 1 hr by Silica gel plate phosphor imaging |
J Med Chem 55: 4990-5002 (2012)
Article DOI: 10.1021/jm300256z
BindingDB Entry DOI: 10.7270/Q2BZ674B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50271622
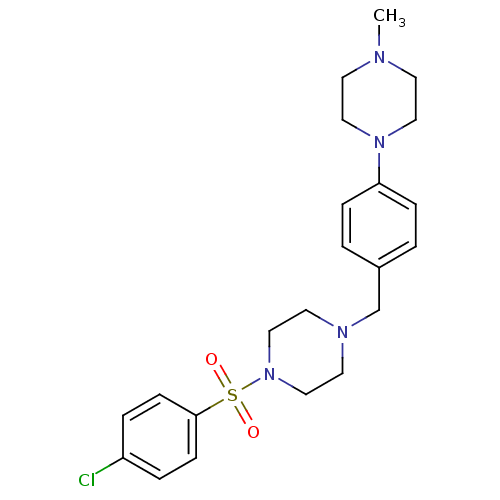
(1-[4-Chlorophenylsulfonyl]-4-[4-(4-methylpiperazin...)Show SMILES CN1CCN(CC1)c1ccc(CN2CCN(CC2)S(=O)(=O)c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C22H29ClN4O2S/c1-24-10-14-26(15-11-24)21-6-2-19(3-7-21)18-25-12-16-27(17-13-25)30(28,29)22-8-4-20(23)5-9-22/h2-9H,10-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of 2,3-oxidosqualene-lanosterol cyclase (unknown origin) |
Bioorg Med Chem 16: 6218-32 (2008)
Article DOI: 10.1016/j.bmc.2008.04.034
BindingDB Entry DOI: 10.7270/Q2M61K14 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130786
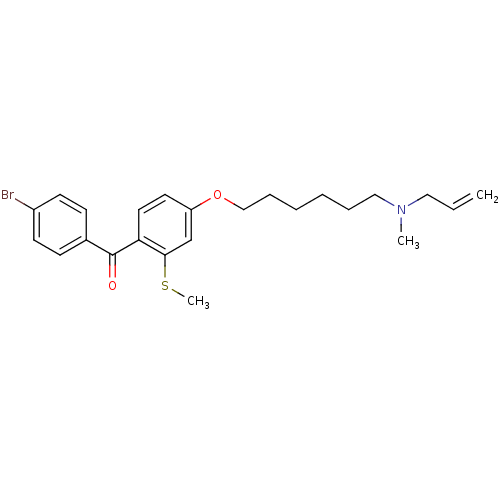
(CHEMBL115923 | CHEMBL611486 | {4-[6-(Allyl-methyl-...)Show SMILES CSc1cc(OCCCCCCN(C)CC=C)ccc1C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C24H30BrNO2S/c1-4-15-26(2)16-7-5-6-8-17-28-21-13-14-22(23(18-21)29-3)24(27)19-9-11-20(25)12-10-19/h4,9-14,18H,1,5-8,15-17H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130786

(CHEMBL115923 | CHEMBL611486 | {4-[6-(Allyl-methyl-...)Show SMILES CSc1cc(OCCCCCCN(C)CC=C)ccc1C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C24H30BrNO2S/c1-4-15-26(2)16-7-5-6-8-17-28-21-13-14-22(23(18-21)29-3)24(27)19-9-11-20(25)12-10-19/h4,9-14,18H,1,5-8,15-17H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Torino
Curated by ChEMBL
| Assay Description
Inhibition of oxidosqualene cyclase |
J Med Chem 50: 5039-42 (2007)
Article DOI: 10.1021/jm0704651
BindingDB Entry DOI: 10.7270/Q2ZP47FF |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130774
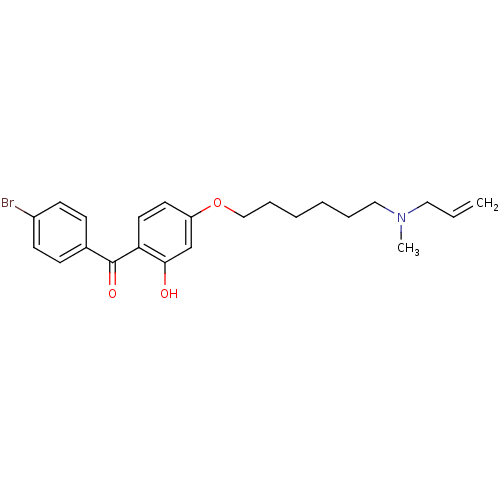
(CHEMBL115375 | CHEMBL611757 | {4-[6-(Allyl-methyl-...)Show SMILES CN(CCCCCCOc1ccc(C(=O)c2ccc(Br)cc2)c(O)c1)CC=C Show InChI InChI=1S/C23H28BrNO3/c1-3-14-25(2)15-6-4-5-7-16-28-20-12-13-21(22(26)17-20)23(27)18-8-10-19(24)11-9-18/h3,8-13,17,26H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130774

(CHEMBL115375 | CHEMBL611757 | {4-[6-(Allyl-methyl-...)Show SMILES CN(CCCCCCOc1ccc(C(=O)c2ccc(Br)cc2)c(O)c1)CC=C Show InChI InChI=1S/C23H28BrNO3/c1-3-14-25(2)15-6-4-5-7-16-28-20-12-13-21(22(26)17-20)23(27)18-8-10-19(24)11-9-18/h3,8-13,17,26H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Torino
Curated by ChEMBL
| Assay Description
Inhibition of oxidosqualene cyclase |
J Med Chem 50: 5039-42 (2007)
Article DOI: 10.1021/jm0704651
BindingDB Entry DOI: 10.7270/Q2ZP47FF |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128072
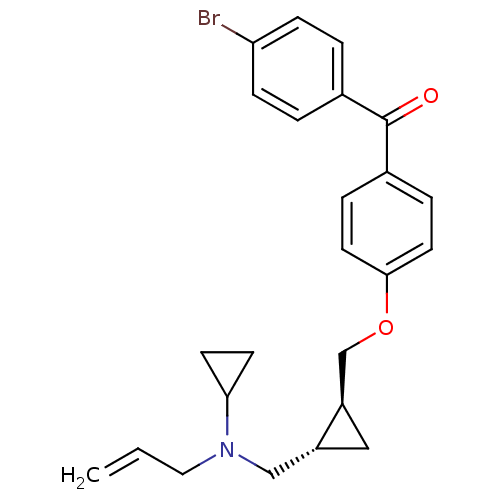
((4-{2-[(Allyl-cyclopropyl-amino)-methyl]-cycloprop...)Show SMILES Brc1ccc(cc1)C(=O)c1ccc(OC[C@H]2C[C@@H]2CN(CC=C)C2CC2)cc1 Show InChI InChI=1S/C24H26BrNO2/c1-2-13-26(22-9-10-22)15-19-14-20(19)16-28-23-11-5-18(6-12-23)24(27)17-3-7-21(25)8-4-17/h2-8,11-12,19-20,22H,1,9-10,13-16H2/t19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.46 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of oxidosqualene cyclase |
Eur J Med Chem 43: 1462-8 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.019
BindingDB Entry DOI: 10.7270/Q2CR5VKG |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128065

(CHEMBL304858 | CHEMBL324162 | N-allyl-6-(4-(4-brom...)Show SMILES CN(CCCCCCOc1ccc(C(=O)c2ccc(Br)cc2)c(F)c1)CC=C Show InChI InChI=1S/C23H27BrFNO2/c1-3-14-26(2)15-6-4-5-7-16-28-20-12-13-21(22(25)17-20)23(27)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128065

(CHEMBL304858 | CHEMBL324162 | N-allyl-6-(4-(4-brom...)Show SMILES CN(CCCCCCOc1ccc(C(=O)c2ccc(Br)cc2)c(F)c1)CC=C Show InChI InChI=1S/C23H27BrFNO2/c1-3-14-26(2)15-6-4-5-7-16-28-20-12-13-21(22(25)17-20)23(27)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Torino
Curated by ChEMBL
| Assay Description
Inhibition of oxidosqualene cyclase |
J Med Chem 50: 5039-42 (2007)
Article DOI: 10.1021/jm0704651
BindingDB Entry DOI: 10.7270/Q2ZP47FF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128065

(CHEMBL304858 | CHEMBL324162 | N-allyl-6-(4-(4-brom...)Show SMILES CN(CCCCCCOc1ccc(C(=O)c2ccc(Br)cc2)c(F)c1)CC=C Show InChI InChI=1S/C23H27BrFNO2/c1-3-14-26(2)15-6-4-5-7-16-28-20-12-13-21(22(25)17-20)23(27)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-Universität
Curated by ChEMBL
| Assay Description
Inhibitory activity against Oxidosqualene-lanosterol cyclase from human liver microsomes |
J Med Chem 46: 2083-92 (2003)
Article DOI: 10.1021/jm0211218
BindingDB Entry DOI: 10.7270/Q2VM4BM9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130775
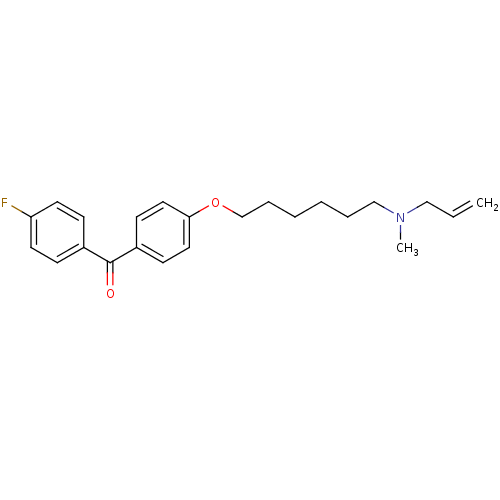
(CHEMBL554209 | CHEMBL611758 | {4-[6-(Allyl-methyl-...)Show InChI InChI=1S/C23H28FNO2/c1-3-16-25(2)17-6-4-5-7-18-27-22-14-10-20(11-15-22)23(26)19-8-12-21(24)13-9-19/h3,8-15H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130775

(CHEMBL554209 | CHEMBL611758 | {4-[6-(Allyl-methyl-...)Show InChI InChI=1S/C23H28FNO2/c1-3-16-25(2)17-6-4-5-7-18-27-22-14-10-20(11-15-22)23(26)19-8-12-21(24)13-9-19/h3,8-15H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Torino
Curated by ChEMBL
| Assay Description
Inhibition of oxidosqualene cyclase |
J Med Chem 50: 5039-42 (2007)
Article DOI: 10.1021/jm0704651
BindingDB Entry DOI: 10.7270/Q2ZP47FF |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128065

(CHEMBL304858 | CHEMBL324162 | N-allyl-6-(4-(4-brom...)Show SMILES CN(CCCCCCOc1ccc(C(=O)c2ccc(Br)cc2)c(F)c1)CC=C Show InChI InChI=1S/C23H27BrFNO2/c1-3-14-26(2)15-6-4-5-7-16-28-20-12-13-21(22(25)17-20)23(27)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His6-tagged OSC expressed in Pichia pastoris GS115 using 2,3-oxidosqualene as substrate after 90 mins by 1H NMR method |
J Med Chem 61: 5047-5053 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00484
BindingDB Entry DOI: 10.7270/Q2NK3HN7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50423108
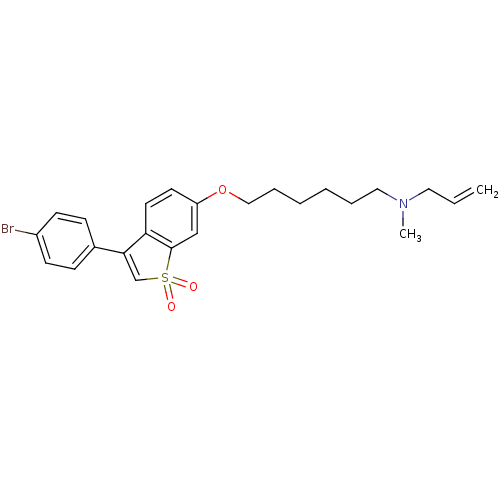
(CHEMBL612024)Show SMILES CN(CCCCCCOc1ccc2C(=CS(=O)(=O)c2c1)c1ccc(Br)cc1)CC=C |c:13| Show InChI InChI=1S/C24H28BrNO3S/c1-3-14-26(2)15-6-4-5-7-16-29-21-12-13-22-23(18-30(27,28)24(22)17-21)19-8-10-20(25)11-9-19/h3,8-13,17-18H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Torino
Curated by ChEMBL
| Assay Description
Inhibition of oxidosqualene cyclase |
J Med Chem 50: 5039-42 (2007)
Article DOI: 10.1021/jm0704651
BindingDB Entry DOI: 10.7270/Q2ZP47FF |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50394835
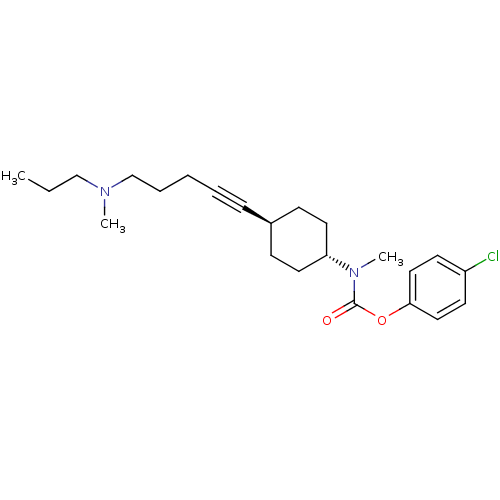
(CHEMBL2164235)Show SMILES CCCN(C)CCCC#C[C@H]1CC[C@@H](CC1)N(C)C(=O)Oc1ccc(Cl)cc1 |r,wU:10.9,wD:13.16,(22.91,-26.91,;24.24,-26.14,;24.24,-24.6,;25.57,-23.83,;25.57,-22.29,;26.9,-24.59,;28.23,-23.82,;29.57,-24.58,;30.9,-23.81,;32.24,-23.03,;33.57,-22.26,;33.57,-20.72,;34.9,-19.94,;36.23,-20.72,;36.23,-22.26,;34.9,-23.02,;37.56,-19.95,;37.57,-18.41,;38.89,-20.73,;38.89,-22.27,;40.23,-19.96,;41.56,-20.74,;41.56,-22.28,;42.89,-23.05,;44.23,-22.29,;45.56,-23.06,;44.23,-20.74,;42.89,-19.97,)| Show InChI InChI=1S/C23H33ClN2O2/c1-4-17-25(2)18-7-5-6-8-19-9-13-21(14-10-19)26(3)23(27)28-22-15-11-20(24)12-16-22/h11-12,15-16,19,21H,4-5,7,9-10,13-14,17-18H2,1-3H3/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL)
Curated by ChEMBL
| Assay Description
Inhibition of human liver microsome 2,3-OSC after 1 hr by Silica gel plate phosphor imaging |
J Med Chem 55: 4990-5002 (2012)
Article DOI: 10.1021/jm300256z
BindingDB Entry DOI: 10.7270/Q2BZ674B |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130772
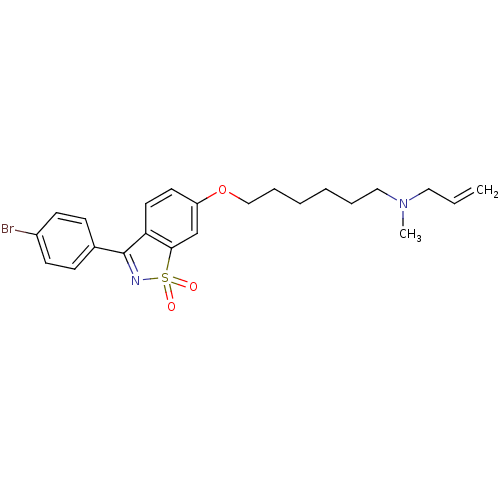
(Allyl-{6-[3-(4-bromo-phenyl)-1,1-dioxo-1H-1lambda*...)Show SMILES CN(CCCCCCOc1ccc2C(=NS(=O)(=O)c2c1)c1ccc(Br)cc1)CC=C |c:13| Show InChI InChI=1S/C23H27BrN2O3S/c1-3-14-26(2)15-6-4-5-7-16-29-20-12-13-21-22(17-20)30(27,28)25-23(21)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130784

(Allyl-{6-[1-(4-bromo-phenyl)-3,4-dihydro-isoquinol...)Show SMILES CN(CCCCCCOc1ccc2C(=NCCc2c1)c1ccc(Br)cc1)CC=C |c:13| Show InChI InChI=1S/C25H31BrN2O/c1-3-16-28(2)17-6-4-5-7-18-29-23-12-13-24-21(19-23)14-15-27-25(24)20-8-10-22(26)11-9-20/h3,8-13,19H,1,4-7,14-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Torino
Curated by ChEMBL
| Assay Description
Inhibition of oxidosqualene cyclase |
J Med Chem 50: 5039-42 (2007)
Article DOI: 10.1021/jm0704651
BindingDB Entry DOI: 10.7270/Q2ZP47FF |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130784

(Allyl-{6-[1-(4-bromo-phenyl)-3,4-dihydro-isoquinol...)Show SMILES CN(CCCCCCOc1ccc2C(=NCCc2c1)c1ccc(Br)cc1)CC=C |c:13| Show InChI InChI=1S/C25H31BrN2O/c1-3-16-28(2)17-6-4-5-7-18-29-23-12-13-24-21(19-23)14-15-27-25(24)20-8-10-22(26)11-9-20/h3,8-13,19H,1,4-7,14-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128070
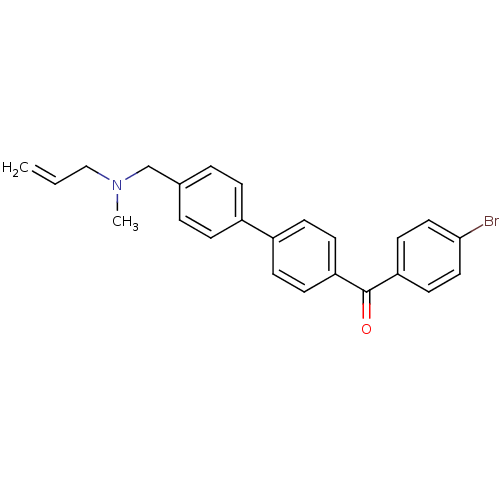
((4''-{[ALLYL(METHYL)AMINO]METHYL}-1,1''-BIPHENYL-4...)Show SMILES CN(CC=C)Cc1ccc(cc1)-c1ccc(cc1)C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C24H22BrNO/c1-3-16-26(2)17-18-4-6-19(7-5-18)20-8-10-21(11-9-20)24(27)22-12-14-23(25)15-13-22/h3-15H,1,16-17H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL)
Curated by ChEMBL
| Assay Description
Inhibition of human liver microsome 2,3-OSC after 1 hr by Silica gel plate phosphor imaging |
J Med Chem 55: 4990-5002 (2012)
Article DOI: 10.1021/jm300256z
BindingDB Entry DOI: 10.7270/Q2BZ674B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data