

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195118 (US10336717, Compound 178 | US9212153, 178,Ex. 139) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195133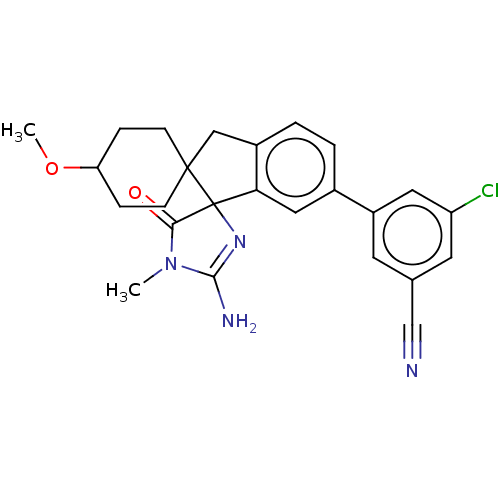 (US10336717, Compound 193 | US9212153, 193,Ex. 154) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195132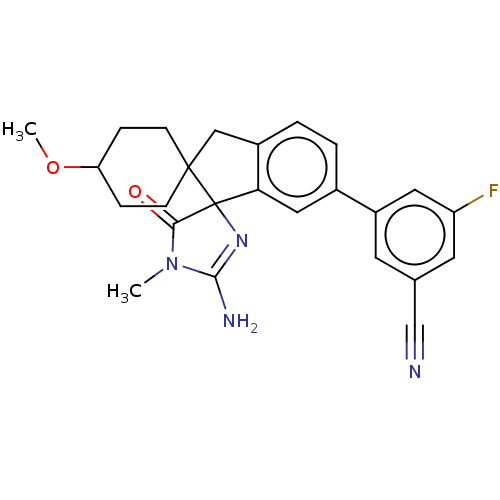 (US10336717, Compound 192 | US9212153, 192,Ex. 153) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195123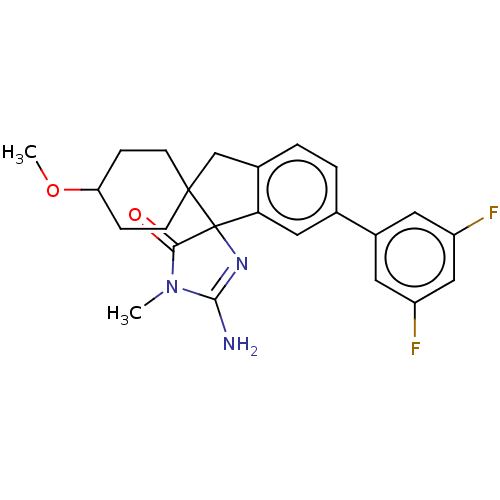 (US10336717, Compound 183 | US9212153, 183,Ex. 144) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195120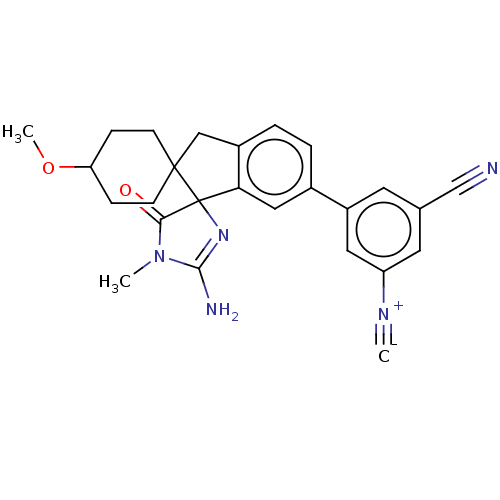 (US9212153, 180,Ex. 141) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195379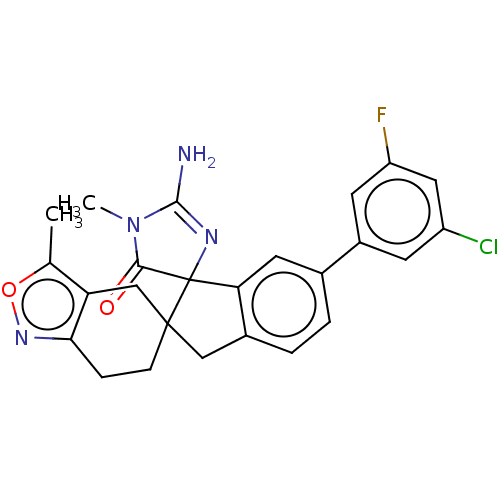 (US10336717, Compound 463 | US9212153, 463,Ex. 397) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195378 (US10336717, Compound 462 | US9212153, 462,Ex. 396 ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195316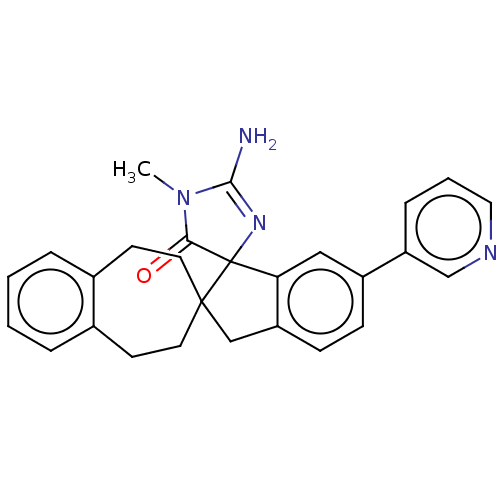 (US9212153, 394,Ex. 335) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195378 (US10336717, Compound 462 | US9212153, 462,Ex. 396 ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195117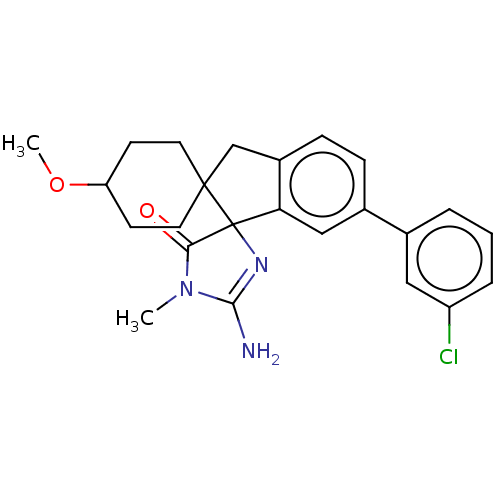 (US10336717, Compound 177 | US9212153, 177,Ex. 138) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195521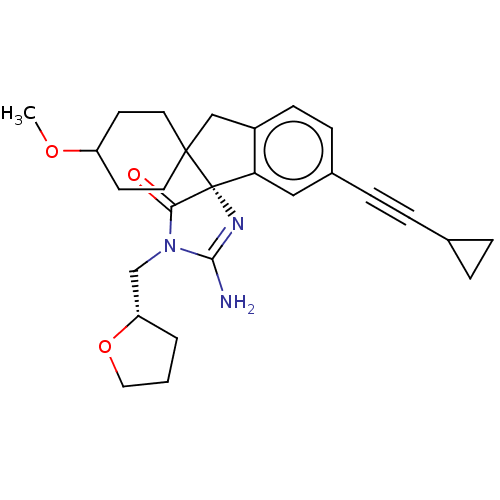 (US9212153, 334,Ex. 282) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195128 (US10336717, Compound 188 | US9212153, 188,Ex. 149) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195093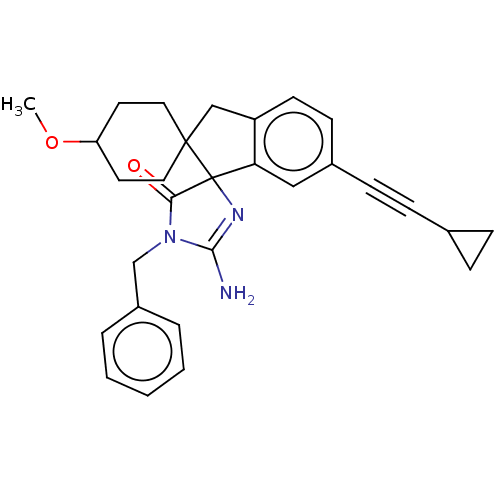 (US10336717, Compound 142 | US9212153, 142,Ex. 107) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195519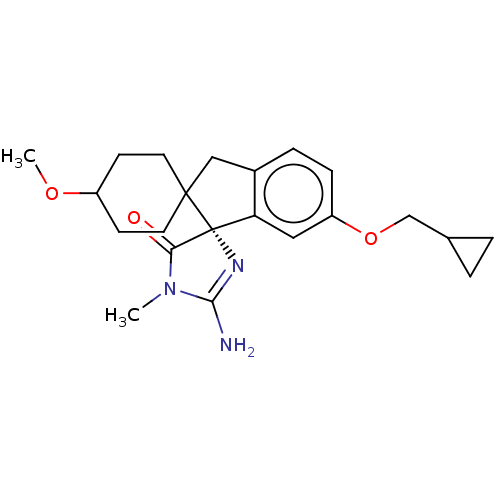 (US10336717, Compound 246 | US9212153, 245,Ex. 199) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM113298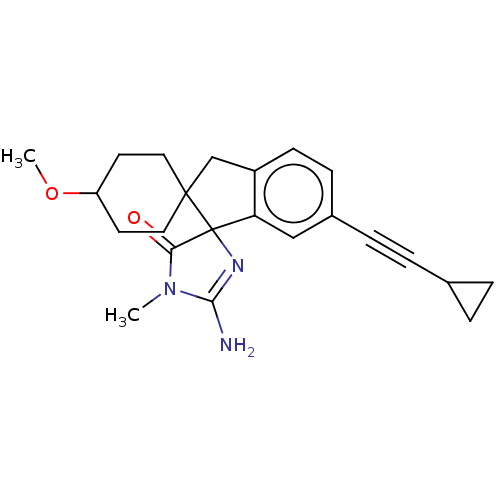 (US10336717, Compound 76 | US8633212, 76 | US921215...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195501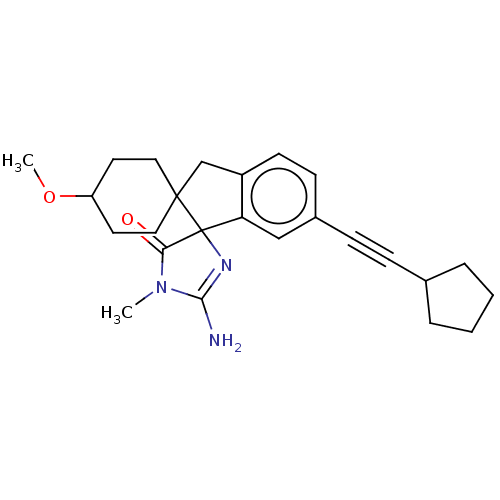 (US9212153, 147,Ex. 111) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195522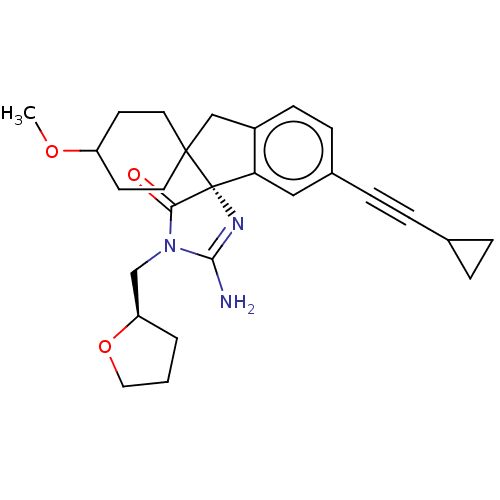 (US9212153, 335,Ex. 282) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195392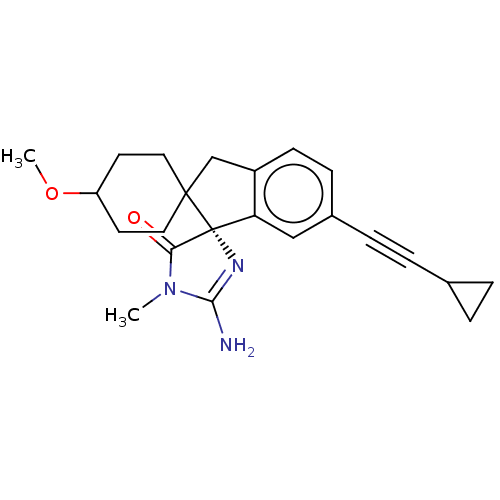 (US10336717, Compound 478 | US9212153, 478,Ex. 409) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195506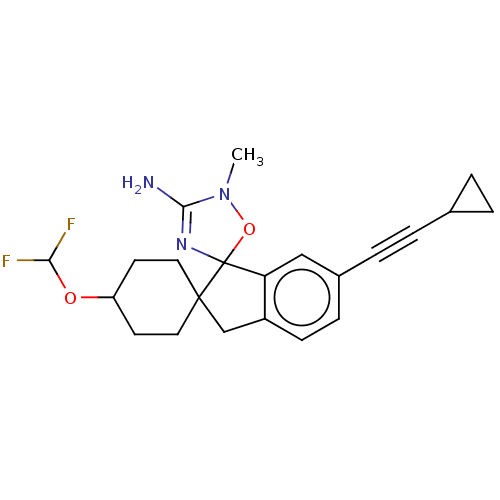 (US10336717, Compound 99 | US9212153, 99,Ex. 65) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195403 (US10336717, Compound 522 | US9212153, 523,Ex. 420) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195127 (US10336717, Compound 187 | US9212153, 187,Ex. 148) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195119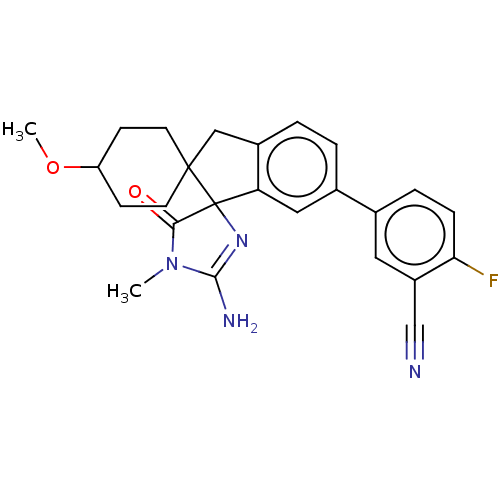 (US10336717, Compound 179 | US9212153, 179,Ex. 140) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195418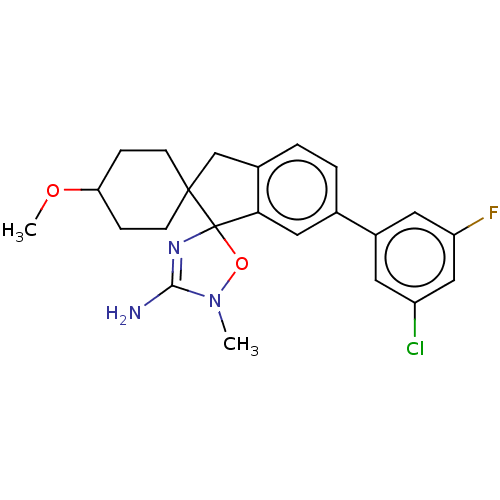 (US10336717, Compound 1 | US9212153, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195125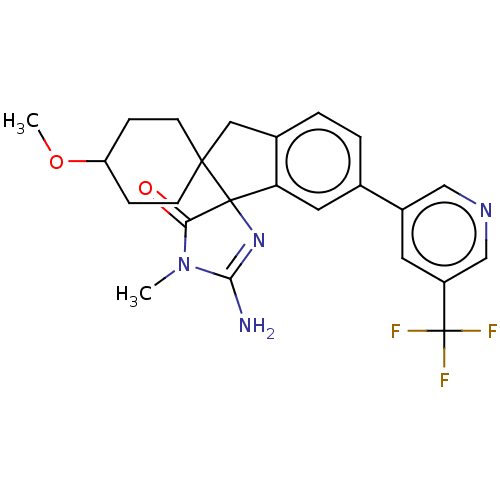 (US10336717, Compound 185 | US9212153, 185,Ex. 146) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195383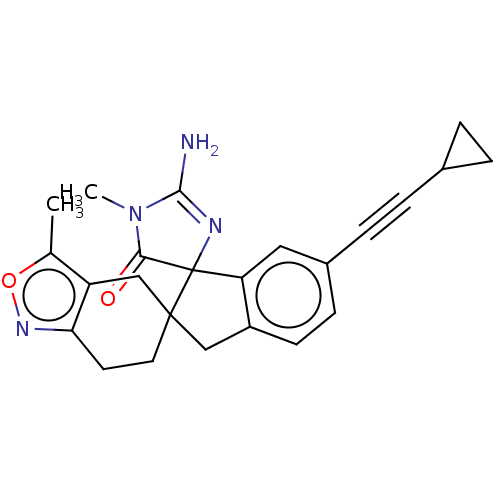 (US9212153, 467,Ex. 401) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195129 (US10336717, Compound 189 | US9212153, 189,Ex. 150) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195099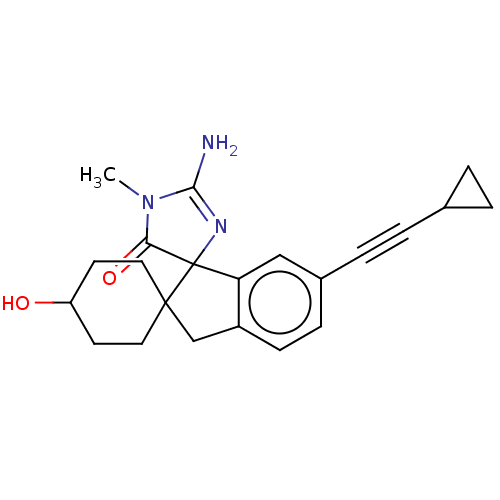 (US9212153, 150 & 151,Ex. 114) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195124 (US9212153, 184,Ex. 145) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195529 (US10336717, Compound 397 | US9212153, 397,Ex. 336) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195419 (US10336717, Compound 2 | US9212153, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195181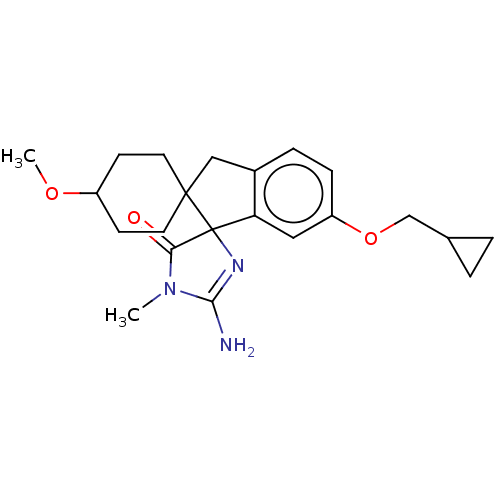 (US10336717, Compound 244 | US9212153, 244,Ex. 199) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195293 (US9212153, 370,Ex. 313) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195061 (US10336717, Compound 115 | US9212153, 115,Ex. 81) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM113392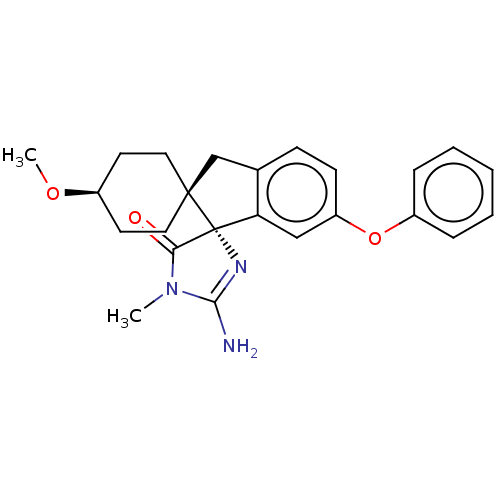 (US10336717, Compound 578 | US8633212, 578 | US9212...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195158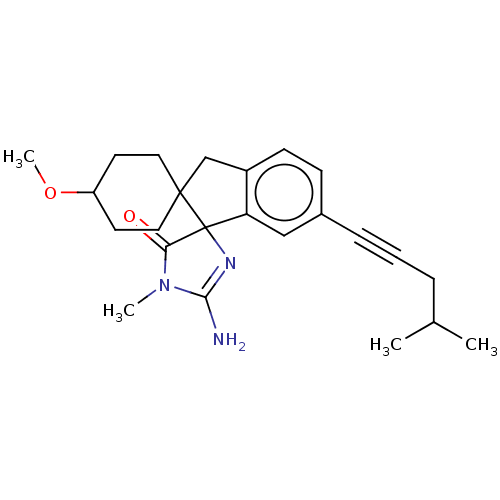 (US10336717, Compound 224 | US9212153, 221,Ex. 179) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195411 (US9212153, 541,Ex. 427) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195421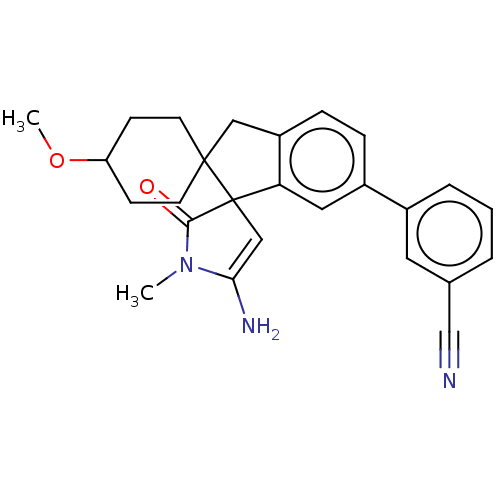 (US9212153, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195342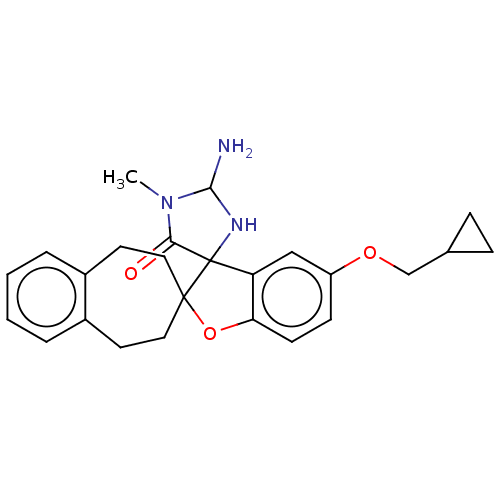 (US9212153, 425,Ex. 362) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195409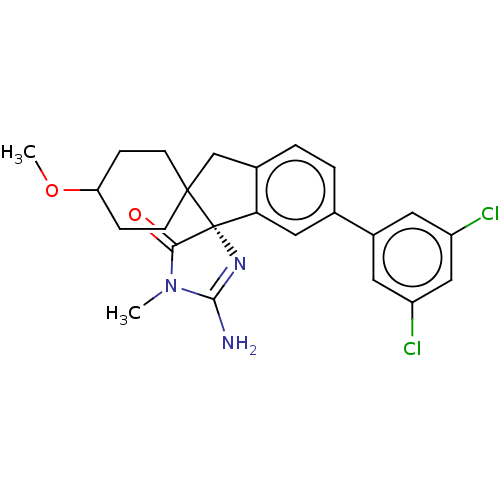 (US10336717, Compound 480 | US9212153, 480,Ex. 424) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195240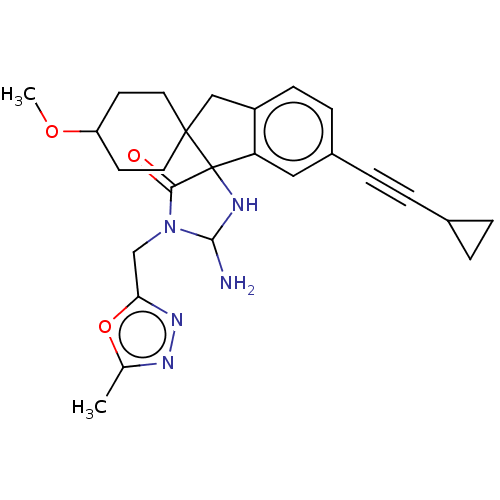 (US9212153, 305,Ex. 254) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195422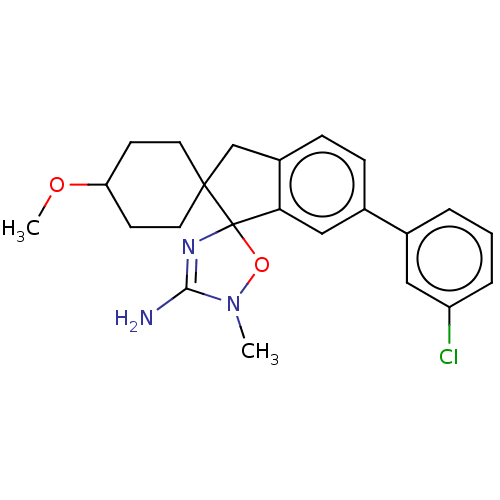 (US10336717, Compound 5 | US9212153, 5) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195420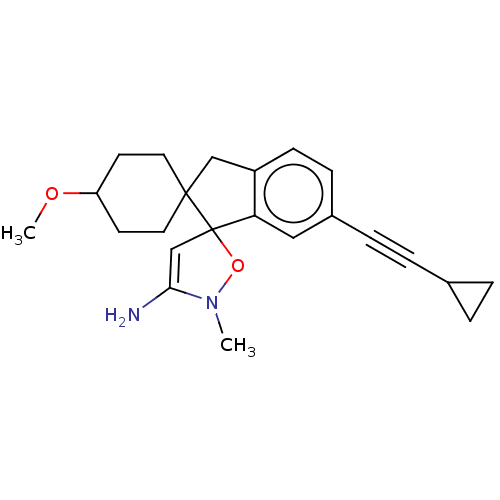 (US9212153, 3) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM113364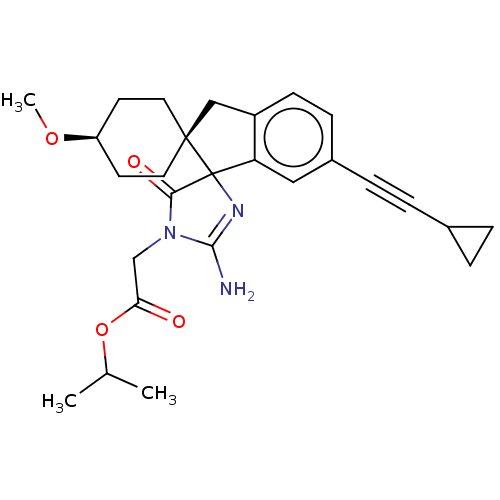 (US10336717, Compound 543 | US8633212, 543 | US9212...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195091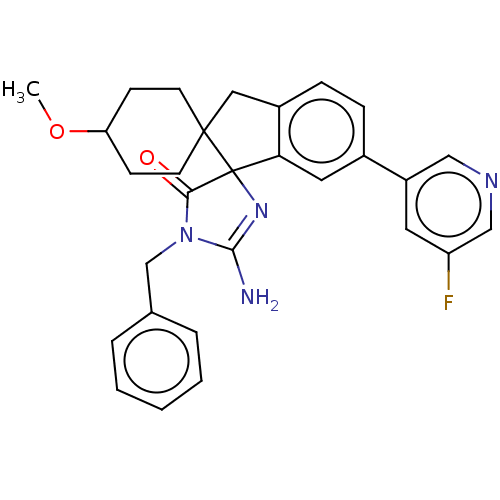 (US10336717, Compound 140 | US9212153, 140,Ex. 105) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195239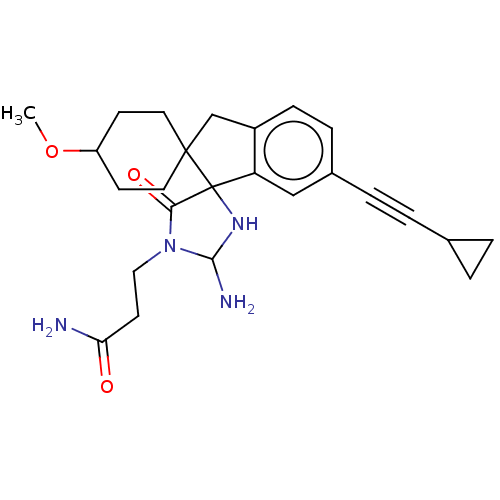 (US9212153, 304,Ex. 253) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195045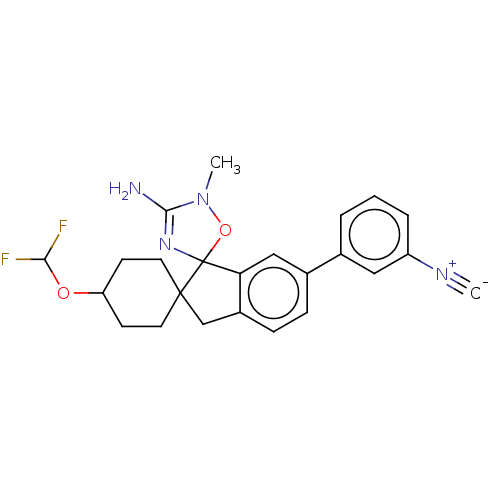 (US9212153, 100,Ex. 66) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195098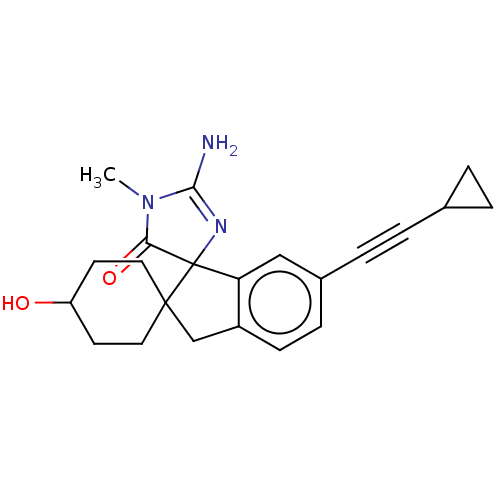 (US9212153, 149,Ex. 113) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195103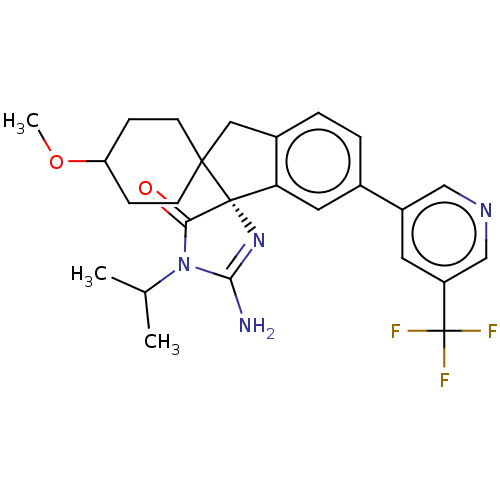 (US10336717, Compound 162 | US9212153, 161,Ex. 124) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | 4.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195423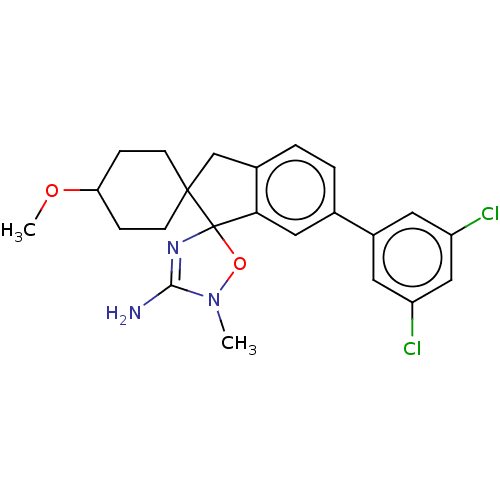 (US10336717, Compound 6 | US9212153, 6) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-458] (Homo sapiens (Human)) | BDBM195143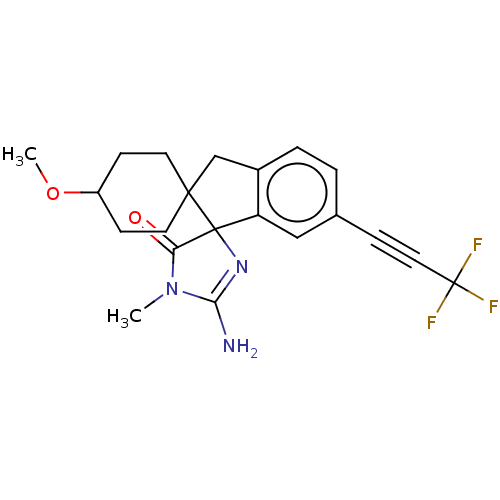 (US9212153, 205,Ex. 165) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488... | US Patent US9212153 (2015) BindingDB Entry DOI: 10.7270/Q2J67FRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 549 total ) | Next | Last >> |