 Found 157 hits of ic50 data for polymerid = 1579
Found 157 hits of ic50 data for polymerid = 1579 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50594973
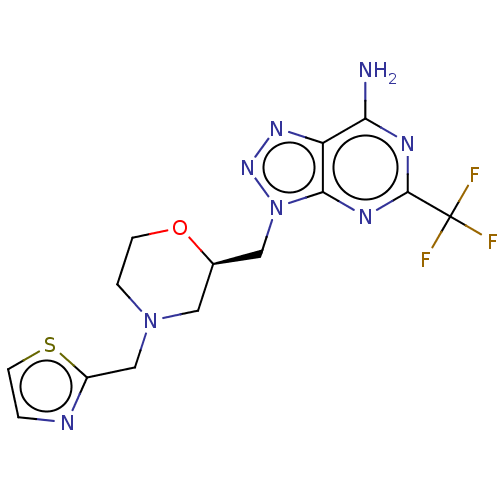
(CHEMBL5169811)Show SMILES Nc1nc(nc2n(C[C@H]3CN(Cc4nccs4)CCO3)nnc12)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114170
BindingDB Entry DOI: 10.7270/Q2RX9H3Q |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50594973

(CHEMBL5169811)Show SMILES Nc1nc(nc2n(C[C@H]3CN(Cc4nccs4)CCO3)nnc12)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human EP1 receptor expressed in CHO cells receptor by NFTA reporter gene assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50594973

(CHEMBL5169811)Show SMILES Nc1nc(nc2n(C[C@H]3CN(Cc4nccs4)CCO3)nnc12)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic affinity tested against isolated Rat Spleen Alpha-1B adrenergic receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50344018
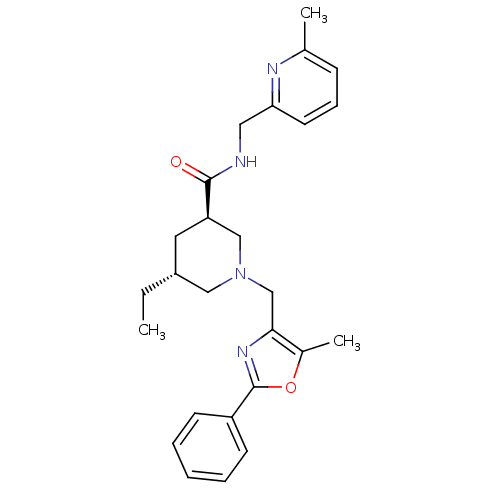
(CHEMBL1779984 | trans-(3R,5R)-5-ethyl-1-((5-methyl...)Show SMILES CC[C@@H]1C[C@H](CN(Cc2nc(oc2C)-c2ccccc2)C1)C(=O)NCc1cccc(C)n1 |r| Show InChI InChI=1S/C26H32N4O2/c1-4-20-13-22(25(31)27-14-23-12-8-9-18(2)28-23)16-30(15-20)17-24-19(3)32-26(29-24)21-10-6-5-7-11-21/h5-12,20,22H,4,13-17H2,1-3H3,(H,27,31)/t20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50344017

(1-((5-methyl-2-phenyloxazol-4-yl)methyl)-N-((6-met...)Show SMILES Cc1oc(nc1CN1CCCC(C1)C(=O)NCc1cccc(C)n1)-c1ccccc1 Show InChI InChI=1S/C24H28N4O2/c1-17-8-6-12-21(26-17)14-25-23(29)20-11-7-13-28(15-20)16-22-18(2)30-24(27-22)19-9-4-3-5-10-19/h3-6,8-10,12,20H,7,11,13-16H2,1-2H3,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50344016

(2-((5-methyl-2-phenyloxazol-4-yl)methyl)-N-((6-met...)Show SMILES Cc1oc(nc1CN1CC(C(=O)NCc2cccc(C)n2)c2ccccc2C1)-c1ccccc1 Show InChI InChI=1S/C28H28N4O2/c1-19-9-8-13-23(30-19)15-29-27(33)25-17-32(16-22-12-6-7-14-24(22)25)18-26-20(2)34-28(31-26)21-10-4-3-5-11-21/h3-14,25H,15-18H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50390334

(CHEMBL2070735)Show SMILES Cc1nc(N)c2nnn(C[C@H]3CN(Cc4nccs4)CCO3)c2n1 |r| Show InChI InChI=1S/C14H18N8OS/c1-9-17-13(15)12-14(18-9)22(20-19-12)7-10-6-21(3-4-23-10)8-11-16-2-5-24-11/h2,5,10H,3-4,6-8H2,1H3,(H2,15,17,18)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A |
Bioorg Med Chem Lett 22: 5721-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.079
BindingDB Entry DOI: 10.7270/Q2J67J0X |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50555231

(CHEMBL4762103)Show SMILES C[C@@H](c1cc(OCC(F)F)ccn1)n1cnc2c(N)nc(Cl)nc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) expressed in Escherichia coli BL21 using [3H]-cAMP substrate incubated for 15 mins by liq... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01573
BindingDB Entry DOI: 10.7270/Q2CZ3BV5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM135584
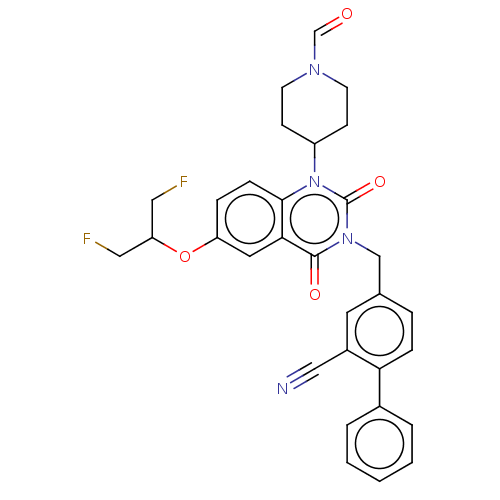
(US8846654, 294)Show SMILES FCC(CF)Oc1ccc2n(C3CCN(CC3)C=O)c(=O)n(Cc3ccc(-c4ccccc4)c(c3)C#N)c(=O)c2c1 Show InChI InChI=1S/C31H28F2N4O4/c32-16-26(17-33)41-25-7-9-29-28(15-25)30(39)36(31(40)37(29)24-10-12-35(20-38)13-11-24)19-21-6-8-27(23(14-21)18-34)22-4-2-1-3-5-22/h1-9,14-15,20,24,26H,10-13,16-17,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Sanofi
US Patent
| Assay Description
By using for PDE8 an enzymatic assay equivalent to that described for PDE7. PDE7 Enzymatic Assay: The assay is carried out in 1.5 ml Eppendorf tube... |
US Patent US8846654 (2014)
BindingDB Entry DOI: 10.7270/Q21V5CPZ |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50555230
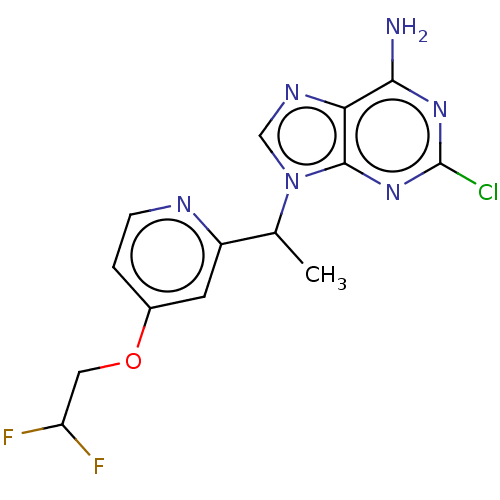
(CHEMBL4753446) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) expressed in Escherichia coli BL21 using [3H]-cAMP substrate incubated for 15 mins by liq... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01573
BindingDB Entry DOI: 10.7270/Q2CZ3BV5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50555224
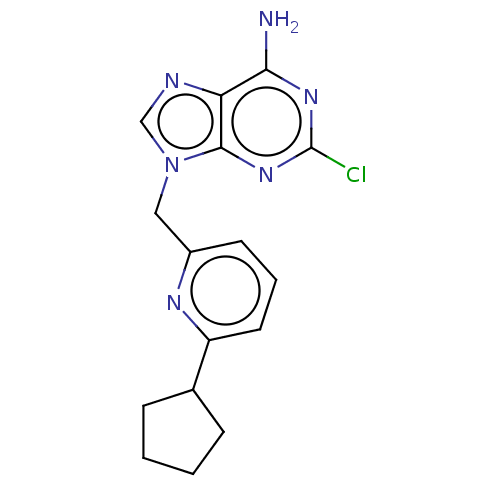
(CHEMBL4790482) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) expressed in Escherichia coli BL21 using [3H]-cAMP substrate incubated for 15 mins by liq... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01573
BindingDB Entry DOI: 10.7270/Q2CZ3BV5 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50555221

(CHEMBL4800434) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) expressed in Escherichia coli BL21 using [3H]-cAMP substrate incubated for 15 mins by liq... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01573
BindingDB Entry DOI: 10.7270/Q2CZ3BV5 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM135577

(US8846654, 11)Show SMILES COc1ccc(Cn2c(=O)n(C3CCN(CC3)C=O)c3ccc(OC(CF)CF)cc3c2=O)cc1OC Show InChI InChI=1S/C26H29F2N3O6/c1-35-23-6-3-17(11-24(23)36-2)15-30-25(33)21-12-19(37-20(13-27)14-28)4-5-22(21)31(26(30)34)18-7-9-29(16-32)10-8-18/h3-6,11-12,16,18,20H,7-10,13-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Sanofi
US Patent
| Assay Description
By using for PDE8 an enzymatic assay equivalent to that described for PDE7. PDE7 Enzymatic Assay: The assay is carried out in 1.5 ml Eppendorf tube... |
US Patent US8846654 (2014)
BindingDB Entry DOI: 10.7270/Q21V5CPZ |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50555225
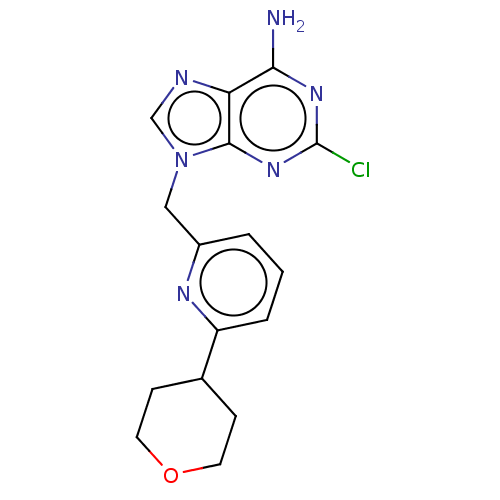
(CHEMBL4789101) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) expressed in Escherichia coli BL21 using [3H]-cAMP substrate incubated for 15 mins by liq... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01573
BindingDB Entry DOI: 10.7270/Q2CZ3BV5 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50555220
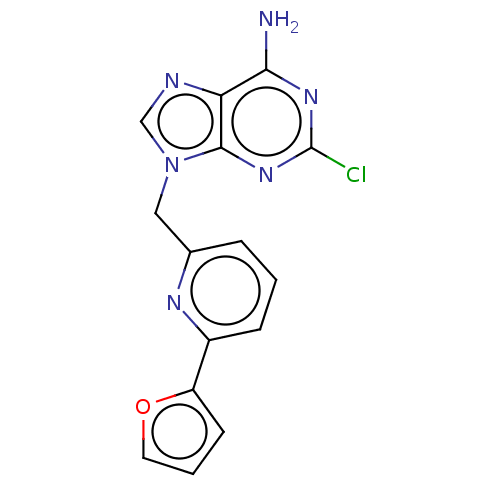
(CHEMBL4778928) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) expressed in Escherichia coli BL21 using [3H]-cAMP substrate incubated for 15 mins by liq... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01573
BindingDB Entry DOI: 10.7270/Q2CZ3BV5 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50555222
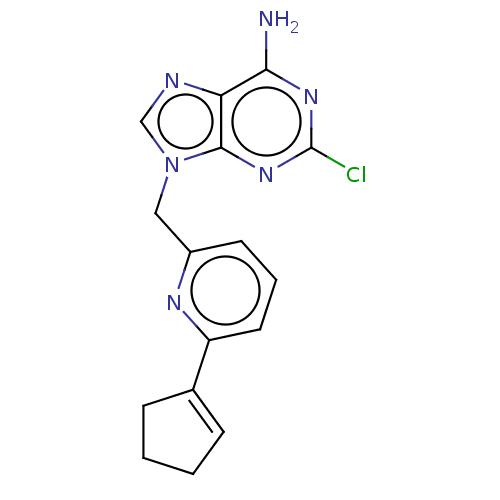
(CHEMBL4798107)Show SMILES Nc1nc(Cl)nc2n(Cc3cccc(n3)C3=CCCC3)cnc12 |t:16| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) expressed in Escherichia coli BL21 using [3H]-cAMP substrate incubated for 15 mins by liq... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01573
BindingDB Entry DOI: 10.7270/Q2CZ3BV5 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50555223

(CHEMBL4745958)Show SMILES Nc1nc(Cl)nc2n(Cc3cccc(n3)C3=CCOCC3)cnc12 |t:16| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) expressed in Escherichia coli BL21 using [3H]-cAMP substrate incubated for 15 mins by liq... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01573
BindingDB Entry DOI: 10.7270/Q2CZ3BV5 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50407428
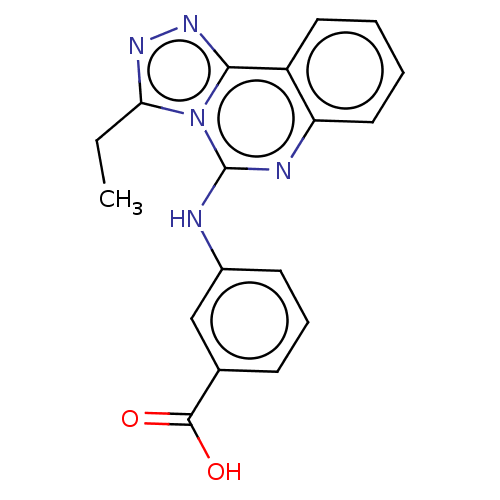
(CHEMBL5288859)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)CC1(S)CCCCC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C50H76N14O12S2/c1-3-27(2)41(63-45(73)33(21-28-24-56-30-12-6-5-11-29(28)30)58-39(67)23-50(78)17-7-4-8-18-50)47(75)60-32(15-16-40(68)69)43(71)61-34(22-37(51)65)44(72)62-35(26-77)48(76)64-20-10-14-36(64)46(74)59-31(13-9-19-55-49(53)54)42(70)57-25-38(52)66/h5-6,11-12,24,27,31-36,41,56,77-78H,3-4,7-10,13-23,25-26H2,1-2H3,(H2,51,65)(H2,52,66)(H,57,70)(H,58,67)(H,59,74)(H,60,75)(H,61,71)(H,62,72)(H,63,73)(H,68,69)(H4,53,54,55)/t27-,31-,32-,33+,34-,35-,36-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic affinity tested against isolated Rat Thoracic Aorta Alpha-1D adrenergic receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50555229
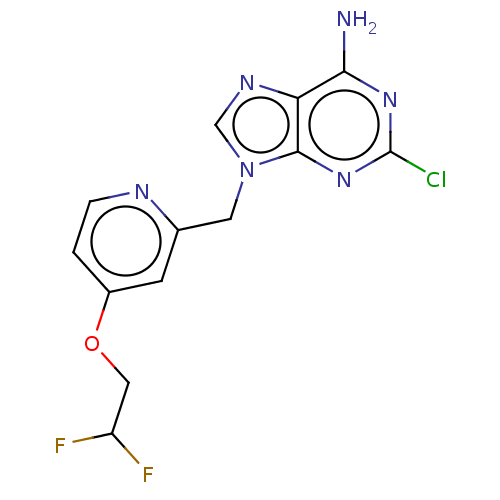
(CHEMBL4757863) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) expressed in Escherichia coli BL21 using [3H]-cAMP substrate incubated for 15 mins by liq... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01573
BindingDB Entry DOI: 10.7270/Q2CZ3BV5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM135583
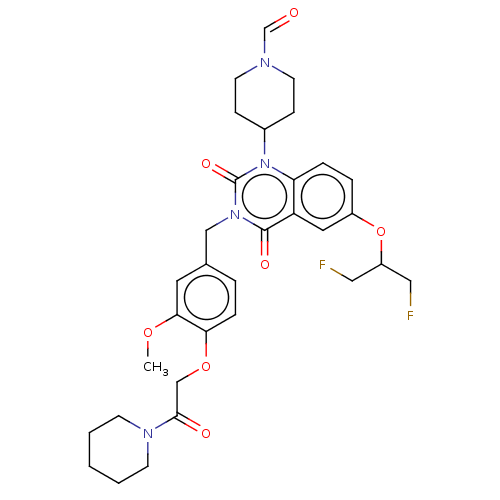
(US8846654, 251)Show SMILES COc1cc(Cn2c(=O)n(C3CCN(CC3)C=O)c3ccc(OC(CF)CF)cc3c2=O)ccc1OCC(=O)N1CCCCC1 Show InChI InChI=1S/C32H38F2N4O7/c1-43-29-15-22(5-8-28(29)44-20-30(40)36-11-3-2-4-12-36)19-37-31(41)26-16-24(45-25(17-33)18-34)6-7-27(26)38(32(37)42)23-9-13-35(21-39)14-10-23/h5-8,15-16,21,23,25H,2-4,9-14,17-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Sanofi
US Patent
| Assay Description
By using for PDE8 an enzymatic assay equivalent to that described for PDE7. PDE7 Enzymatic Assay: The assay is carried out in 1.5 ml Eppendorf tube... |
US Patent US8846654 (2014)
BindingDB Entry DOI: 10.7270/Q21V5CPZ |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50555227

(CHEMBL4747327) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) expressed in Escherichia coli BL21 using [3H]-cAMP substrate incubated for 15 mins by liq... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01573
BindingDB Entry DOI: 10.7270/Q2CZ3BV5 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM23620
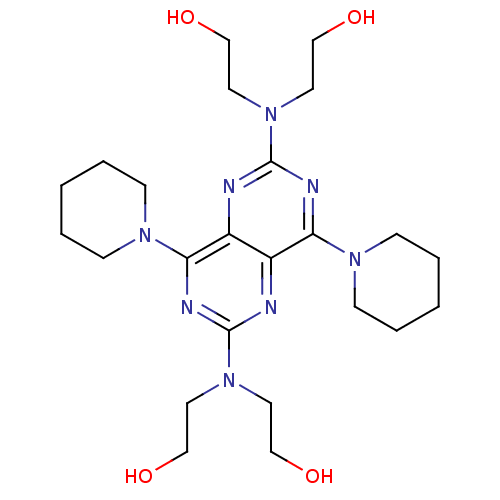
(2-({6-[bis(2-hydroxyethyl)amino]-4,8-bis(piperidin...)Show SMILES OCCN(CCO)c1nc(N2CCCCC2)c2nc(nc(N3CCCCC3)c2n1)N(CCO)CCO Show InChI InChI=1S/C24H40N8O4/c33-15-11-31(12-16-34)23-26-20-19(21(27-23)29-7-3-1-4-8-29)25-24(32(13-17-35)14-18-36)28-22(20)30-9-5-2-6-10-30/h33-36H,1-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | 7.5 | 24 |
University of North Carolina
| Assay Description
Enzymatic activities were assayed using [3H] cAMP and [3H]cGMP as substrate. |
J Biol Chem 287: 11788-97 (2012)
Article DOI: 10.1074/jbc.M111.326777
BindingDB Entry DOI: 10.7270/Q2K9364D |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50357870

(CHEMBL1916130)Show SMILES COc1cc(cc2c1nc(C)c1c(C)nc(-c3ccncc3C)n21)C(F)(F)F Show InChI InChI=1S/C20H17F3N4O/c1-10-9-24-6-5-14(10)19-26-12(3)18-11(2)25-17-15(27(18)19)7-13(20(21,22)23)8-16(17)28-4/h5-9H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE8A using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50555228

(CHEMBL4750412) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) expressed in Escherichia coli BL21 using [3H]-cAMP substrate incubated for 15 mins by liq... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01573
BindingDB Entry DOI: 10.7270/Q2CZ3BV5 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50575152
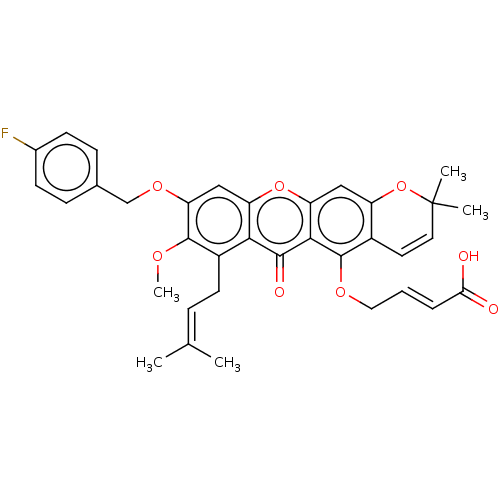
(CHEMBL4877549)Show SMILES [#6]-[#8]-c1c(-[#8]-[#6]-c2ccc(F)cc2)cc2oc3cc4-[#8]C([#6])([#6])[#6]=[#6]-c4c(-[#8]-[#6]\[#6]=[#6]\[#6](-[#8])=O)c3c(=O)c2c1-[#6]\[#6]=[#6](\[#6])-[#6] |c:24| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE8A1 (480-828) (unknown origin) expressed in Escherichia coli BL21 assessed as using [3H]-cAMP as substrate measured for 15 mins by l... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01085
BindingDB Entry DOI: 10.7270/Q29G5RM9 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50603811
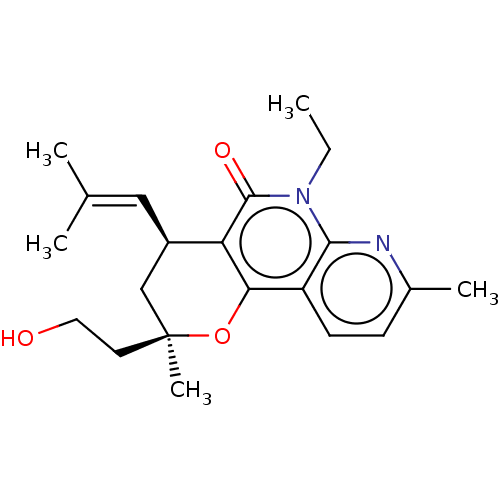
(CHEMBL5171560)Show SMILES [#6]-[#6]-n1c2nc(-[#6])ccc2c2-[#8][C@@]([#6])([#6]-[#6]-[#8])[#6]-[#6@@H](\[#6]=[#6](\[#6])-[#6])-c2c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02058
BindingDB Entry DOI: 10.7270/Q2DF6W9W |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50555226

(CHEMBL4786234) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) expressed in Escherichia coli BL21 using [3H]-cAMP substrate incubated for 15 mins by liq... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01573
BindingDB Entry DOI: 10.7270/Q2CZ3BV5 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50357872
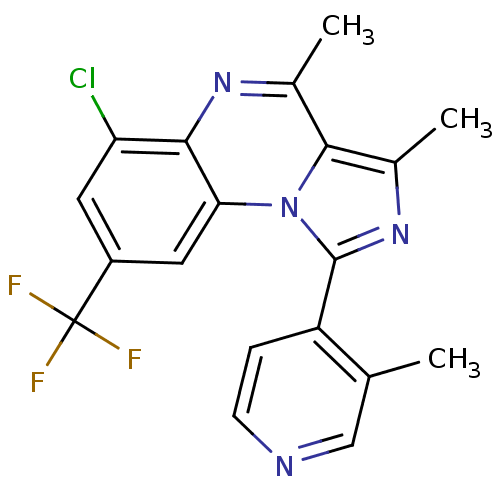
(CHEMBL1916132)Show SMILES Cc1nc(-c2ccncc2C)n2c1c(C)nc1c(Cl)cc(cc21)C(F)(F)F Show InChI InChI=1S/C19H14ClF3N4/c1-9-8-24-5-4-13(9)18-26-11(3)17-10(2)25-16-14(20)6-12(19(21,22)23)7-15(16)27(17)18/h4-8H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE8A using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50357826
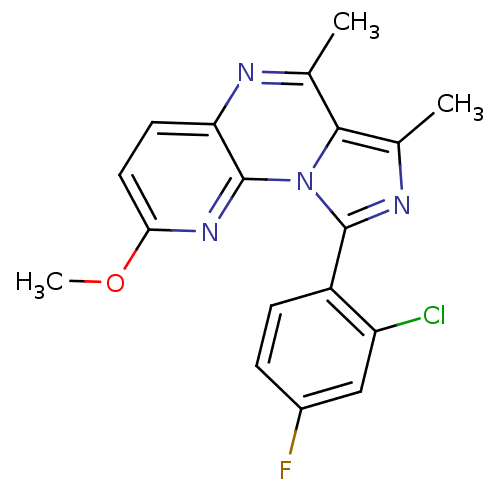
(CHEMBL1915892)Show SMILES COc1ccc2nc(C)c3c(C)nc(-c4ccc(F)cc4Cl)n3c2n1 Show InChI InChI=1S/C18H14ClFN4O/c1-9-16-10(2)22-17(12-5-4-11(20)8-13(12)19)24(16)18-14(21-9)6-7-15(23-18)25-3/h4-8H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE8A using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50357825
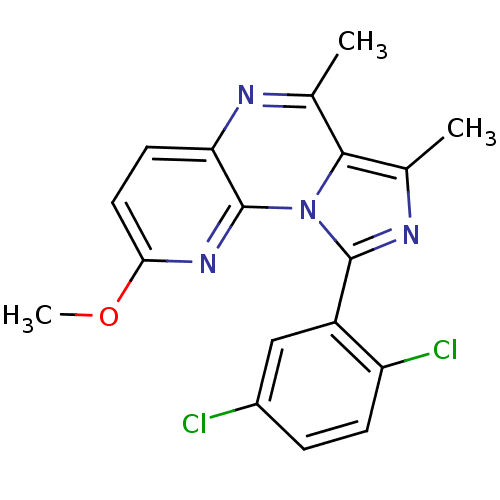
(CHEMBL1915891)Show SMILES COc1ccc2nc(C)c3c(C)nc(-c4cc(Cl)ccc4Cl)n3c2n1 Show InChI InChI=1S/C18H14Cl2N4O/c1-9-16-10(2)22-17(12-8-11(19)4-5-13(12)20)24(16)18-14(21-9)6-7-15(23-18)25-3/h4-8H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE8A using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50356427
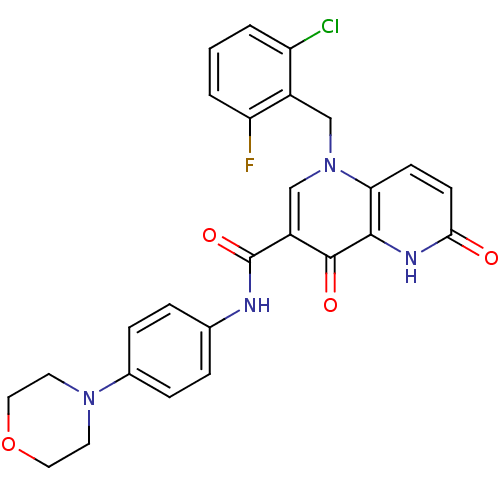
(CHEMBL1911559)Show SMILES Fc1cccc(Cl)c1Cn1cc(C(=O)Nc2ccc(cc2)N2CCOCC2)c(=O)c2[nH]c(=O)ccc12 Show InChI InChI=1S/C26H22ClFN4O4/c27-20-2-1-3-21(28)18(20)14-32-15-19(25(34)24-22(32)8-9-23(33)30-24)26(35)29-16-4-6-17(7-5-16)31-10-12-36-13-11-31/h1-9,15H,10-14H2,(H,29,35)(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
biocrea GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE8A |
Bioorg Med Chem Lett 21: 6652-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.065
BindingDB Entry DOI: 10.7270/Q2KD1Z91 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50319163
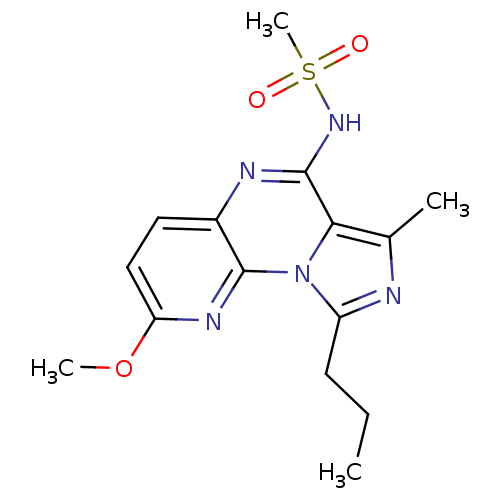
(8-Methoxy-3-methyl-4-methylsulfonylamino-1-propyl-...)Show InChI InChI=1S/C15H19N5O3S/c1-5-6-11-16-9(2)13-14(19-24(4,21)22)17-10-7-8-12(23-3)18-15(10)20(11)13/h7-8H,5-6H2,1-4H3,(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Biotie Therapies GmbH
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A |
J Med Chem 53: 4399-411 (2010)
Article DOI: 10.1021/jm1002793
BindingDB Entry DOI: 10.7270/Q2MK6D3N |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50259148
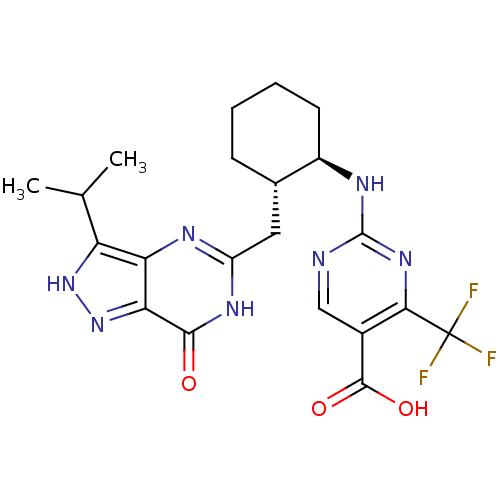
((-)2-({(trans)-2-[(3-isopropyl-7-oxo-6,7-dihydro-1...)Show SMILES CC(C)c1[nH]nc2c1nc(C[C@@H]1CCCC[C@H]1Nc1ncc(C(O)=O)c(n1)C(F)(F)F)[nH]c2=O |r| Show InChI InChI=1S/C21H24F3N7O3/c1-9(2)14-15-16(31-30-14)18(32)28-13(27-15)7-10-5-3-4-6-12(10)26-20-25-8-11(19(33)34)17(29-20)21(22,23)24/h8-10,12H,3-7H2,1-2H3,(H,30,31)(H,33,34)(H,25,26,29)(H,27,28,32)/t10-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE8a |
Bioorg Med Chem Lett 19: 2537-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.024
BindingDB Entry DOI: 10.7270/Q2RB74GG |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50357871
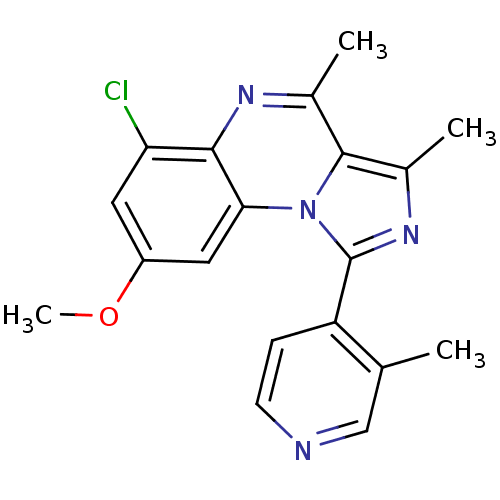
(CHEMBL1916131)Show SMILES COc1cc(Cl)c2nc(C)c3c(C)nc(-c4ccncc4C)n3c2c1 Show InChI InChI=1S/C19H17ClN4O/c1-10-9-21-6-5-14(10)19-23-12(3)18-11(2)22-17-15(20)7-13(25-4)8-16(17)24(18)19/h5-9H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE8A using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50357234
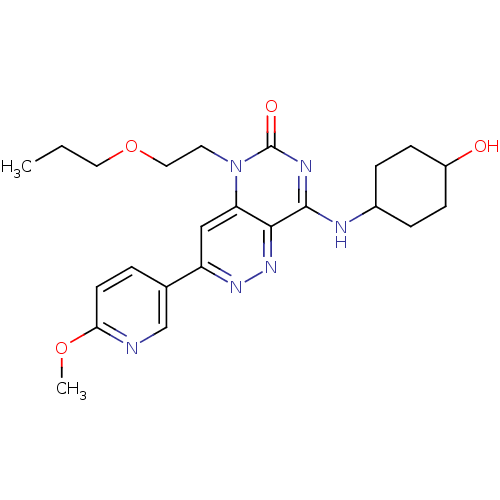
(CHEMBL1916475)Show SMILES CCCOCCn1c2cc(nnc2c(NC2CCC(O)CC2)nc1=O)-c1ccc(OC)nc1 |(22.74,-11.54,;21.41,-12.31,;20.07,-11.54,;18.74,-12.31,;18.74,-13.85,;17.41,-14.62,;17.41,-16.16,;18.74,-16.94,;20.06,-16.18,;21.39,-16.94,;21.39,-18.48,;20.06,-19.25,;18.73,-18.48,;17.41,-19.24,;17.41,-20.78,;16.08,-21.56,;14.75,-20.78,;13.42,-21.54,;13.41,-23.08,;12.08,-23.85,;14.75,-23.86,;16.09,-23.09,;16.08,-18.48,;16.08,-16.94,;14.74,-16.18,;22.71,-16.16,;22.71,-14.63,;24.03,-13.85,;25.38,-14.61,;26.71,-13.84,;28.04,-14.6,;25.38,-16.16,;24.05,-16.93,)| Show InChI InChI=1S/C23H30N6O4/c1-3-11-33-12-10-29-19-13-18(15-4-9-20(32-2)24-14-15)27-28-21(19)22(26-23(29)31)25-16-5-7-17(30)8-6-16/h4,9,13-14,16-17,30H,3,5-8,10-12H2,1-2H3,(H,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50296256
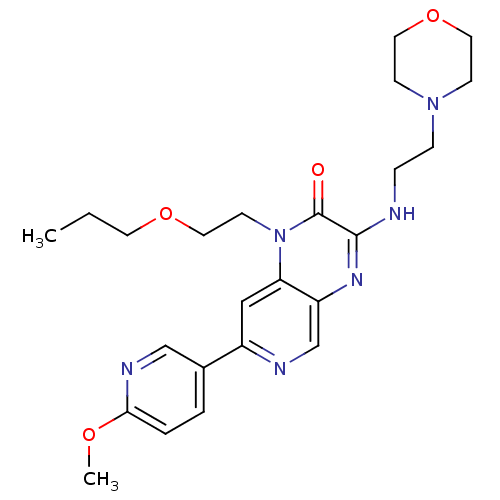
(7-(6-methoxypyridin-3-yl)-3-(2-morpholinoethylamin...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCCN2CCOCC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H32N6O4/c1-3-11-33-14-10-30-21-15-19(18-4-5-22(32-2)27-16-18)26-17-20(21)28-23(24(30)31)25-6-7-29-8-12-34-13-9-29/h4-5,15-17H,3,6-14H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50300953
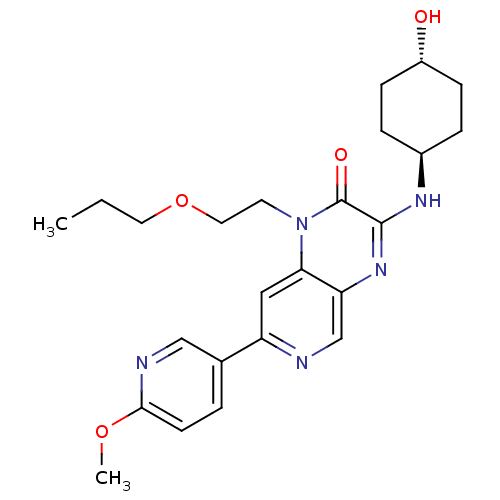
(3-[(trans-4-hydroxycyclohexyl)amino]-7-(6-methoxyp...)Show SMILES CCCOCCn1c2cc(ncc2nc(N[C@H]2CC[C@H](O)CC2)c1=O)-c1ccc(OC)nc1 |r,wU:16.16,wD:19.20,(24.97,-.51,;23.64,-1.28,;23.64,-2.82,;22.3,-3.59,;22.3,-5.13,;20.97,-5.9,;20.97,-7.44,;22.3,-8.21,;23.62,-7.45,;24.95,-8.21,;24.95,-9.75,;23.62,-10.52,;22.3,-9.75,;20.97,-10.52,;19.62,-9.75,;18.29,-10.51,;16.96,-9.74,;15.63,-10.51,;14.29,-9.73,;14.3,-8.19,;12.97,-7.41,;15.64,-7.43,;16.97,-8.2,;19.64,-8.21,;18.31,-7.44,;26.28,-7.43,;27.61,-8.2,;28.94,-7.43,;28.94,-5.89,;30.27,-5.11,;31.61,-5.87,;27.59,-5.12,;26.27,-5.9,)| Show InChI InChI=1S/C24H31N5O4/c1-3-11-33-12-10-29-21-13-19(16-4-9-22(32-2)26-14-16)25-15-20(21)28-23(24(29)31)27-17-5-7-18(30)8-6-17/h4,9,13-15,17-18,30H,3,5-8,10-12H2,1-2H3,(H,27,28)/t17-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50308557
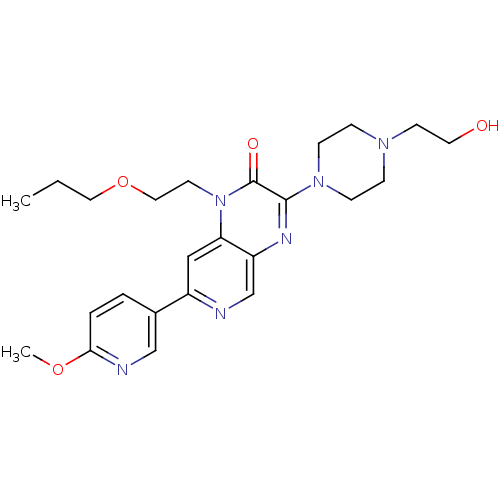
(3-(4-(2-hydroxyethyl)piperazin-1-yl)-1-(2-propoxye...)Show SMILES CCCOCCn1c2cc(ncc2nc(N2CCN(CCO)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H32N6O4/c1-3-13-34-14-11-30-21-15-19(18-4-5-22(33-2)26-16-18)25-17-20(21)27-23(24(30)32)29-8-6-28(7-9-29)10-12-31/h4-5,15-17,31H,3,6-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A |
J Med Chem 53: 2656-60 (2010)
Article DOI: 10.1021/jm901781q
BindingDB Entry DOI: 10.7270/Q2VM4CCK |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50540044
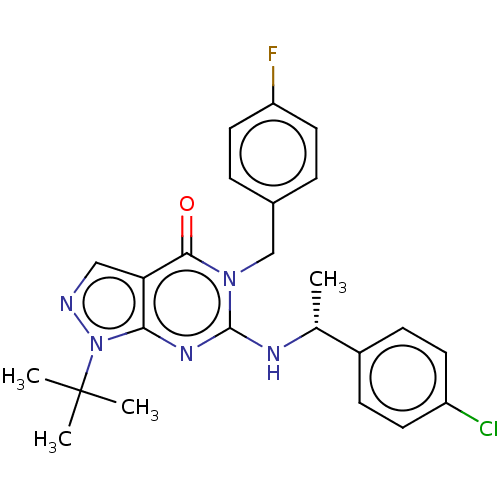
(CHEMBL4644729)Show SMILES C[C@@H](Nc1nc2n(ncc2c(=O)n1Cc1ccc(F)cc1)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H25ClFN5O/c1-15(17-7-9-18(25)10-8-17)28-23-29-21-20(13-27-31(21)24(2,3)4)22(32)30(23)14-16-5-11-19(26)12-6-16/h5-13,15H,14H2,1-4H3,(H,28,29)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) using [3H]-cAMP substrate incubated for 15 mins by liquid scintillation counting method r... |
J Med Chem 63: 7867-7879 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00711
BindingDB Entry DOI: 10.7270/Q20V8HBZ |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50555232
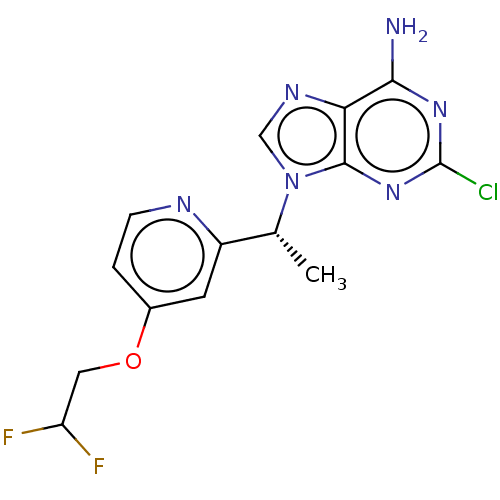
(CHEMBL4790086)Show SMILES C[C@H](c1cc(OCC(F)F)ccn1)n1cnc2c(N)nc(Cl)nc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) expressed in Escherichia coli BL21 using [3H]-cAMP substrate incubated for 15 mins by liq... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01573
BindingDB Entry DOI: 10.7270/Q2CZ3BV5 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50467472

(CHEMBL4281766)Show SMILES [H][C@@]12Cc3c([nH]c4ccccc34)[C@H](N1C(=O)N(CC)C2=O)c1ccc(CC)o1 |r| Show InChI InChI=1S/C21H21N3O3/c1-3-12-9-10-17(27-12)19-18-14(13-7-5-6-8-15(13)22-18)11-16-20(25)23(4-2)21(26)24(16)19/h5-10,16,19,22H,3-4,11H2,1-2H3/t16-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal GST-tagged PDE8A1 expressed in baculovirus infected sf9 cells using FAM-cyclic-3',5-AMP as sub... |
Eur J Med Chem 158: 767-780 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.028
BindingDB Entry DOI: 10.7270/Q2JS9T4N |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50246909
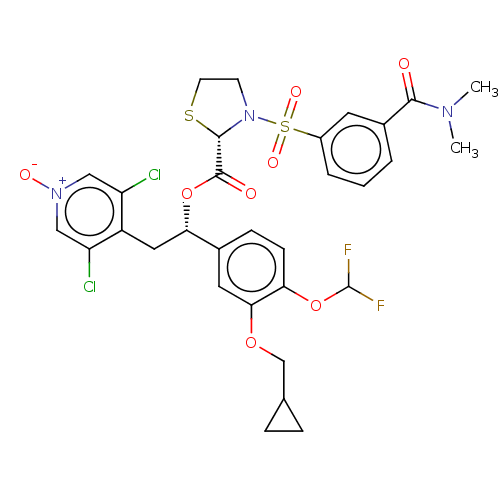
(CHEMBL4075951)Show SMILES CN(C)C(=O)c1cccc(c1)S(=O)(=O)N1CCS[C@H]1C(=O)O[C@@H](Cc1c(Cl)c[n+]([O-])cc1Cl)c1ccc(OC(F)F)c(OCC2CC2)c1 |r| Show InChI InChI=1S/C31H31Cl2F2N3O8S2/c1-36(2)28(39)20-4-3-5-21(12-20)48(42,43)38-10-11-47-29(38)30(40)45-26(14-22-23(32)15-37(41)16-24(22)33)19-8-9-25(46-31(34)35)27(13-19)44-17-18-6-7-18/h3-5,8-9,12-13,15-16,18,26,29,31H,6-7,10-11,14,17H2,1-2H3/t26-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiesi Farmaceutici S.p.A.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE8A1 expressed insect sf9 cells by radiometric assay |
J Med Chem 60: 10026-10046 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01044
BindingDB Entry DOI: 10.7270/Q25H7JN4 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50467488

(CHEMBL4286052)Show SMILES [H][C@]12Cc3c([nH]c4ccccc34)[C@H](N1C(=O)CN(C)C2=O)c1ccc(CC)s1 |r| Show InChI InChI=1S/C21H21N3O2S/c1-3-12-8-9-17(27-12)20-19-14(13-6-4-5-7-15(13)22-19)10-16-21(26)23(2)11-18(25)24(16)20/h4-9,16,20,22H,3,10-11H2,1-2H3/t16-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal GST-tagged PDE8A1 expressed in baculovirus infected sf9 cells using FAM-cyclic-3',5-AMP as sub... |
Eur J Med Chem 158: 767-780 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.028
BindingDB Entry DOI: 10.7270/Q2JS9T4N |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM23620

(2-({6-[bis(2-hydroxyethyl)amino]-4,8-bis(piperidin...)Show SMILES OCCN(CCO)c1nc(N2CCCCC2)c2nc(nc(N3CCCCC3)c2n1)N(CCO)CCO Show InChI InChI=1S/C24H40N8O4/c33-15-11-31(12-16-34)23-26-20-19(21(27-23)29-7-3-1-4-8-29)25-24(32(13-17-35)14-18-36)28-22(20)30-9-5-2-6-10-30/h33-36H,1-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic affinity tested against isolated Rat Thoracic Aorta Alpha-1D adrenergic receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM123687
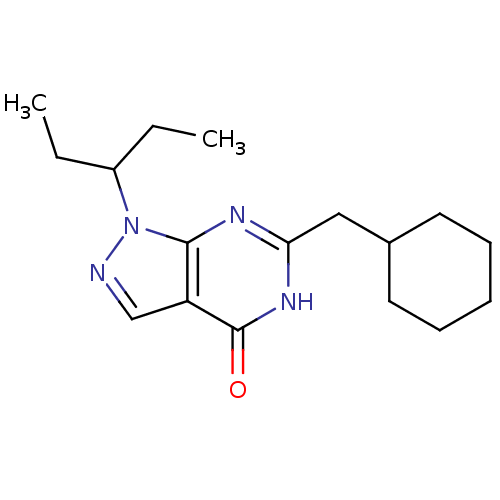
(US8741907, 3 | US9067945, 2)Show InChI InChI=1S/C17H26N4O/c1-3-13(4-2)21-16-14(11-18-21)17(22)20-15(19-16)10-12-8-6-5-7-9-12/h11-13H,3-10H2,1-2H3,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
The test substances are dissolved in 100% DMSO and serially diluted to determine their in vitro effect on PDE 9A. 2 uL portions of the diluted substa... |
US Patent US8741907 (2014)
BindingDB Entry DOI: 10.7270/Q21V5CNH |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM123687

(US8741907, 3 | US9067945, 2)Show InChI InChI=1S/C17H26N4O/c1-3-13(4-2)21-16-14(11-18-21)17(22)20-15(19-16)10-12-8-6-5-7-9-12/h11-13H,3-10H2,1-2H3,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingehleim International GmbH
US Patent
| Assay Description
The test substances are dissolved in 100% DMSO and serially diluted to determine their in vitro effect on PDE 9A. Typically, serial dilutions from 20... |
US Patent US9067945 (2015)
BindingDB Entry DOI: 10.7270/Q2708052 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50357849
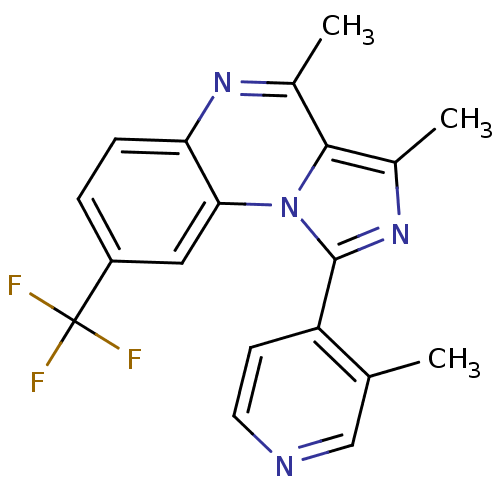
(CHEMBL1916108)Show SMILES Cc1nc(-c2ccncc2C)n2c1c(C)nc1ccc(cc21)C(F)(F)F Show InChI InChI=1S/C19H15F3N4/c1-10-9-23-7-6-14(10)18-25-12(3)17-11(2)24-15-5-4-13(19(20,21)22)8-16(15)26(17)18/h4-9H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE8A using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50319165
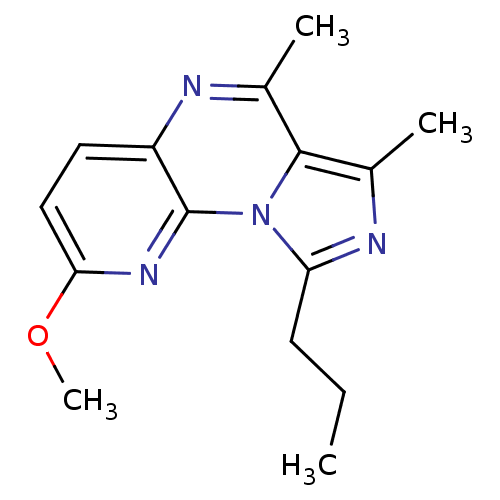
(2-methoxy-6,7-dimethyl-9-propylimidazo[1,5-a]pyrid...)Show InChI InChI=1S/C15H18N4O/c1-5-6-12-17-10(3)14-9(2)16-11-7-8-13(20-4)18-15(11)19(12)14/h7-8H,5-6H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE8A using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50356428
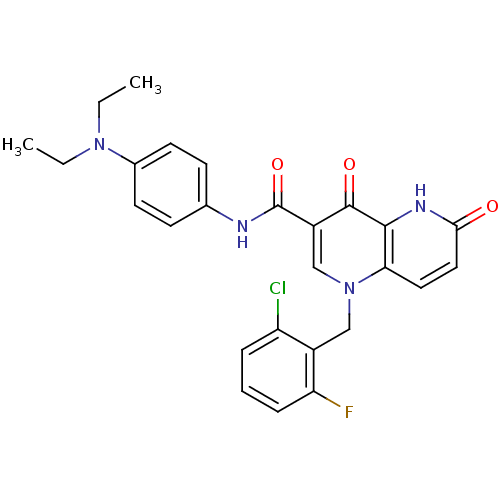
(CHEMBL1911572)Show SMILES CCN(CC)c1ccc(NC(=O)c2cn(Cc3c(F)cccc3Cl)c3ccc(=O)[nH]c3c2=O)cc1 Show InChI InChI=1S/C26H24ClFN4O3/c1-3-31(4-2)17-10-8-16(9-11-17)29-26(35)19-15-32(14-18-20(27)6-5-7-21(18)28)22-12-13-23(33)30-24(22)25(19)34/h5-13,15H,3-4,14H2,1-2H3,(H,29,35)(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
biocrea GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE8A |
Bioorg Med Chem Lett 21: 6652-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.065
BindingDB Entry DOI: 10.7270/Q2KD1Z91 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50319165

(2-methoxy-6,7-dimethyl-9-propylimidazo[1,5-a]pyrid...)Show InChI InChI=1S/C15H18N4O/c1-5-6-12-17-10(3)14-9(2)16-11-7-8-13(20-4)18-15(11)19(12)14/h7-8H,5-6H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Biotie Therapies GmbH
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A |
J Med Chem 53: 4399-411 (2010)
Article DOI: 10.1021/jm1002793
BindingDB Entry DOI: 10.7270/Q2MK6D3N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data