 Found 9957 hits of ic50 for UniProtKB: P08684
Found 9957 hits of ic50 for UniProtKB: P08684 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM144637
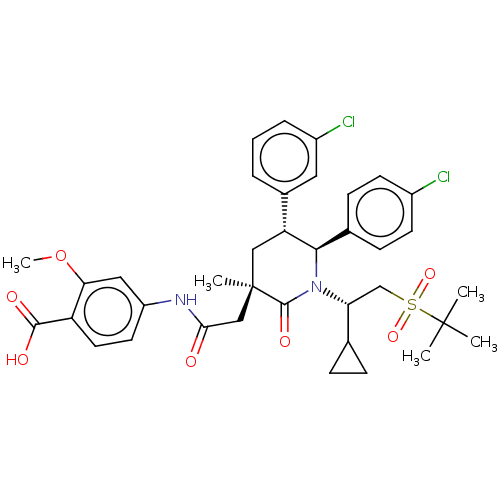
(US8952036, Ex. 5)Show SMILES COc1cc(NC(=O)C[C@@]2(C)C[C@@H]([C@H](N([C@H](CS(=O)(=O)C(C)(C)C)C3CC3)C2=O)c2ccc(Cl)cc2)c2cccc(Cl)c2)ccc1C(O)=O |r| Show InChI InChI=1S/C37H42Cl2N2O7S/c1-36(2,3)49(46,47)21-30(22-9-10-22)41-33(23-11-13-25(38)14-12-23)29(24-7-6-8-26(39)17-24)19-37(4,35(41)45)20-32(42)40-27-15-16-28(34(43)44)31(18-27)48-5/h6-8,11-18,22,29-30,33H,9-10,19-21H2,1-5H3,(H,40,42)(H,43,44)/t29-,30-,33-,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0503 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen Inc.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US8952036 (2015)
BindingDB Entry DOI: 10.7270/Q27P8X4P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50448963
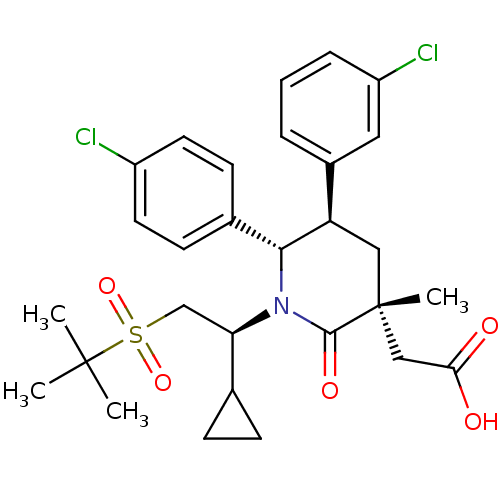
(CHEMBL3125537 | US9296736, 351 | US9593129, Exampl...)Show SMILES CC(C)(C)S(=O)(=O)C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H35Cl2NO5S/c1-28(2,3)38(36,37)17-24(18-8-9-18)32-26(19-10-12-21(30)13-11-19)23(20-6-5-7-22(31)14-20)15-29(4,27(32)35)16-25(33)34/h5-7,10-14,18,23-24,26H,8-9,15-17H2,1-4H3,(H,33,34)/t23-,24-,26-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0962 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen Inc.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US8952036 (2015)
BindingDB Entry DOI: 10.7270/Q27P8X4P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50337733
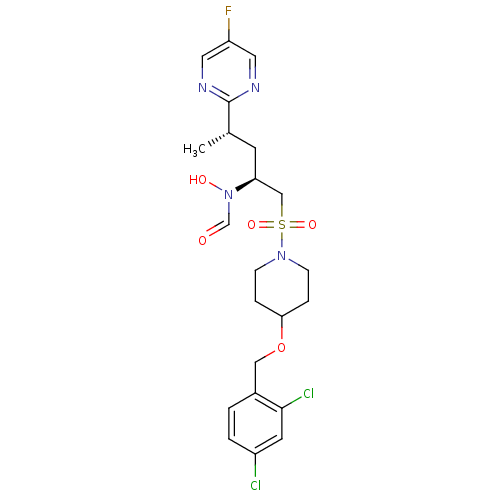
(CHEMBL1683444 | N-((2S,4S)-1-(4-(2,4-dichlorobenzy...)Show SMILES C[C@@H](C[C@@H](CS(=O)(=O)N1CCC(CC1)OCc1ccc(Cl)cc1Cl)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C22H27Cl2FN4O5S/c1-15(22-26-10-18(25)11-27-22)8-19(29(31)14-30)13-35(32,33)28-6-4-20(5-7-28)34-12-16-2-3-17(23)9-21(16)24/h2-3,9-11,14-15,19-20,31H,4-8,12-13H2,1H3/t15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM144636

(US8952036, Ex. 4)Show SMILES CC(C)(C)S(=O)(=O)C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)c(F)c1 |r| Show InChI InChI=1S/C29H34Cl2FNO5S/c1-28(2,3)39(37,38)16-24(17-8-9-17)33-26(19-10-11-22(31)23(32)13-19)21(18-6-5-7-20(30)12-18)14-29(4,27(33)36)15-25(34)35/h5-7,10-13,17,21,24,26H,8-9,14-16H2,1-4H3,(H,34,35)/t21-,24-,26-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.102 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen Inc.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US8952036 (2015)
BindingDB Entry DOI: 10.7270/Q27P8X4P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Eur J Med Chem 101: 627-39 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.029
BindingDB Entry DOI: 10.7270/Q2QF8VPZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330011
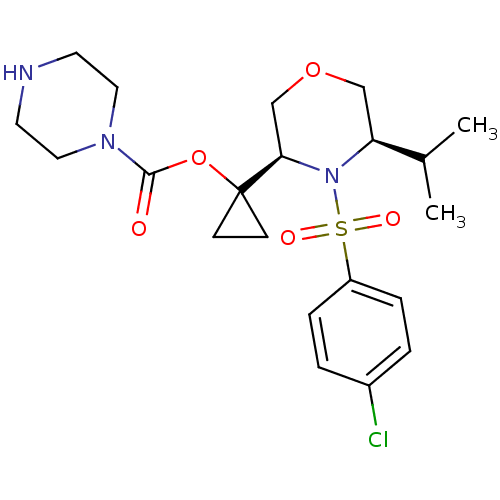
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-isopropylm...)Show SMILES CC(C)[C@@H]1COC[C@@H](N1S(=O)(=O)c1ccc(Cl)cc1)C1(CC1)OC(=O)N1CCNCC1 |r| Show InChI InChI=1S/C21H30ClN3O5S/c1-15(2)18-13-29-14-19(25(18)31(27,28)17-5-3-16(22)4-6-17)21(7-8-21)30-20(26)24-11-9-23-10-12-24/h3-6,15,18-19,23H,7-14H2,1-2H3/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM144638
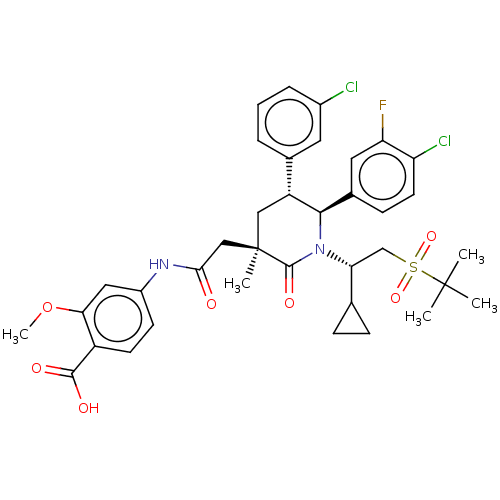
(US8952036, Ex. 3)Show SMILES COc1cc(NC(=O)C[C@@]2(C)C[C@@H]([C@H](N([C@H](CS(=O)(=O)C(C)(C)C)C3CC3)C2=O)c2ccc(Cl)c(F)c2)c2cccc(Cl)c2)ccc1C(O)=O |r| Show InChI InChI=1S/C37H41Cl2FN2O7S/c1-36(2,3)50(47,48)20-30(21-9-10-21)42-33(23-11-14-28(39)29(40)16-23)27(22-7-6-8-24(38)15-22)18-37(4,35(42)46)19-32(43)41-25-12-13-26(34(44)45)31(17-25)49-5/h6-8,11-17,21,27,30,33H,9-10,18-20H2,1-5H3,(H,41,43)(H,44,45)/t27-,30-,33-,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen Inc.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US8952036 (2015)
BindingDB Entry DOI: 10.7270/Q27P8X4P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM143355
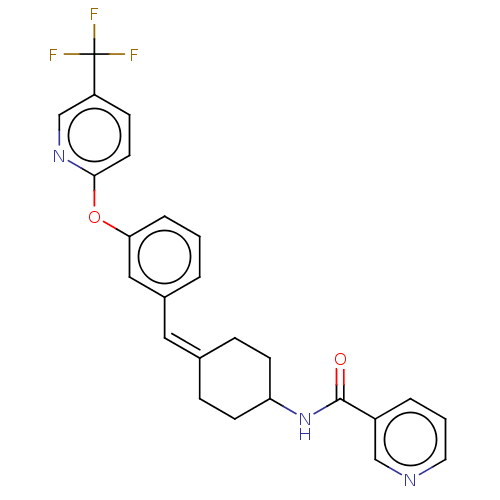
(US9682953, 20.A-1)Show SMILES CCOC(=O)Cn1nc2C(=O)N(C(c2c1C(C)C)c1ccc(Cl)cc1C)c1cc(Cl)ccc1C Show InChI InChI=1S/C25H22F3N3O2/c26-25(27,28)20-8-11-23(30-16-20)33-22-5-1-3-18(14-22)13-17-6-9-21(10-7-17)31-24(32)19-4-2-12-29-15-19/h1-5,8,11-16,21H,6-7,9-10H2,(H,31,32)/b17-13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using testosterone as substrate preincubated for 5 mins followed by NADPH cofactor addition and measur... |
Bioorg Med Chem Lett 29: 238-243 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.048
BindingDB Entry DOI: 10.7270/Q2125X14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252094
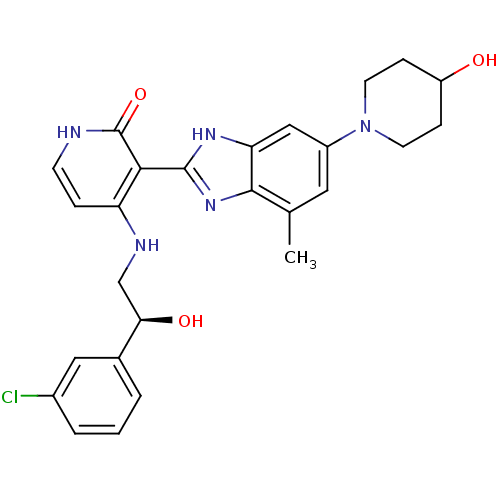
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(O)CC1 |r| Show InChI InChI=1S/C26H28ClN5O3/c1-15-11-18(32-9-6-19(33)7-10-32)13-21-24(15)31-25(30-21)23-20(5-8-28-26(23)35)29-14-22(34)16-3-2-4-17(27)12-16/h2-5,8,11-13,19,22,33-34H,6-7,9-10,14H2,1H3,(H,30,31)(H2,28,29,35)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252237

((S)-methyl 1-(2-(4-(2-(3-chlorophenyl)-2-hydroxyet...)Show SMILES COC(=O)NC1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C28H31ClN6O4/c1-16-12-20(35-10-7-19(8-11-35)32-28(38)39-2)14-22-25(16)34-26(33-22)24-21(6-9-30-27(24)37)31-15-23(36)17-4-3-5-18(29)13-17/h3-6,9,12-14,19,23,36H,7-8,10-11,15H2,1-2H3,(H,32,38)(H,33,34)(H2,30,31,37)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330006
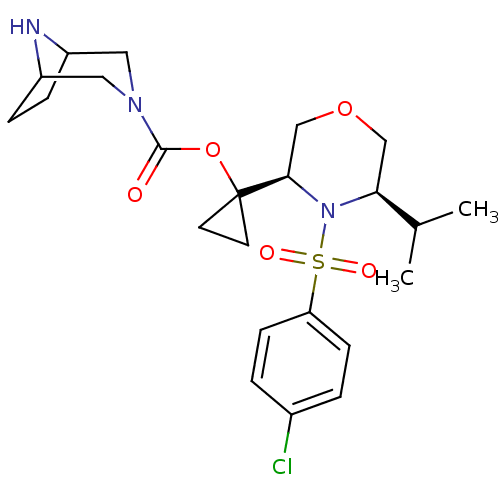
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-isopropylm...)Show SMILES CC(C)[C@@H]1COC[C@@H](N1S(=O)(=O)c1ccc(Cl)cc1)C1(CC1)OC(=O)N1CC2CCC(C1)N2 |r,TLB:23:25:32:28.29| Show InChI InChI=1S/C23H32ClN3O5S/c1-15(2)20-13-31-14-21(27(20)33(29,30)19-7-3-16(24)4-8-19)23(9-10-23)32-22(28)26-11-17-5-6-18(12-26)25-17/h3-4,7-8,15,17-18,20-21,25H,5-6,9-14H2,1-2H3/t17?,18?,20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252295

(3-(6-(4-((1R,4S)-5-oxa-2-aza-bicyclo[2.2.1]heptan-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)N1C[C@@H]2C[C@@H]1CO2 |r,THB:31:34:39.40:37| Show InChI InChI=1S/C31H35ClN6O3/c1-18-11-22(37-9-6-21(7-10-37)38-16-24-13-23(38)17-41-24)14-26-29(18)36-30(35-26)28-25(5-8-33-31(28)40)34-15-27(39)19-3-2-4-20(32)12-19/h2-5,8,11-12,14,21,23-24,27,39H,6-7,9-10,13,15-17H2,1H3,(H,35,36)(H2,33,34,40)/t23-,24+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330016
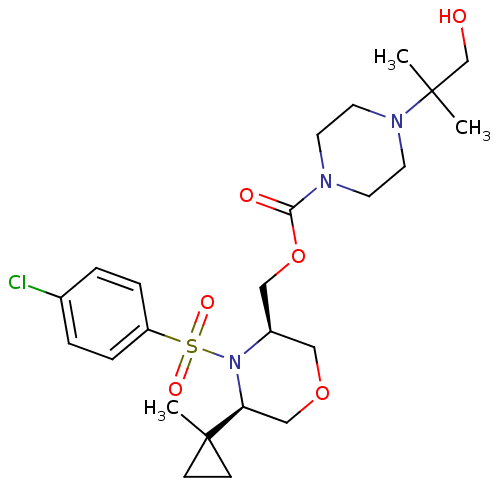
(((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(1-methylcyc...)Show SMILES CC(C)(CO)N1CCN(CC1)C(=O)OC[C@H]1COC[C@H](N1S(=O)(=O)c1ccc(Cl)cc1)C1(C)CC1 |r| Show InChI InChI=1S/C24H36ClN3O6S/c1-23(2,17-29)27-12-10-26(11-13-27)22(30)34-15-19-14-33-16-21(24(3)8-9-24)28(19)35(31,32)20-6-4-18(25)5-7-20/h4-7,19,21,29H,8-17H2,1-3H3/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM27879

(4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCOCC1 |r| Show InChI InChI=1S/C25H26ClN5O3/c1-15-11-18(31-7-9-34-10-8-31)13-20-23(15)30-24(29-20)22-19(5-6-27-25(22)33)28-14-21(32)16-3-2-4-17(26)12-16/h2-6,11-13,21,32H,7-10,14H2,1H3,(H,29,30)(H2,27,28,33)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252193
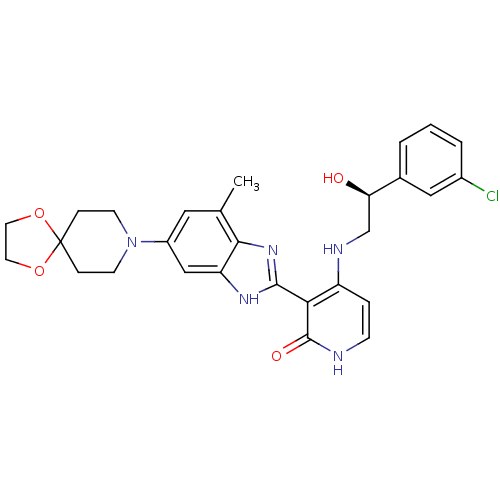
(4-((S)-2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC2(CC1)OCCO2 |r| Show InChI InChI=1S/C28H30ClN5O4/c1-17-13-20(34-9-6-28(7-10-34)37-11-12-38-28)15-22-25(17)33-26(32-22)24-21(5-8-30-27(24)36)31-16-23(35)18-3-2-4-19(29)14-18/h2-5,8,13-15,23,35H,6-7,9-12,16H2,1H3,(H,32,33)(H2,30,31,36)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252236
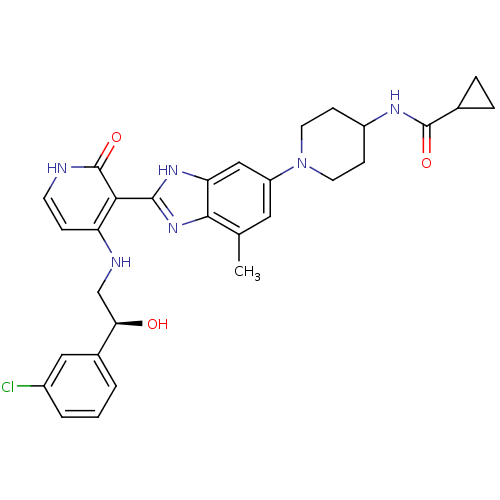
((S)-N-(1-(2-(4-(2-(3-chlorophenyl)-2-hydroxyethyla...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)NC(=O)C1CC1 |r| Show InChI InChI=1S/C30H33ClN6O3/c1-17-13-22(37-11-8-21(9-12-37)34-29(39)18-5-6-18)15-24-27(17)36-28(35-24)26-23(7-10-32-30(26)40)33-16-25(38)19-3-2-4-20(31)14-19/h2-4,7,10,13-15,18,21,25,38H,5-6,8-9,11-12,16H2,1H3,(H,34,39)(H,35,36)(H2,32,33,40)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252297
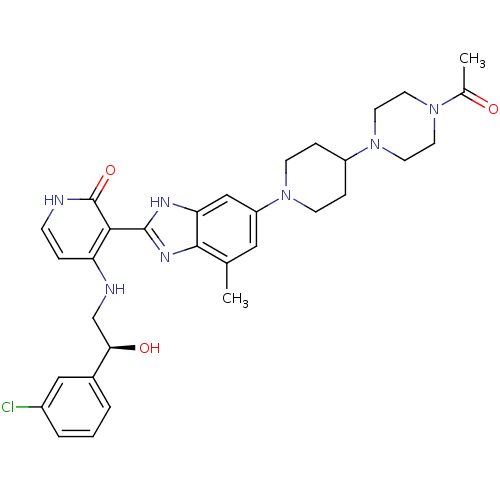
((S)-3-(6-(4-(4-acetylpiperazin-1-yl)piperidin-1-yl...)Show SMILES CC(=O)N1CCN(CC1)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C32H38ClN7O3/c1-20-16-25(39-10-7-24(8-11-39)40-14-12-38(13-15-40)21(2)41)18-27-30(20)37-31(36-27)29-26(6-9-34-32(29)43)35-19-28(42)22-4-3-5-23(33)17-22/h3-6,9,16-18,24,28,42H,7-8,10-15,19H2,1-2H3,(H,36,37)(H2,34,35,43)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330017
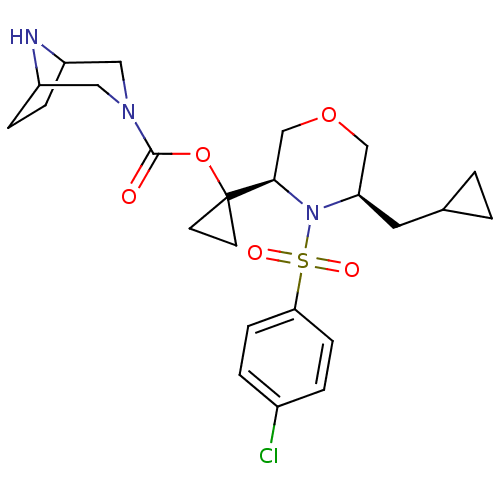
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(cycloprop...)Show SMILES Clc1ccc(cc1)S(=O)(=O)N1[C@H](CC2CC2)COC[C@@H]1C1(CC1)OC(=O)N1CC2CCC(C1)N2 |r,TLB:24:26:33:29.30| Show InChI InChI=1S/C24H32ClN3O5S/c25-17-3-7-21(8-4-17)34(30,31)28-20(11-16-1-2-16)14-32-15-22(28)24(9-10-24)33-23(29)27-12-18-5-6-19(13-27)26-18/h3-4,7-8,16,18-20,22,26H,1-2,5-6,9-15H2/t18?,19?,20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50544198
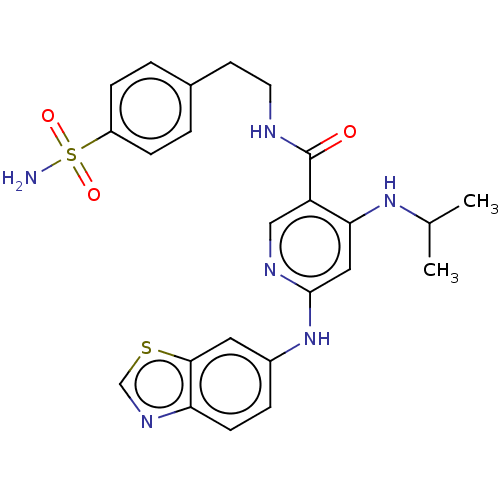
(CHEMBL4636136)Show SMILES CC(C)Nc1cc(Nc2ccc3ncsc3c2)ncc1C(=O)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C24H26N6O3S2/c1-15(2)29-21-12-23(30-17-5-8-20-22(11-17)34-14-28-20)27-13-19(21)24(31)26-10-9-16-3-6-18(7-4-16)35(25,32)33/h3-8,11-15H,9-10H2,1-2H3,(H,26,31)(H2,25,32,33)(H2,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Biocon Bristol Myers Squibb Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 11: 1402-1409 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00082
BindingDB Entry DOI: 10.7270/Q2542S4M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252143
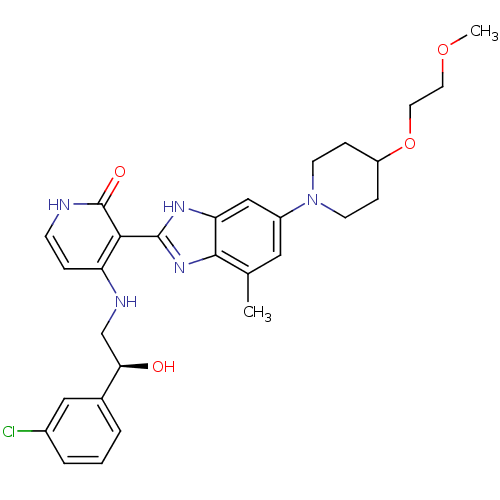
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES COCCOC1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C29H34ClN5O4/c1-18-14-21(35-10-7-22(8-11-35)39-13-12-38-2)16-24-27(18)34-28(33-24)26-23(6-9-31-29(26)37)32-17-25(36)19-4-3-5-20(30)15-19/h3-6,9,14-16,22,25,36H,7-8,10-13,17H2,1-2H3,(H,33,34)(H2,31,32,37)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM31772
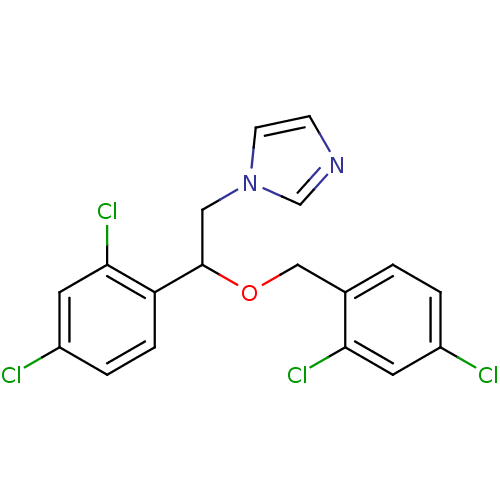
(1-[2-(2,4-dichlorobenzyl)oxy-2-(2,4-dichlorophenyl...)Show SMILES Clc1ccc(COC(Cn2ccnc2)c2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C18H14Cl4N2O/c19-13-2-1-12(16(21)7-13)10-25-18(9-24-6-5-23-11-24)15-4-3-14(20)8-17(15)22/h1-8,11,18H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01170
BindingDB Entry DOI: 10.7270/Q2DZ0D8J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252238

((S)-methyl 1-(2-(4-(2-(3-chlorophenyl)-2-hydroxyet...)Show SMILES COCCN(CCOC)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C32H41ClN6O4/c1-21-17-25(38-11-8-24(9-12-38)39(13-15-42-2)14-16-43-3)19-27-30(21)37-31(36-27)29-26(7-10-34-32(29)41)35-20-28(40)22-5-4-6-23(33)18-22/h4-7,10,17-19,24,28,40H,8-9,11-16,20H2,1-3H3,(H,36,37)(H2,34,35,41)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50586268
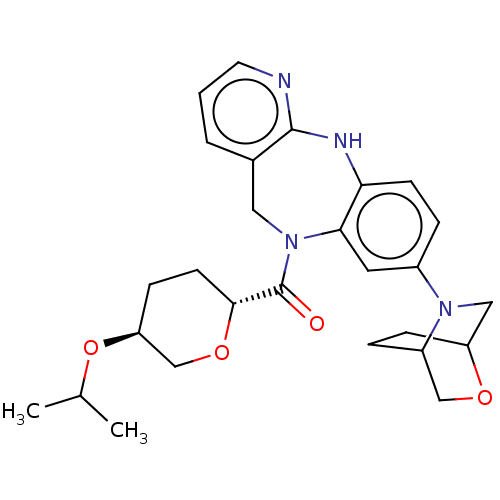
(CHEMBL5079148)Show SMILES CC(C)O[C@H]1CC[C@@H](OC1)C(=O)N1Cc2cccnc2Nc2ccc(cc12)N1CC2CCC1CO2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00089
BindingDB Entry DOI: 10.7270/Q2RJ4PCP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using testosterone as substrate after 5 mins by LC-MS analysis |
J Med Chem 59: 3340-52 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00030
BindingDB Entry DOI: 10.7270/Q29K4D50 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252142

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES COC1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C27H30ClN5O3/c1-16-12-19(33-10-7-20(36-2)8-11-33)14-22-25(16)32-26(31-22)24-21(6-9-29-27(24)35)30-15-23(34)17-4-3-5-18(28)13-17/h3-6,9,12-14,20,23,34H,7-8,10-11,15H2,1-2H3,(H,31,32)(H2,29,30,35)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50376470
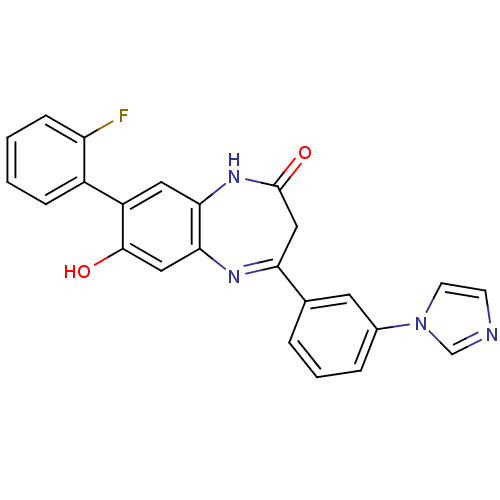
(CHEMBL405895)Show SMILES Oc1cc2N=C(CC(=O)Nc2cc1-c1ccccc1F)c1cccc(c1)-n1ccnc1 |c:4| Show InChI InChI=1S/C24H17FN4O2/c25-19-7-2-1-6-17(19)18-11-21-22(12-23(18)30)27-20(13-24(31)28-21)15-4-3-5-16(10-15)29-9-8-26-14-29/h1-12,14,30H,13H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 18: 2725-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.076
BindingDB Entry DOI: 10.7270/Q2S75H7D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50376467
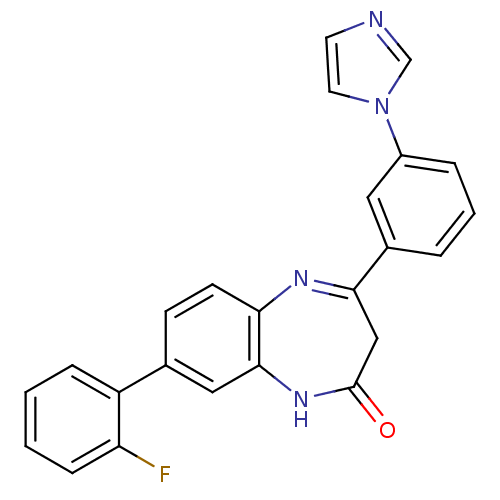
(CHEMBL260636)Show SMILES Fc1ccccc1-c1ccc2N=C(CC(=O)Nc2c1)c1cccc(c1)-n1ccnc1 |c:12| Show InChI InChI=1S/C24H17FN4O/c25-20-7-2-1-6-19(20)16-8-9-21-23(13-16)28-24(30)14-22(27-21)17-4-3-5-18(12-17)29-11-10-26-15-29/h1-13,15H,14H2,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 18: 2725-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.076
BindingDB Entry DOI: 10.7270/Q2S75H7D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50428877
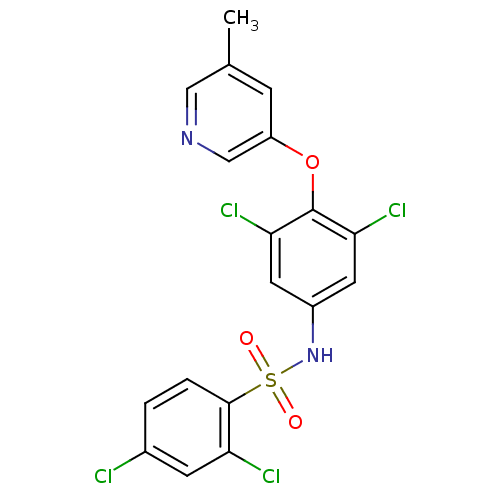
(CHEMBL2338480)Show SMILES Cc1cncc(Oc2c(Cl)cc(NS(=O)(=O)c3ccc(Cl)cc3Cl)cc2Cl)c1 Show InChI InChI=1S/C18H12Cl4N2O3S/c1-10-4-13(9-23-8-10)27-18-15(21)6-12(7-16(18)22)24-28(25,26)17-3-2-11(19)5-14(17)20/h2-9,24H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252144

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)OCCO |r| Show InChI InChI=1S/C28H32ClN5O4/c1-17-13-20(34-9-6-21(7-10-34)38-12-11-35)15-23-26(17)33-27(32-23)25-22(5-8-30-28(25)37)31-16-24(36)18-3-2-4-19(29)14-18/h2-5,8,13-15,21,24,35-36H,6-7,9-12,16H2,1H3,(H,32,33)(H2,30,31,37)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50297370

(3-(3-(tert-butylthio)-5-(pyridin-2-ylmethoxy)-1-(4...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C(O)=O)n(Cc2ccc(cc2)-c2nccs2)c2ccc(OCc3ccccn3)cc12 Show InChI InChI=1S/C33H35N3O3S2/c1-32(2,3)41-29-26-18-25(39-21-24-8-6-7-15-34-24)13-14-27(26)36(28(29)19-33(4,5)31(37)38)20-22-9-11-23(12-10-22)30-35-16-17-40-30/h6-18H,19-21H2,1-5H3,(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 52: 5803-15 (2009)
Article DOI: 10.1021/jm900945d
BindingDB Entry DOI: 10.7270/Q2G44QB1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330023
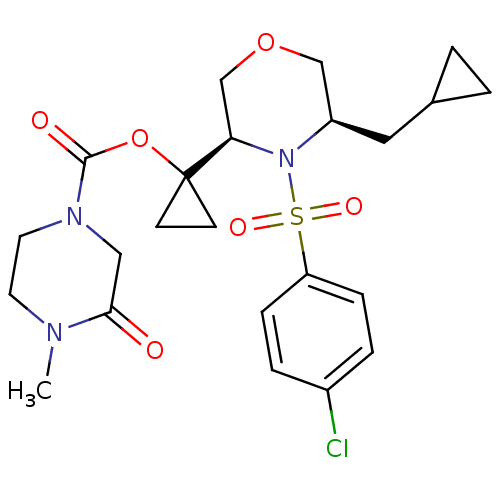
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(cycloprop...)Show SMILES CN1CCN(CC1=O)C(=O)OC1(CC1)[C@H]1COC[C@@H](CC2CC2)N1S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H30ClN3O6S/c1-25-10-11-26(13-21(25)28)22(29)33-23(8-9-23)20-15-32-14-18(12-16-2-3-16)27(20)34(30,31)19-6-4-17(24)5-7-19/h4-7,16,18,20H,2-3,8-15H2,1H3/t18-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50337730
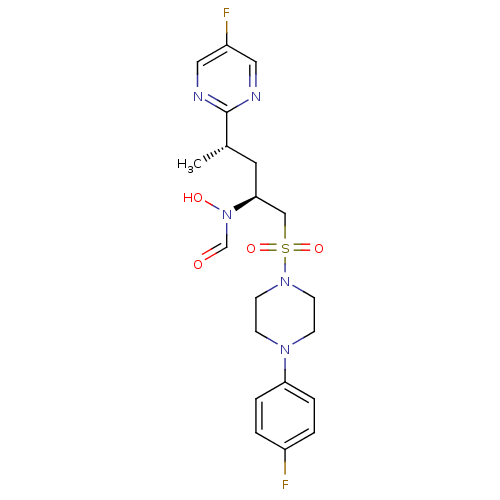
(CHEMBL1683443 | N-((2S,4S)-1-(4-(4-fluorophenyl)pi...)Show SMILES C[C@@H](C[C@@H](CS(=O)(=O)N1CCN(CC1)c1ccc(F)cc1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C20H25F2N5O4S/c1-15(20-23-11-17(22)12-24-20)10-19(27(29)14-28)13-32(30,31)26-8-6-25(7-9-26)18-4-2-16(21)3-5-18/h2-5,11-12,14-15,19,29H,6-10,13H2,1H3/t15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330015
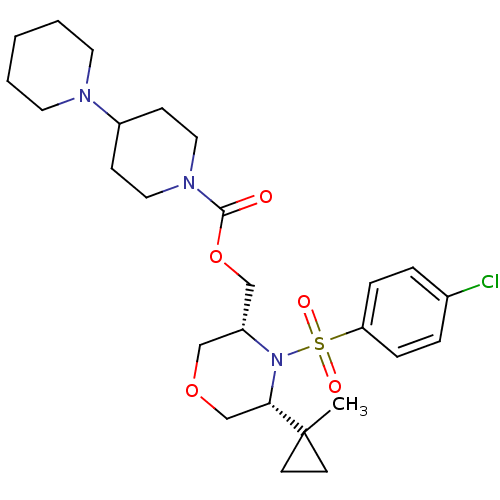
(((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(1-methylcyc...)Show SMILES CC1(CC1)[C@@H]1COC[C@H](COC(=O)N2CCC(CC2)N2CCCCC2)N1S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C26H38ClN3O5S/c1-26(11-12-26)24-19-34-17-22(30(24)36(32,33)23-7-5-20(27)6-8-23)18-35-25(31)29-15-9-21(10-16-29)28-13-3-2-4-14-28/h5-8,21-22,24H,2-4,9-19H2,1H3/t22-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252300
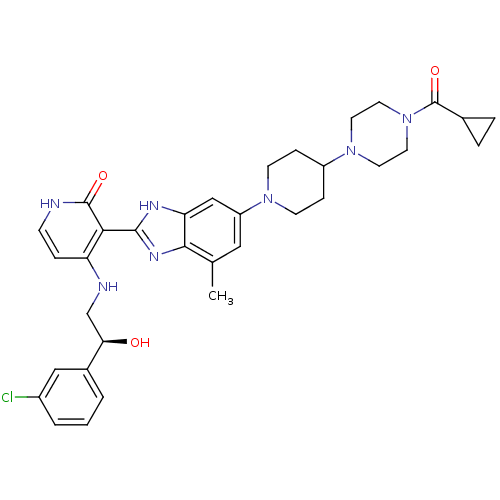
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)N1CCN(CC1)C(=O)C1CC1 |r| Show InChI InChI=1S/C34H40ClN7O3/c1-21-17-26(40-11-8-25(9-12-40)41-13-15-42(16-14-41)34(45)22-5-6-22)19-28-31(21)39-32(38-28)30-27(7-10-36-33(30)44)37-20-29(43)23-3-2-4-24(35)18-23/h2-4,7,10,17-19,22,25,29,43H,5-6,8-9,11-16,20H2,1H3,(H,38,39)(H2,36,37,44)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252303
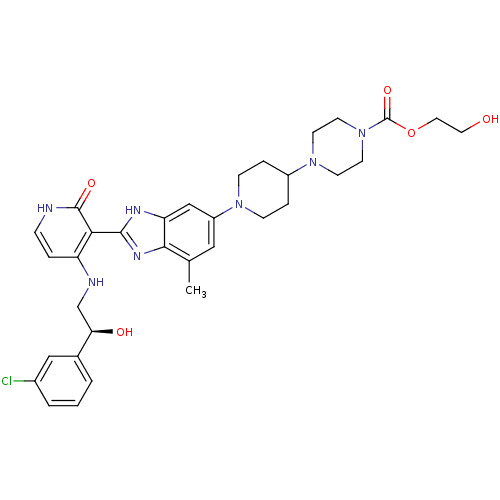
((S)-2-hydroxyethyl 4-(1-(2-(4-(2-(3-chlorophenyl)-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)N1CCN(CC1)C(=O)OCCO |r| Show InChI InChI=1S/C33H40ClN7O5/c1-21-17-25(39-9-6-24(7-10-39)40-11-13-41(14-12-40)33(45)46-16-15-42)19-27-30(21)38-31(37-27)29-26(5-8-35-32(29)44)36-20-28(43)22-3-2-4-23(34)18-22/h2-5,8,17-19,24,28,42-43H,6-7,9-16,20H2,1H3,(H,37,38)(H2,35,36,44)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50008760
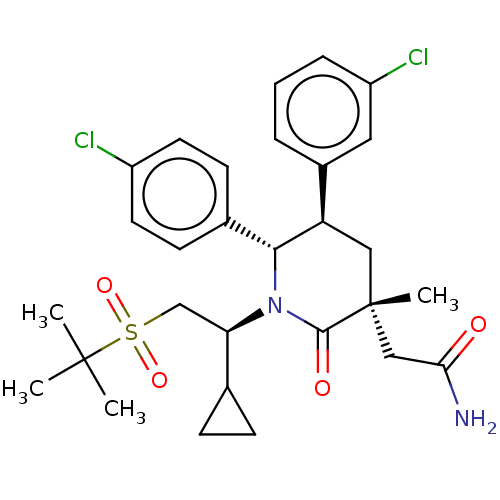
(CHEMBL3236364)Show SMILES CC(C)(C)S(=O)(=O)C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(N)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H36Cl2N2O4S/c1-28(2,3)38(36,37)17-24(18-8-9-18)33-26(19-10-12-21(30)13-11-19)23(20-6-5-7-22(31)14-20)15-29(4,27(33)35)16-25(32)34/h5-7,10-14,18,23-24,26H,8-9,15-17H2,1-4H3,(H2,32,34)/t23-,24-,26-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 1.83 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen Inc.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US8952036 (2015)
BindingDB Entry DOI: 10.7270/Q27P8X4P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330022
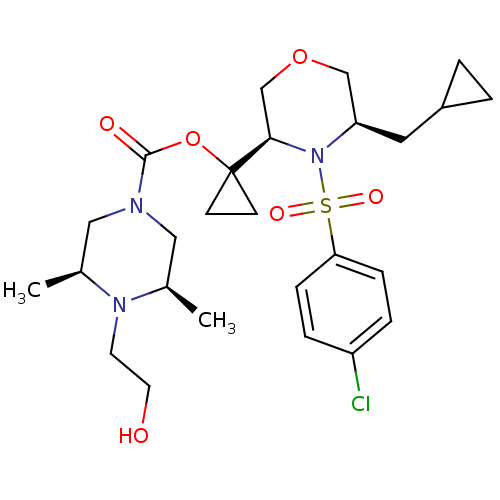
((3S,5R)-1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(c...)Show SMILES C[C@H]1CN(C[C@@H](C)N1CCO)C(=O)OC1(CC1)[C@H]1COC[C@@H](CC2CC2)N1S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C26H38ClN3O6S/c1-18-14-28(15-19(2)29(18)11-12-31)25(32)36-26(9-10-26)24-17-35-16-22(13-20-3-4-20)30(24)37(33,34)23-7-5-21(27)6-8-23/h5-8,18-20,22,24,31H,3-4,9-17H2,1-2H3/t18-,19+,22-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50335954

(CHEMBL1668898 | Sodium [2'-[(cyclopropanecarbonyl-...)Show SMILES CCOc1ccc(cn1)-c1ccc(c(CN(CC)C(=O)C2CC2)c1)-c1cc(CC([O-])=O)ccc1OC Show InChI InChI=1S/C29H32N2O5/c1-4-31(29(34)20-7-8-20)18-23-16-21(22-10-13-27(30-17-22)36-5-2)9-11-24(23)25-14-19(15-28(32)33)6-12-26(25)35-3/h6,9-14,16-17,20H,4-5,7-8,15,18H2,1-3H3,(H,32,33)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by time-dependent inhibition assay |
Bioorg Med Chem Lett 21: 1036-40 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.016
BindingDB Entry DOI: 10.7270/Q27P8ZNT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330018
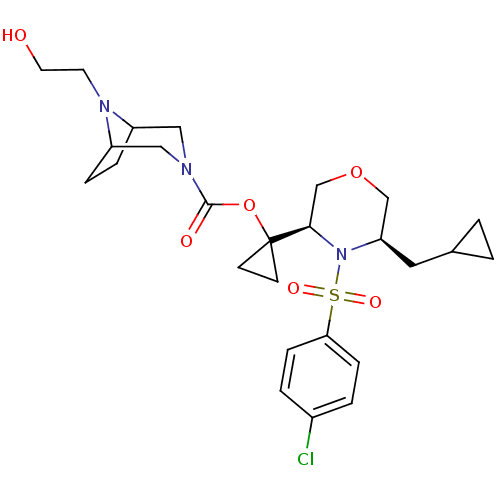
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(cycloprop...)Show SMILES OCCN1C2CCC1CN(C2)C(=O)OC1(CC1)[C@H]1COC[C@@H](CC2CC2)N1S(=O)(=O)c1ccc(Cl)cc1 |r,THB:11:9:3:5.6| Show InChI InChI=1S/C26H36ClN3O6S/c27-19-3-7-23(8-4-19)37(33,34)30-22(13-18-1-2-18)16-35-17-24(30)26(9-10-26)36-25(32)28-14-20-5-6-21(15-28)29(20)11-12-31/h3-4,7-8,18,20-22,24,31H,1-2,5-6,9-17H2/t20?,21?,22-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252296
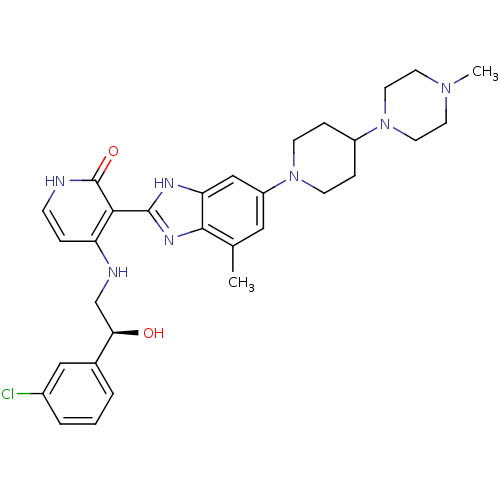
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES CN1CCN(CC1)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C31H38ClN7O2/c1-20-16-24(38-10-7-23(8-11-38)39-14-12-37(2)13-15-39)18-26-29(20)36-30(35-26)28-25(6-9-33-31(28)41)34-19-27(40)21-4-3-5-22(32)17-21/h3-6,9,16-18,23,27,40H,7-8,10-15,19H2,1-2H3,(H,35,36)(H2,33,34,41)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252302

((S)-2-methoxyethyl 4-(1-(2-(4-(2-(3-chlorophenyl)-...)Show SMILES COCCOC(=O)N1CCN(CC1)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C34H42ClN7O5/c1-22-18-26(40-10-7-25(8-11-40)41-12-14-42(15-13-41)34(45)47-17-16-46-2)20-28-31(22)39-32(38-28)30-27(6-9-36-33(30)44)37-21-29(43)23-4-3-5-24(35)19-23/h3-6,9,18-20,25,29,43H,7-8,10-17,21H2,1-2H3,(H,38,39)(H2,36,37,44)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) using BOMCC as a substrate incubated for 20 mins by fluorescence based analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113674
BindingDB Entry DOI: 10.7270/Q28K7DVS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM143355

(US9682953, 20.A-1)Show SMILES CCOC(=O)Cn1nc2C(=O)N(C(c2c1C(C)C)c1ccc(Cl)cc1C)c1cc(Cl)ccc1C Show InChI InChI=1S/C25H22F3N3O2/c26-25(27,28)20-8-11-23(30-16-20)33-22-5-1-3-18(14-22)13-17-6-9-21(10-7-17)31-24(32)19-4-2-12-29-15-19/h1-5,8,11-16,21H,6-7,9-10H2,(H,31,32)/b17-13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 5 mins followed by NADPH cofactor addition and measured ... |
Bioorg Med Chem Lett 29: 238-243 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.048
BindingDB Entry DOI: 10.7270/Q2125X14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252194
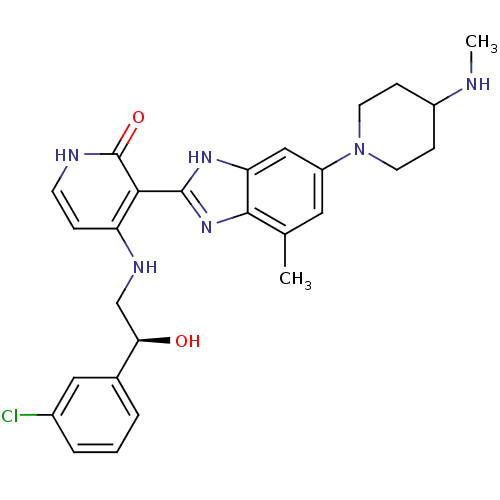
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES CNC1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C27H31ClN6O2/c1-16-12-20(34-10-7-19(29-2)8-11-34)14-22-25(16)33-26(32-22)24-21(6-9-30-27(24)36)31-15-23(35)17-4-3-5-18(28)13-17/h3-6,9,12-14,19,23,29,35H,7-8,10-11,15H2,1-2H3,(H,32,33)(H2,30,31,36)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50234534
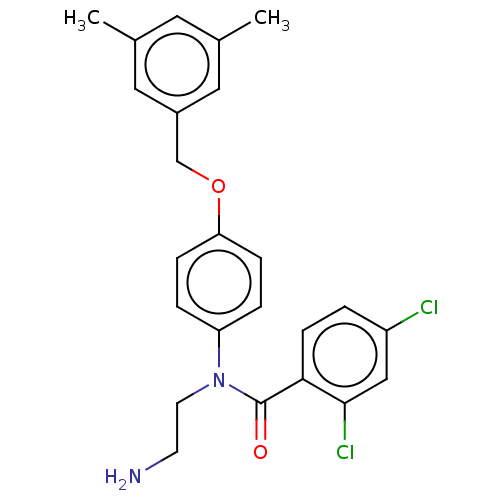
(CHEMBL4088945)Show SMILES Cl.Cc1cc(C)cc(COc2ccc(cc2)N(CCN)C(=O)c2ccc(Cl)cc2Cl)c1 Show InChI InChI=1S/C24H24Cl2N2O2/c1-16-11-17(2)13-18(12-16)15-30-21-6-4-20(5-7-21)28(10-9-27)24(29)22-8-3-19(25)14-23(22)26/h3-8,11-14H,9-10,15,27H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem 25: 1571-1584 (2017)
Article DOI: 10.1016/j.bmc.2016.11.019
BindingDB Entry DOI: 10.7270/Q20004C6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252299

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES COCCOCC(=O)N1CCN(CC1)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C35H44ClN7O5/c1-23-18-27(41-10-7-26(8-11-41)42-12-14-43(15-13-42)31(45)22-48-17-16-47-2)20-29-33(23)40-34(39-29)32-28(6-9-37-35(32)46)38-21-30(44)24-4-3-5-25(36)19-24/h3-6,9,18-20,26,30,44H,7-8,10-17,21-22H2,1-2H3,(H,39,40)(H2,37,38,46)/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252294
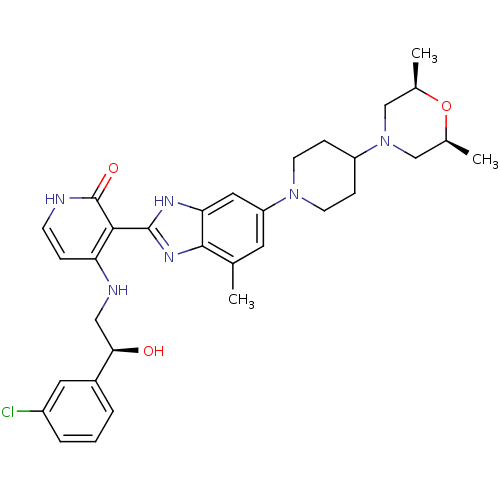
(4-((S)-2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES C[C@H]1CN(C[C@@H](C)O1)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C32H39ClN6O3/c1-19-13-25(38-11-8-24(9-12-38)39-17-20(2)42-21(3)18-39)15-27-30(19)37-31(36-27)29-26(7-10-34-32(29)41)35-16-28(40)22-5-4-6-23(33)14-22/h4-7,10,13-15,20-21,24,28,40H,8-9,11-12,16-18H2,1-3H3,(H,36,37)(H2,34,35,41)/t20-,21+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
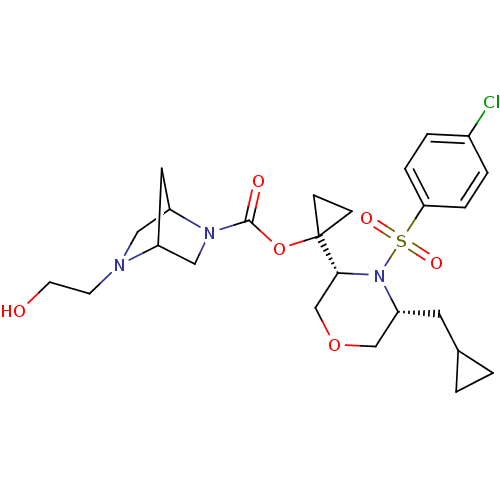
(Homo sapiens (Human)) | BDBM50330021
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(cycloprop...)Show SMILES OCCN1CC2CC1CN2C(=O)OC1(CC1)[C@H]1COC[C@@H](CC2CC2)N1S(=O)(=O)c1ccc(Cl)cc1 |r,TLB:2:3:9.8:6| Show InChI InChI=1S/C25H34ClN3O6S/c26-18-3-5-22(6-4-18)36(32,33)29-21(11-17-1-2-17)15-34-16-23(29)25(7-8-25)35-24(31)28-14-19-12-20(28)13-27(19)9-10-30/h3-6,17,19-21,23,30H,1-2,7-16H2/t19?,20?,21-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
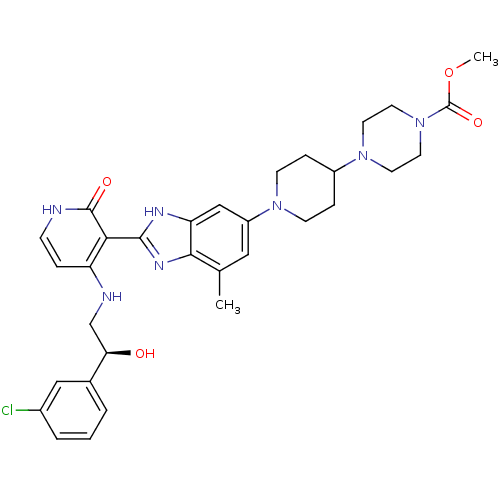
(Homo sapiens (Human)) | BDBM50252301
((S)-methyl 4-(1-(2-(4-(2-(3-chlorophenyl)-2-hydrox...)Show SMILES COC(=O)N1CCN(CC1)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C32H38ClN7O4/c1-20-16-24(38-10-7-23(8-11-38)39-12-14-40(15-13-39)32(43)44-2)18-26-29(20)37-30(36-26)28-25(6-9-34-31(28)42)35-19-27(41)21-4-3-5-22(33)17-21/h3-6,9,16-18,23,27,41H,7-8,10-15,19H2,1-2H3,(H,36,37)(H2,34,35,42)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
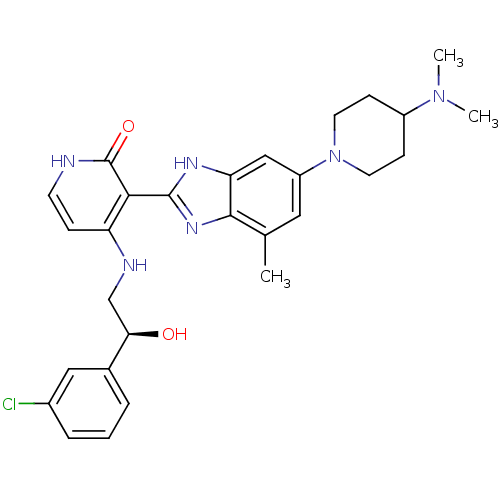
(Homo sapiens (Human)) | BDBM50252195
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES CN(C)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C28H33ClN6O2/c1-17-13-21(35-11-8-20(9-12-35)34(2)3)15-23-26(17)33-27(32-23)25-22(7-10-30-28(25)37)31-16-24(36)18-5-4-6-19(29)14-18/h4-7,10,13-15,20,24,36H,8-9,11-12,16H2,1-3H3,(H,32,33)(H2,30,31,37)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data