

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054718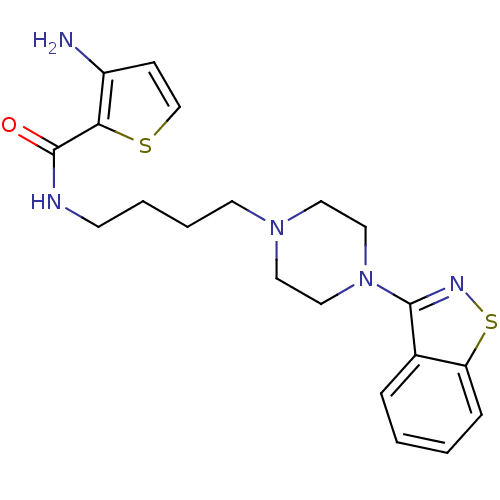 (3-Amino-thiophene-2-carboxylic acid [4-(4-benzo[d]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054710 (1,2,3,4-Tetrahydro-quinoline-8-carboxylic acid [4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054713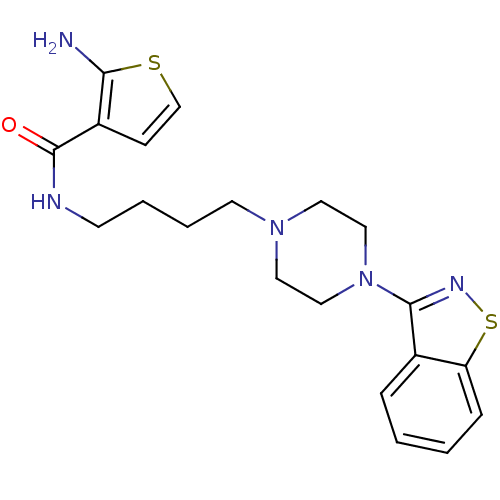 (2-Amino-thiophene-3-carboxylic acid [4-(4-benzo[d]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054706 (1H-Indole-7-carboxylic acid [4-(4-benzo[d]isothiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054707 (CHEMBL344994 | Pyridine-2-carboxylic acid [4-(4-be...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054705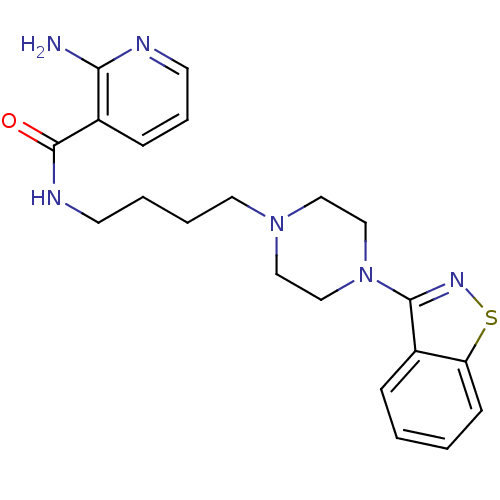 (2-Amino-N-[4-(4-benzo[d]isothiazol-3-yl-piperazin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054715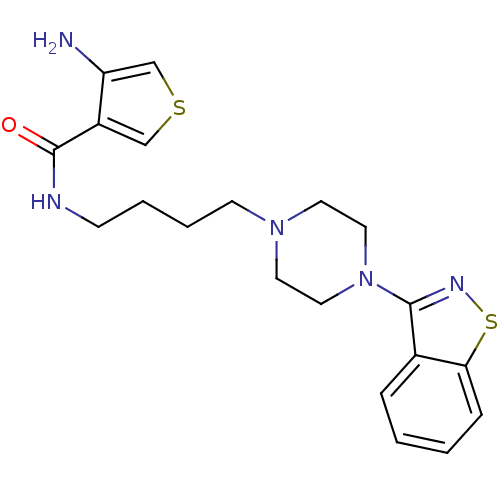 (4-Amino-thiophene-3-carboxylic acid [4-(4-benzo[d]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054716 (1H-Indazole-3-carboxylic acid [4-(4-benzo[d]isothi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054714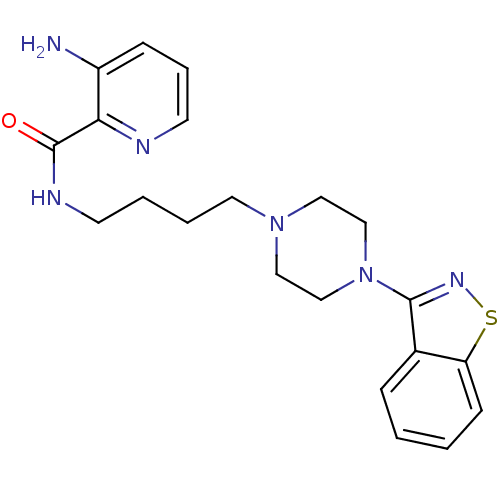 (3-Amino-pyridine-2-carboxylic acid [4-(4-benzo[d]i...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054712 (3H-Benzoimidazole-4-carboxylic acid [4-(4-benzo[d]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054704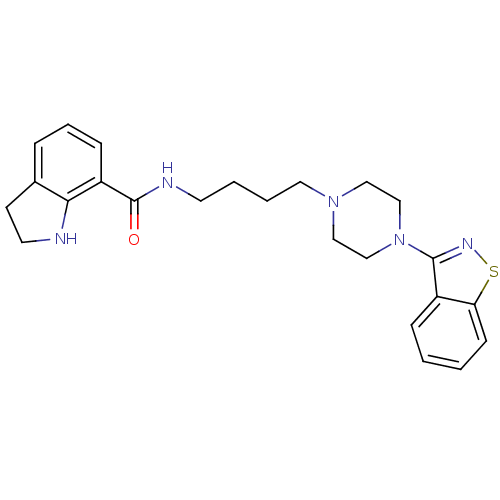 (2,3-Dihydro-1H-indole-7-carboxylic acid [4-(4-benz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054703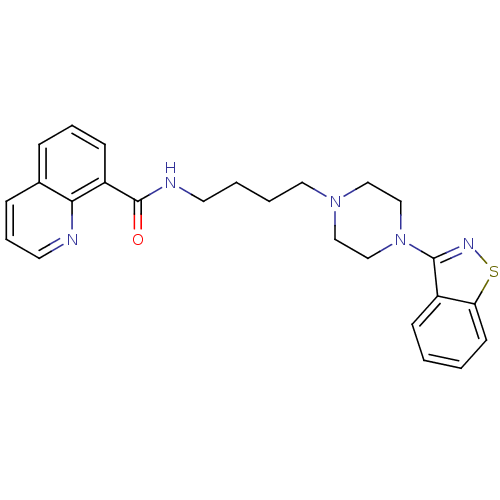 (CHEMBL358268 | Quinoline-8-carboxylic acid [4-(4-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054719 (CHEMBL356498 | N-[4-(4-Benzo[d]isothiazol-3-yl-pip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Polytechnic University Curated by ChEMBL | Assay Description Inhibitory activity towards 5-hydroxytryptamine 1A receptor by the displacement of [3H]-8-OH-DPAT in mouse hippocampus | J Med Chem 33: 386-94 (1990) BindingDB Entry DOI: 10.7270/Q23T9G58 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054711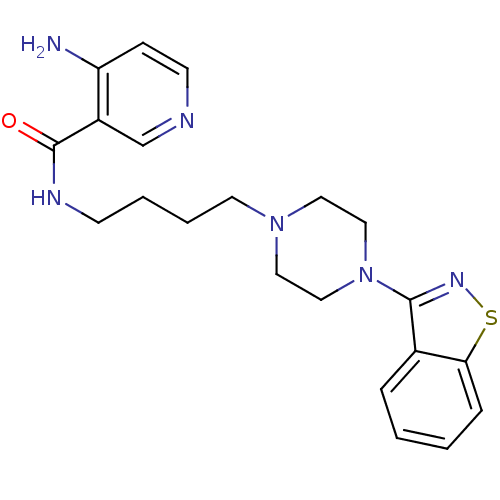 (4-Amino-N-[4-(4-benzo[d]isothiazol-3-yl-piperazin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054708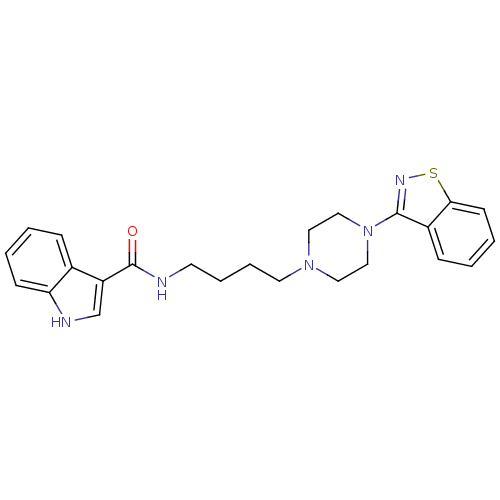 (1H-Indole-3-carboxylic acid [4-(4-benzo[d]isothiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054702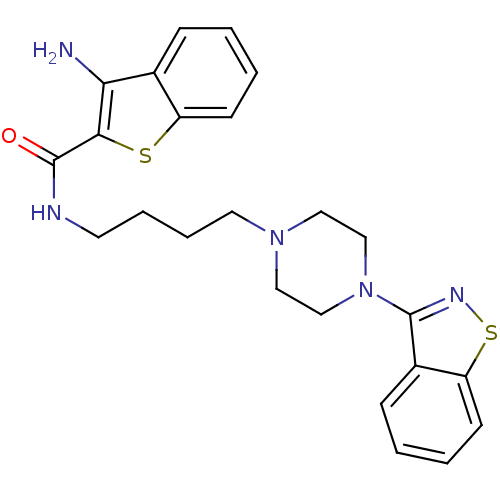 (3-Amino-benzo[b]thiophene-2-carboxylic acid [4-(4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054709 (CHEMBL141494 | N-[4-(4-Benzo[d]isothiazol-3-yl-pip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054717 (3-Amino-N-[4-(4-benzo[d]isothiazol-3-yl-piperazin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50060437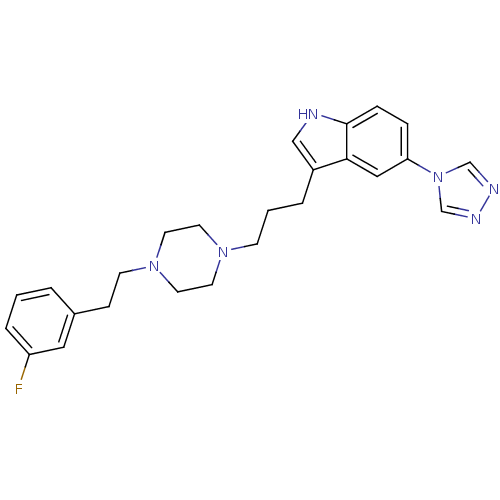 (3-(3-{4-[2-(3-Fluoro-phenyl)-ethyl]-piperazin-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the affinity at 5-hydroxytryptamine 1A receptor | J Med Chem 40: 3501-3 (1997) Article DOI: 10.1021/jm9704560 BindingDB Entry DOI: 10.7270/Q2DZ07D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50069041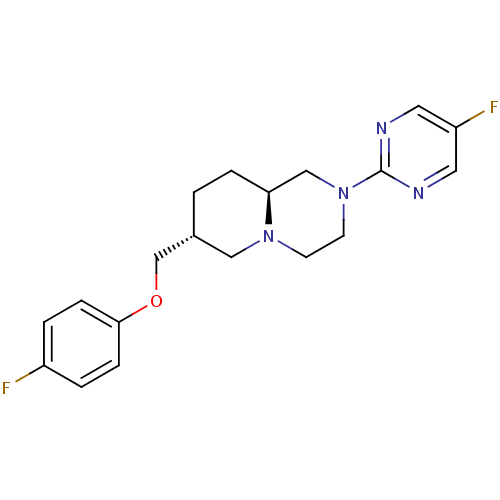 ((7R,9aS)-7-(4-Fluoro-phenoxymethyl)-2-(5-fluoro-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro binding affinity against 5-hydroxytryptamine 1A receptor in CHO cells | Bioorg Med Chem Lett 8: 725-30 (1999) BindingDB Entry DOI: 10.7270/Q29Z941K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50034043 (1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor was determined using [3H]-8-OH-DPAT radioligand | J Med Chem 38: 1119-31 (1995) BindingDB Entry DOI: 10.7270/Q2S75H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50007674 ((+)-erythro 4-[2-(4-Benzyl-piperidin-1-yl)-1-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Compound was tested for the binding affinity against 5-hydroxytryptamine 1A receptor by using [3H]-DPAT as radioligand | J Med Chem 34: 3085-90 (1991) BindingDB Entry DOI: 10.7270/Q2FN155W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the affinity at 5-hydroxytryptamine 1A receptor | J Med Chem 40: 3501-3 (1997) Article DOI: 10.1021/jm9704560 BindingDB Entry DOI: 10.7270/Q2DZ07D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50108654 (1-[3-methoxyspiro[3,4-dihydro-1H-isochromene-1,4'-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmazeutisches Institut der Universität Freiburg Curated by ChEMBL | Assay Description Inhibition of 5-OH-DPAT binding to 5-hydroxytryptamine 1A receptor | J Med Chem 45: 438-48 (2002) BindingDB Entry DOI: 10.7270/Q2VT1RDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50108653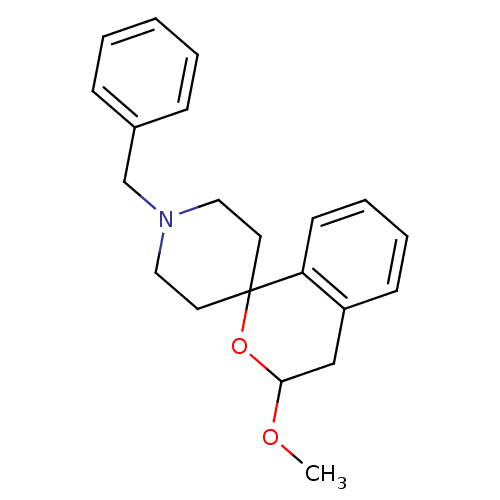 (1'-benzyl-3-methoxyspiro[3,4-dihydro-1H-isochromen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmazeutisches Institut der Universität Freiburg Curated by ChEMBL | Assay Description Inhibition of 5-OH-DPAT binding to 5-hydroxytryptamine 1A receptor | J Med Chem 45: 438-48 (2002) BindingDB Entry DOI: 10.7270/Q2VT1RDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50108659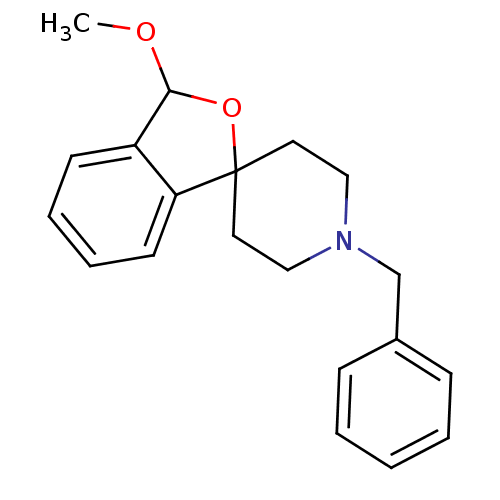 (1'-benzyl-3-methoxy-3H-spiro[2-benzofuran-1,4'-pip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmazeutisches Institut der Universität Freiburg Curated by ChEMBL | Assay Description Inhibition of 5-OH-DPAT binding to 5-hydroxytryptamine 1A receptor | J Med Chem 45: 438-48 (2002) BindingDB Entry DOI: 10.7270/Q2VT1RDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50122716 (5-(Piperidin-4-ylmethoxy)-9-propyl-1,2,3,4-tetrahy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen Curated by ChEMBL | Assay Description Ability to displace [3H]8-OH-DPAT from 5-hydroxytryptamine 1A receptor | J Med Chem 46: 138-47 (2002) Article DOI: 10.1021/jm020954v BindingDB Entry DOI: 10.7270/Q2J67G8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50012983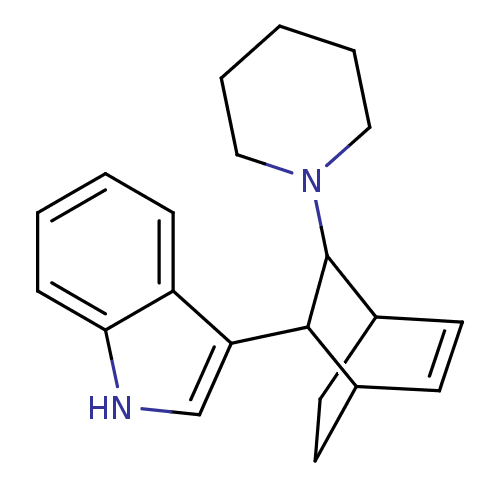 (3-(3-Piperidin-1-yl-bicyclo[2.2.2]oct-5-en-2-yl)-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Polytechnic University Curated by ChEMBL | Assay Description Inhibitory activity towards 5-hydroxytryptamine 1A receptor by the displacement of [3H]-8-OH-DPAT in mouse hippocampus | J Med Chem 33: 386-94 (1990) BindingDB Entry DOI: 10.7270/Q23T9G58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50012983 (3-(3-Piperidin-1-yl-bicyclo[2.2.2]oct-5-en-2-yl)-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Polytechnic University Curated by ChEMBL | Assay Description Inhibitory activity towards 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in mouse hippocampus | J Med Chem 33: 386-94 (1990) BindingDB Entry DOI: 10.7270/Q23T9G58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50012980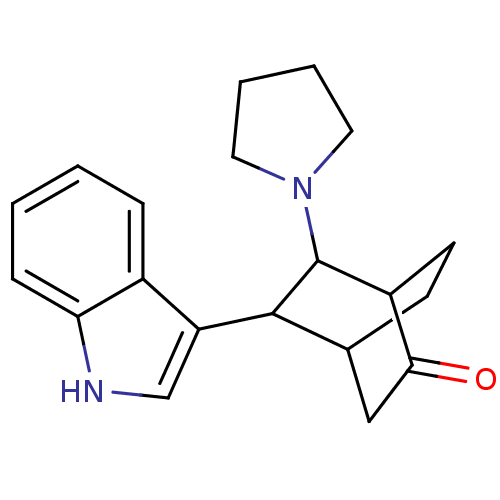 (5-(1H-Indol-3-yl)-6-pyrrolidin-1-yl-bicyclo[2.2.2]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Polytechnic University Curated by ChEMBL | Assay Description Inhibitory activity towards 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in mouse hippocampus | J Med Chem 33: 386-94 (1990) BindingDB Entry DOI: 10.7270/Q23T9G58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50012980 (5-(1H-Indol-3-yl)-6-pyrrolidin-1-yl-bicyclo[2.2.2]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Polytechnic University Curated by ChEMBL | Assay Description Inhibitory activity towards 5-hydroxytryptamine 1A receptor by the displacement of [3H]-8-OH-DPAT in mouse hippocampus | J Med Chem 33: 386-94 (1990) BindingDB Entry DOI: 10.7270/Q23T9G58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50012982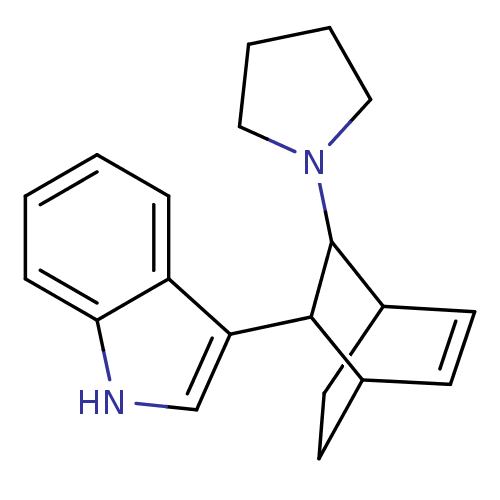 (3-(3-Pyrrolidin-1-yl-bicyclo[2.2.2]oct-5-en-2-yl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Polytechnic University Curated by ChEMBL | Assay Description Inhibitory activity towards 5-hydroxytryptamine 1A receptor by the displacement of [3H]-8-OH-DPAT in mouse hippocampus | J Med Chem 33: 386-94 (1990) BindingDB Entry DOI: 10.7270/Q23T9G58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50012982 (3-(3-Pyrrolidin-1-yl-bicyclo[2.2.2]oct-5-en-2-yl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Polytechnic University Curated by ChEMBL | Assay Description Inhibitory activity towards 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in mouse hippocampus | J Med Chem 33: 386-94 (1990) BindingDB Entry DOI: 10.7270/Q23T9G58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50012981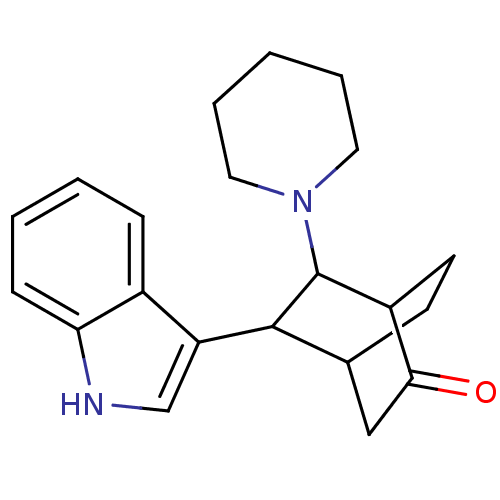 (5-(1H-Indol-3-yl)-6-piperidin-1-yl-bicyclo[2.2.2]o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Polytechnic University Curated by ChEMBL | Assay Description Inhibitory activity towards 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in mouse hippocampus | J Med Chem 33: 386-94 (1990) BindingDB Entry DOI: 10.7270/Q23T9G58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50012981 (5-(1H-Indol-3-yl)-6-piperidin-1-yl-bicyclo[2.2.2]o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Polytechnic University Curated by ChEMBL | Assay Description Inhibitory activity towards 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in mouse hippocampus | J Med Chem 33: 386-94 (1990) BindingDB Entry DOI: 10.7270/Q23T9G58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50012984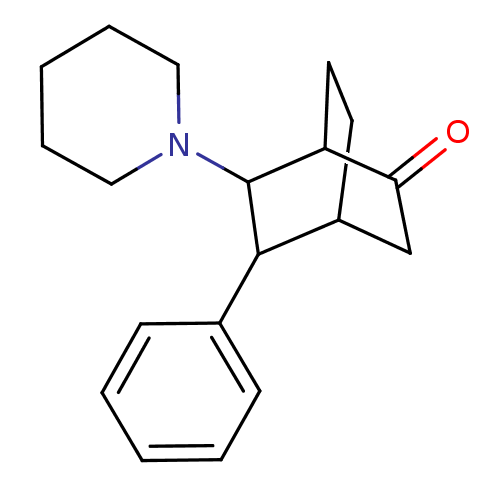 (5-Phenyl-6-piperidin-1-yl-bicyclo[2.2.2]octan-2-on...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Polytechnic University Curated by ChEMBL | Assay Description Inhibitory activity towards 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in mouse hippocampus | J Med Chem 33: 386-94 (1990) BindingDB Entry DOI: 10.7270/Q23T9G58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||