 Found 100 hits Enz. Inhib. hit(s) with all data for entry = 50031543
Found 100 hits Enz. Inhib. hit(s) with all data for entry = 50031543 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315080

(4-(3-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6...)Show SMILES C[C@@H]1COCCN1c1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)NCCN(C)C)cc1)N1C2CCC1COC2 |r| Show InChI InChI=1S/C32H41N9O4/c1-21-18-44-17-16-40(21)30-36-28(37-31(38-30)41-26-12-13-27(41)20-45-19-26)22-4-8-24(9-5-22)34-32(43)35-25-10-6-23(7-11-25)29(42)33-14-15-39(2)3/h4-11,21,26-27H,12-20H2,1-3H3,(H,33,42)(H2,34,35,43)/t21-,26?,27?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315071

(4-(3-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6...)Show SMILES CN(C)CCNC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)C2CCOCC2)cc1 Show InChI InChI=1S/C32H40N8O4/c1-39(2)16-15-33-30(41)23-5-9-25(10-6-23)35-32(42)34-24-7-3-21(4-8-24)28-36-29(22-13-17-43-18-14-22)38-31(37-28)40-26-11-12-27(40)20-44-19-26/h3-10,22,26-27H,11-20H2,1-2H3,(H,33,41)(H2,34,35,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315056

(1-(4-(4,6-di(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-...)Show SMILES OCc1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)N2C3CCC2COC3)cc1 Show InChI InChI=1S/C29H33N7O4/c37-13-18-1-5-20(6-2-18)30-29(38)31-21-7-3-19(4-8-21)26-32-27(35-22-9-10-23(35)15-39-14-22)34-28(33-26)36-24-11-12-25(36)17-40-16-24/h1-8,22-25,37H,9-17H2,(H2,30,31,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315079
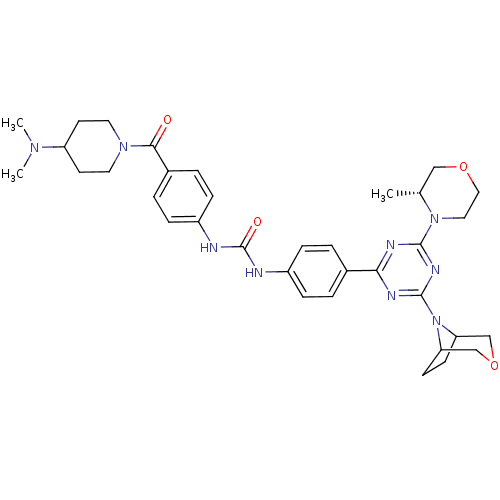
(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-((...)Show SMILES C[C@@H]1COCCN1c1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N2CCC(CC2)N(C)C)cc1)N1C2CCC1COC2 |r| Show InChI InChI=1S/C35H45N9O4/c1-23-20-47-19-18-43(23)33-38-31(39-34(40-33)44-29-12-13-30(44)22-48-21-29)24-4-8-26(9-5-24)36-35(46)37-27-10-6-25(7-11-27)32(45)42-16-14-28(15-17-42)41(2)3/h4-11,23,28-30H,12-22H2,1-3H3,(H2,36,37,46)/t23-,29?,30?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315055
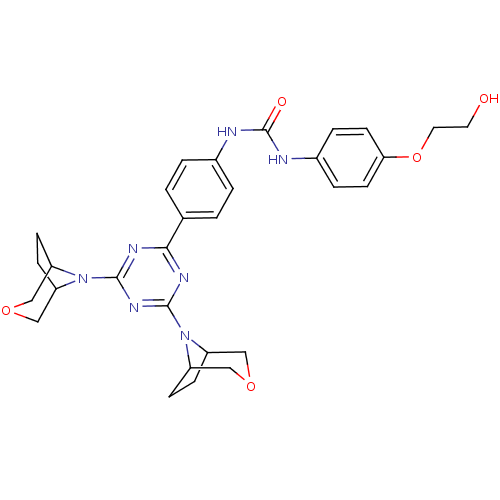
(1-(4-(4,6-di(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-...)Show SMILES OCCOc1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)N2C3CCC2COC3)cc1 Show InChI InChI=1S/C30H35N7O5/c38-13-14-42-26-11-5-21(6-12-26)32-30(39)31-20-3-1-19(2-4-20)27-33-28(36-22-7-8-23(36)16-40-15-22)35-29(34-27)37-24-9-10-25(37)18-41-17-24/h1-6,11-12,22-25,38H,7-10,13-18H2,(H2,31,32,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315070
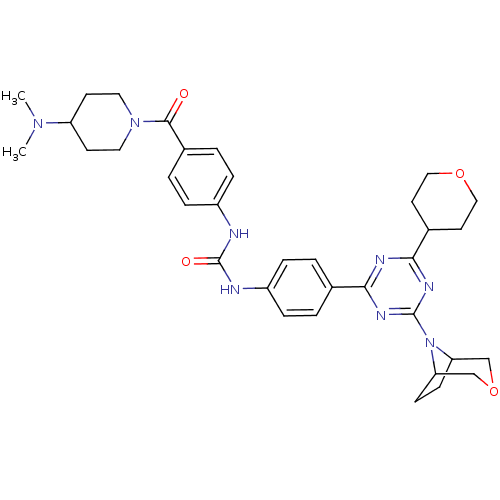
(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-(t...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)C2CCOCC2)cc1 Show InChI InChI=1S/C35H44N8O4/c1-41(2)28-13-17-42(18-14-28)33(44)25-5-9-27(10-6-25)37-35(45)36-26-7-3-23(4-8-26)31-38-32(24-15-19-46-20-16-24)40-34(39-31)43-29-11-12-30(43)22-47-21-29/h3-10,24,28-30H,11-22H2,1-2H3,(H2,36,37,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315073
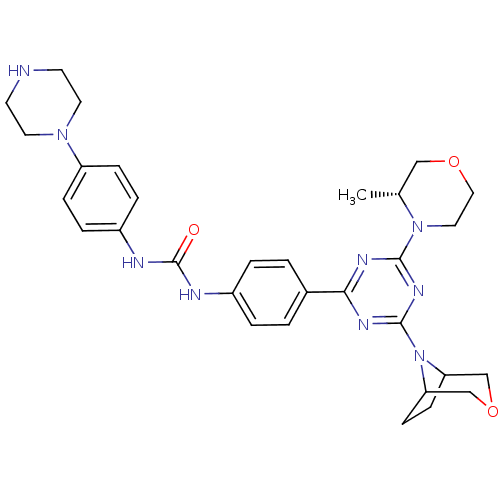
(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-((...)Show SMILES C[C@@H]1COCCN1c1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)N2CCNCC2)cc1)N1C2CCC1COC2 |r| Show InChI InChI=1S/C31H39N9O3/c1-21-18-42-17-16-39(21)29-35-28(36-30(37-29)40-26-10-11-27(40)20-43-19-26)22-2-4-23(5-3-22)33-31(41)34-24-6-8-25(9-7-24)38-14-12-32-13-15-38/h2-9,21,26-27,32H,10-20H2,1H3,(H2,33,34,41)/t21-,26?,27?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315078

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-((...)Show SMILES CC(C)N1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOC[C@H]2C)N2C3CCC2COC3)cc1 |r| Show InChI InChI=1S/C35H45N9O4/c1-23(2)41-14-16-42(17-15-41)32(45)26-6-10-28(11-7-26)37-35(46)36-27-8-4-25(5-9-27)31-38-33(43-18-19-47-20-24(43)3)40-34(39-31)44-29-12-13-30(44)22-48-21-29/h4-11,23-24,29-30H,12-22H2,1-3H3,(H2,36,37,46)/t24-,29?,30?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315069
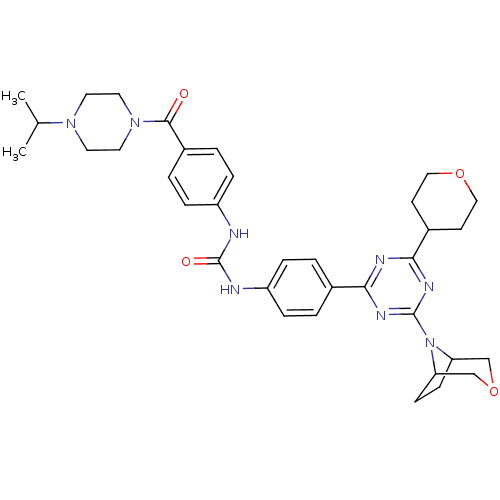
(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-(t...)Show SMILES CC(C)N1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)C2CCOCC2)cc1 Show InChI InChI=1S/C35H44N8O4/c1-23(2)41-15-17-42(18-16-41)33(44)26-5-9-28(10-6-26)37-35(45)36-27-7-3-24(4-8-27)31-38-32(25-13-19-46-20-14-25)40-34(39-31)43-29-11-12-30(43)22-47-21-29/h3-10,23,25,29-30H,11-22H2,1-2H3,(H2,36,37,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315063

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-(t...)Show SMILES CN1CCN(CC1)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)C2CCOCC2)cc1 Show InChI InChI=1S/C32H40N8O3/c1-38-14-16-39(17-15-38)26-8-6-25(7-9-26)34-32(41)33-24-4-2-22(3-5-24)29-35-30(23-12-18-42-19-13-23)37-31(36-29)40-27-10-11-28(40)21-43-20-27/h2-9,23,27-28H,10-21H2,1H3,(H2,33,34,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315064

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-(t...)Show SMILES O=C(Nc1ccc(cc1)N1CCNCC1)Nc1ccc(cc1)-c1nc(nc(n1)N1C2CCC1COC2)C1CCOCC1 Show InChI InChI=1S/C31H38N8O3/c40-31(34-24-5-7-25(8-6-24)38-15-13-32-14-16-38)33-23-3-1-21(2-4-23)28-35-29(22-11-17-41-18-12-22)37-30(36-28)39-26-9-10-27(39)20-42-19-26/h1-8,22,26-27,32H,9-20H2,(H2,33,34,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315067
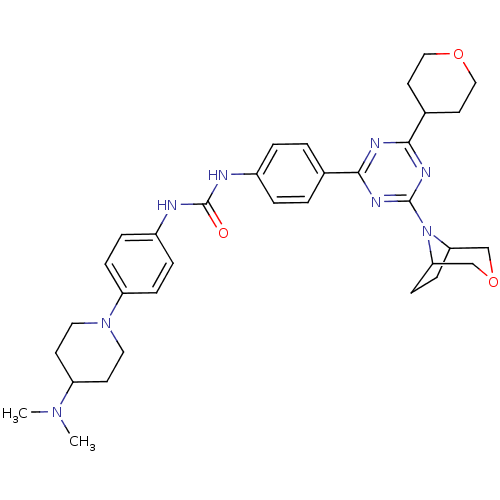
(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-(t...)Show SMILES CN(C)C1CCN(CC1)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)C2CCOCC2)cc1 Show InChI InChI=1S/C34H44N8O3/c1-40(2)27-13-17-41(18-14-27)28-9-7-26(8-10-28)36-34(43)35-25-5-3-23(4-6-25)31-37-32(24-15-19-44-20-16-24)39-33(38-31)42-29-11-12-30(42)22-45-21-29/h3-10,24,27,29-30H,11-22H2,1-2H3,(H2,35,36,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315065

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-(t...)Show SMILES CCN1CCN(CC1)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)C2CCOCC2)cc1 Show InChI InChI=1S/C33H42N8O3/c1-2-39-15-17-40(18-16-39)27-9-7-26(8-10-27)35-33(42)34-25-5-3-23(4-6-25)30-36-31(24-13-19-43-20-14-24)38-32(37-30)41-28-11-12-29(41)22-44-21-28/h3-10,24,28-29H,2,11-22H2,1H3,(H2,34,35,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315077

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-((...)Show SMILES C[C@@H]1COCCN1c1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N2CCN(C)CC2)cc1)N1C2CCC1COC2 |r| Show InChI InChI=1S/C33H41N9O4/c1-22-19-45-18-17-41(22)31-36-29(37-32(38-31)42-27-11-12-28(42)21-46-20-27)23-3-7-25(8-4-23)34-33(44)35-26-9-5-24(6-10-26)30(43)40-15-13-39(2)14-16-40/h3-10,22,27-28H,11-21H2,1-2H3,(H2,34,35,44)/t22-,27?,28?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315048
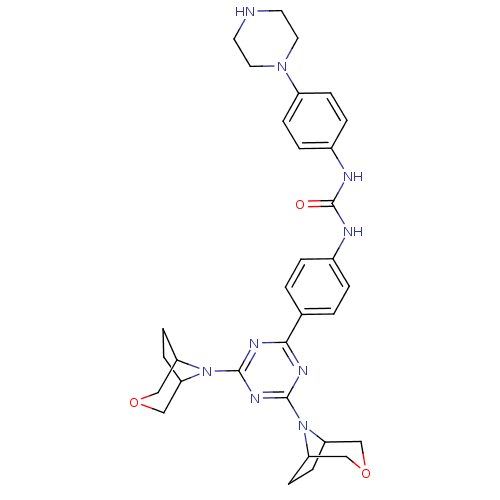
(1-(4-(4,6-di(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-...)Show SMILES O=C(Nc1ccc(cc1)N1CCNCC1)Nc1ccc(cc1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C32H39N9O3/c42-32(35-23-5-7-24(8-6-23)39-15-13-33-14-16-39)34-22-3-1-21(2-4-22)29-36-30(40-25-9-10-26(40)18-43-17-25)38-31(37-29)41-27-11-12-28(41)20-44-19-27/h1-8,25-28,33H,9-20H2,(H2,34,35,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315062

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-(3...)Show SMILES CN1CCN(CC1)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)C2=CCOCC2)N2C3CCC2COC3)cc1 |t:30| Show InChI InChI=1S/C32H38N8O3/c1-38-14-16-39(17-15-38)26-8-6-25(7-9-26)34-32(41)33-24-4-2-22(3-5-24)29-35-30(23-12-18-42-19-13-23)37-31(36-29)40-27-10-11-28(40)21-43-20-27/h2-9,12,27-28H,10-11,13-21H2,1H3,(H2,33,34,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315057
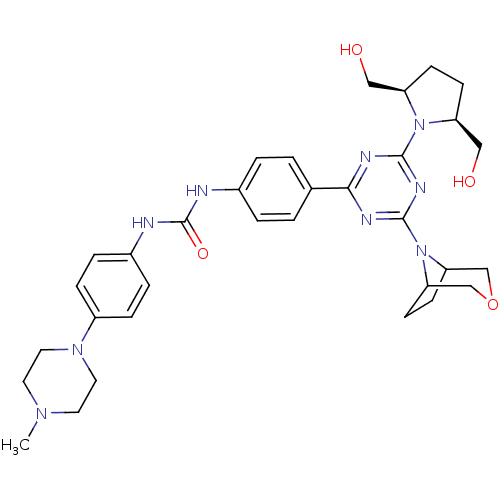
(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-((...)Show SMILES CN1CCN(CC1)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)N2[C@@H](CO)CC[C@H]2CO)cc1 |r| Show InChI InChI=1S/C33H43N9O4/c1-39-14-16-40(17-15-39)25-8-6-24(7-9-25)35-33(45)34-23-4-2-22(3-5-23)30-36-31(41-26(18-43)10-11-27(41)19-44)38-32(37-30)42-28-12-13-29(42)21-46-20-28/h2-9,26-29,43-44H,10-21H2,1H3,(H2,34,35,45)/t26-,27+,28?,29? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315072

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-(t...)Show SMILES CN(C)CCOc1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)C2CCOCC2)cc1 Show InChI InChI=1S/C31H39N7O4/c1-37(2)15-18-42-27-11-7-24(8-12-27)33-31(39)32-23-5-3-21(4-6-23)28-34-29(22-13-16-40-17-14-22)36-30(35-28)38-25-9-10-26(38)20-41-19-25/h3-8,11-12,22,25-26H,9-10,13-20H2,1-2H3,(H2,32,33,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315054
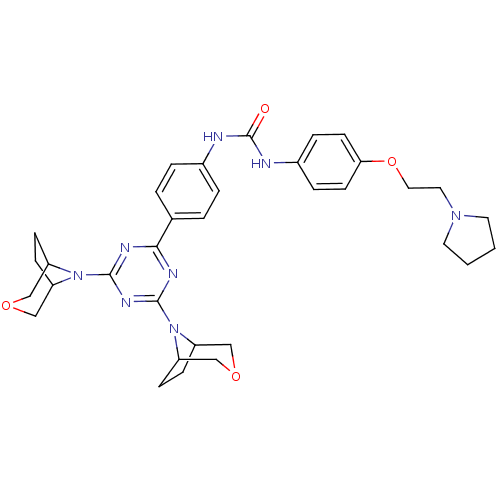
(1-(4-(4,6-di(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-...)Show SMILES O=C(Nc1ccc(OCCN2CCCC2)cc1)Nc1ccc(cc1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C34H42N8O4/c43-34(36-25-7-13-30(14-8-25)46-18-17-40-15-1-2-16-40)35-24-5-3-23(4-6-24)31-37-32(41-26-9-10-27(41)20-44-19-26)39-33(38-31)42-28-11-12-29(42)22-45-21-28/h3-8,13-14,26-29H,1-2,9-12,15-22H2,(H2,35,36,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315068

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-(t...)Show SMILES CN1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)C2CCOCC2)cc1 Show InChI InChI=1S/C33H40N8O4/c1-39-14-16-40(17-15-39)31(42)24-4-8-26(9-5-24)35-33(43)34-25-6-2-22(3-7-25)29-36-30(23-12-18-44-19-13-23)38-32(37-29)41-27-10-11-28(41)21-45-20-27/h2-9,23,27-28H,10-21H2,1H3,(H2,34,35,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315066
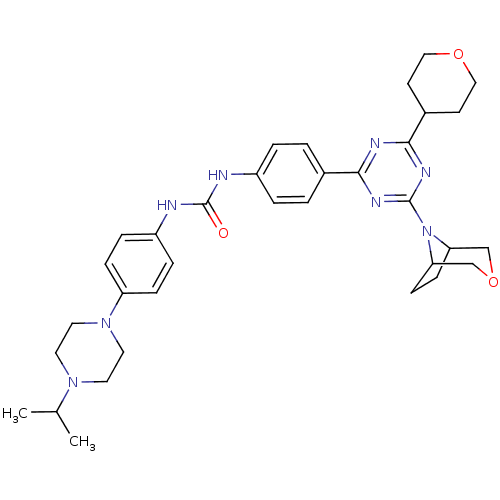
(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-(t...)Show SMILES CC(C)N1CCN(CC1)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)C2CCOCC2)cc1 Show InChI InChI=1S/C34H44N8O3/c1-23(2)40-15-17-41(18-16-40)28-9-7-27(8-10-28)36-34(43)35-26-5-3-24(4-6-26)31-37-32(25-13-19-44-20-14-25)39-33(38-31)42-29-11-12-30(42)22-45-21-29/h3-10,23,25,29-30H,11-22H2,1-2H3,(H2,35,36,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315051
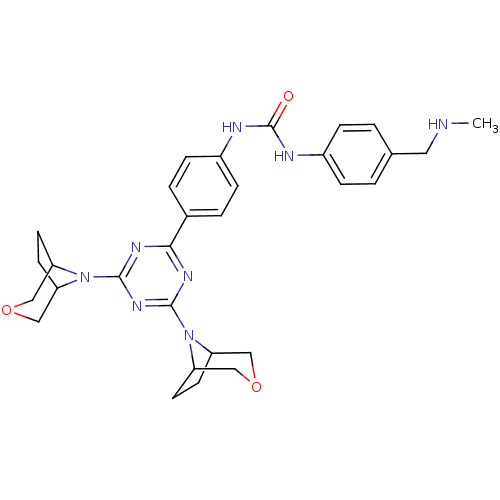
(1-(4-(4,6-di(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-...)Show SMILES CNCc1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)N2C3CCC2COC3)cc1 Show InChI InChI=1S/C30H36N8O3/c1-31-14-19-2-6-21(7-3-19)32-30(39)33-22-8-4-20(5-9-22)27-34-28(37-23-10-11-24(37)16-40-15-23)36-29(35-27)38-25-12-13-26(38)18-41-17-25/h2-9,23-26,31H,10-18H2,1H3,(H2,32,33,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315076

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-((...)Show SMILES C[C@@H]1COCCN1c1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)N2CCC(CC2)N(C)C)cc1)N1C2CCC1COC2 |r| Show InChI InChI=1S/C34H45N9O3/c1-23-20-45-19-18-42(23)32-37-31(38-33(39-32)43-29-12-13-30(43)22-46-21-29)24-4-6-25(7-5-24)35-34(44)36-26-8-10-28(11-9-26)41-16-14-27(15-17-41)40(2)3/h4-11,23,27,29-30H,12-22H2,1-3H3,(H2,35,36,44)/t23-,29?,30?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315053
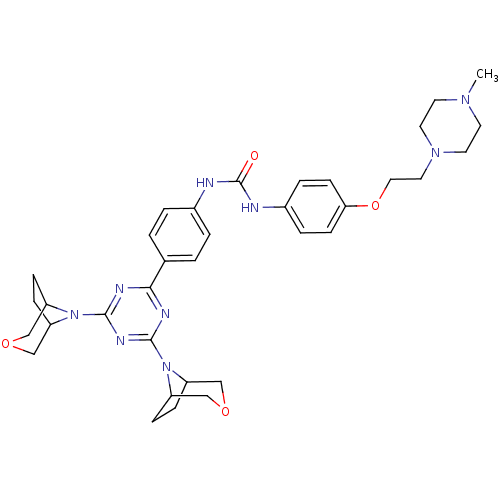
(1-(4-(4,6-di(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-...)Show SMILES CN1CCN(CCOc2ccc(NC(=O)Nc3ccc(cc3)-c3nc(nc(n3)N3C4CCC3COC4)N3C4CCC3COC4)cc2)CC1 Show InChI InChI=1S/C35H45N9O4/c1-41-14-16-42(17-15-41)18-19-48-31-12-6-26(7-13-31)37-35(45)36-25-4-2-24(3-5-25)32-38-33(43-27-8-9-28(43)21-46-20-27)40-34(39-32)44-29-10-11-30(44)23-47-22-29/h2-7,12-13,27-30H,8-11,14-23H2,1H3,(H2,36,37,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315049

(1-(4-(4,6-di(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-...)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3ccc(cc3)-c3nc(nc(n3)N3C4CCC3COC4)N3C4CCC3COC4)cc2)CC1 Show InChI InChI=1S/C34H43N9O3/c1-40-14-16-41(17-15-40)18-23-2-6-25(7-3-23)35-34(44)36-26-8-4-24(5-9-26)31-37-32(42-27-10-11-28(42)20-45-19-27)39-33(38-31)43-29-12-13-30(43)22-46-21-29/h2-9,27-30H,10-22H2,1H3,(H2,35,36,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315047
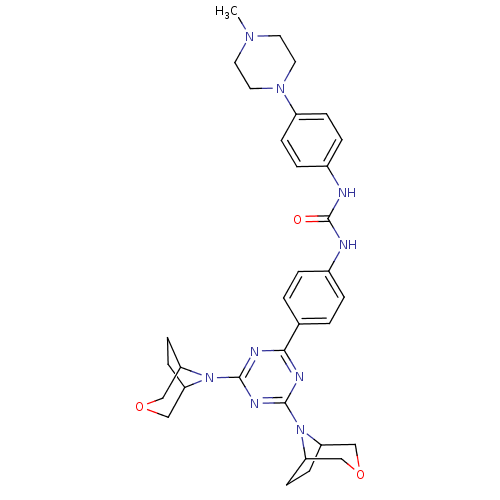
(1-(4-(4,6-di(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-...)Show SMILES CN1CCN(CC1)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)N2C3CCC2COC3)cc1 Show InChI InChI=1S/C33H41N9O3/c1-39-14-16-40(17-15-39)25-8-6-24(7-9-25)35-33(43)34-23-4-2-22(3-5-23)30-36-31(41-26-10-11-27(41)19-44-18-26)38-32(37-30)42-28-12-13-29(42)21-45-20-28/h2-9,26-29H,10-21H2,1H3,(H2,34,35,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315050
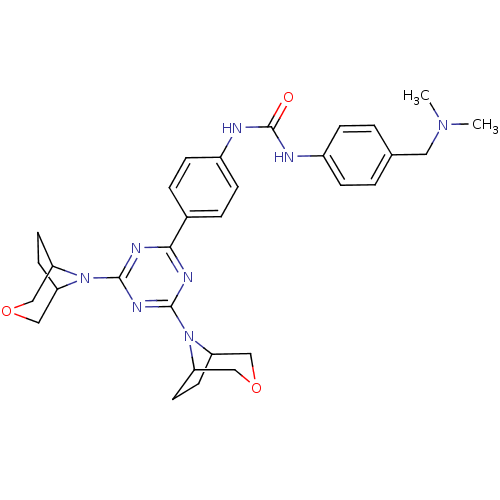
(1-(4-(4,6-di(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-...)Show SMILES CN(C)Cc1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)N2C3CCC2COC3)cc1 Show InChI InChI=1S/C31H38N8O3/c1-37(2)15-20-3-7-22(8-4-20)32-31(40)33-23-9-5-21(6-10-23)28-34-29(38-24-11-12-25(38)17-41-16-24)36-30(35-28)39-26-13-14-27(39)19-42-18-26/h3-10,24-27H,11-19H2,1-2H3,(H2,32,33,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315061
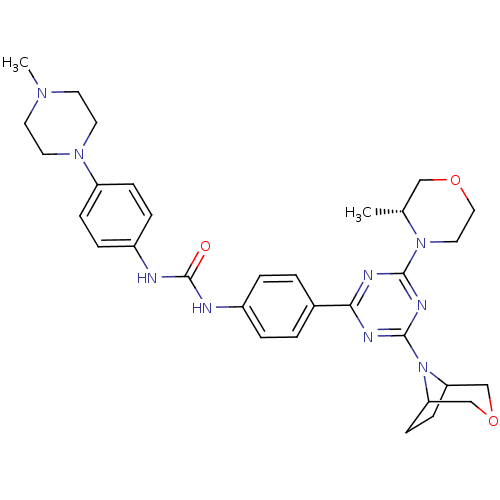
(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-((...)Show SMILES C[C@@H]1COCCN1c1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)N2CCN(C)CC2)cc1)N1C2CCC1COC2 |r| Show InChI InChI=1S/C32H41N9O3/c1-22-19-43-18-17-40(22)30-35-29(36-31(37-30)41-27-11-12-28(41)21-44-20-27)23-3-5-24(6-4-23)33-32(42)34-25-7-9-26(10-8-25)39-15-13-38(2)14-16-39/h3-10,22,27-28H,11-21H2,1-2H3,(H2,33,34,42)/t22-,27?,28?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315074
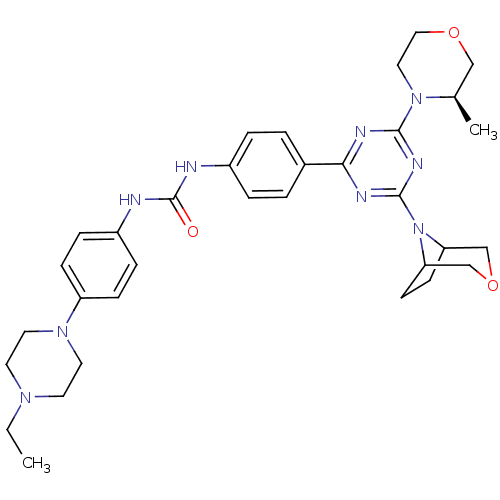
(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-((...)Show SMILES CCN1CCN(CC1)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOC[C@H]2C)N2C3CCC2COC3)cc1 |r| Show InChI InChI=1S/C33H43N9O3/c1-3-39-14-16-40(17-15-39)27-10-8-26(9-11-27)35-33(43)34-25-6-4-24(5-7-25)30-36-31(41-18-19-44-20-23(41)2)38-32(37-30)42-28-12-13-29(42)22-45-21-28/h4-11,23,28-29H,3,12-22H2,1-2H3,(H2,34,35,43)/t23-,28?,29?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315075

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-((...)Show SMILES CC(C)N1CCN(CC1)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOC[C@H]2C)N2C3CCC2COC3)cc1 |r| Show InChI InChI=1S/C34H45N9O3/c1-23(2)40-14-16-41(17-15-40)28-10-8-27(9-11-28)36-34(44)35-26-6-4-25(5-7-26)31-37-32(42-18-19-45-20-24(42)3)39-33(38-31)43-29-12-13-30(43)22-46-21-29/h4-11,23-24,29-30H,12-22H2,1-3H3,(H2,35,36,44)/t24-,29?,30?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315052
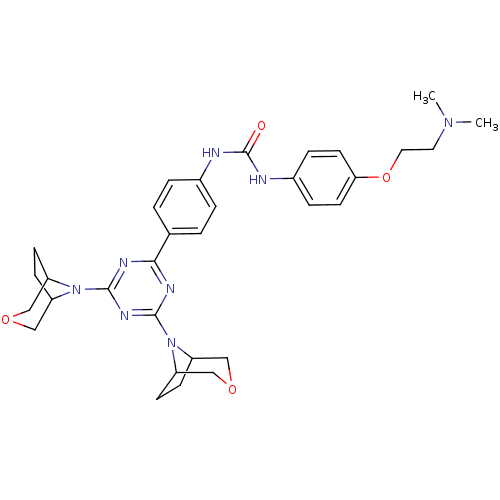
(1-(4-(4,6-di(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-...)Show SMILES CN(C)CCOc1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)N2C3CCC2COC3)cc1 Show InChI InChI=1S/C32H40N8O4/c1-38(2)15-16-44-28-13-7-23(8-14-28)34-32(41)33-22-5-3-21(4-6-22)29-35-30(39-24-9-10-25(39)18-42-17-24)37-31(36-29)40-26-11-12-27(40)20-43-19-26/h3-8,13-14,24-27H,9-12,15-20H2,1-2H3,(H2,33,34,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315081

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-((...)Show SMILES C[C@@H]1COCCN1c1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(OCCN(C)C)cc2)cc1)N1C2CCC1COC2 |r| Show InChI InChI=1S/C31H40N8O4/c1-21-18-41-16-15-38(21)29-34-28(35-30(36-29)39-25-10-11-26(39)20-42-19-25)22-4-6-23(7-5-22)32-31(40)33-24-8-12-27(13-9-24)43-17-14-37(2)3/h4-9,12-13,21,25-26H,10-11,14-20H2,1-3H3,(H2,32,33,40)/t21-,25?,26?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315058

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-(i...)Show SMILES CC(C)Nc1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)N2CCN(C)CC2)cc1)N1C2CCC1COC2 Show InChI InChI=1S/C30H39N9O2/c1-20(2)31-28-34-27(35-29(36-28)39-25-12-13-26(39)19-41-18-25)21-4-6-22(7-5-21)32-30(40)33-23-8-10-24(11-9-23)38-16-14-37(3)15-17-38/h4-11,20,25-26H,12-19H2,1-3H3,(H2,32,33,40)(H,31,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315059
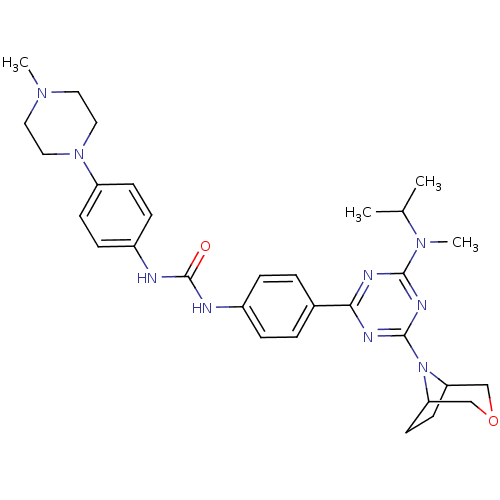
(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-(i...)Show SMILES CC(C)N(C)c1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)N2CCN(C)CC2)cc1)N1C2CCC1COC2 Show InChI InChI=1S/C31H41N9O2/c1-21(2)38(4)29-34-28(35-30(36-29)40-26-13-14-27(40)20-42-19-26)22-5-7-23(8-6-22)32-31(41)33-24-9-11-25(12-10-24)39-17-15-37(3)16-18-39/h5-12,21,26-27H,13-20H2,1-4H3,(H2,32,33,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315060
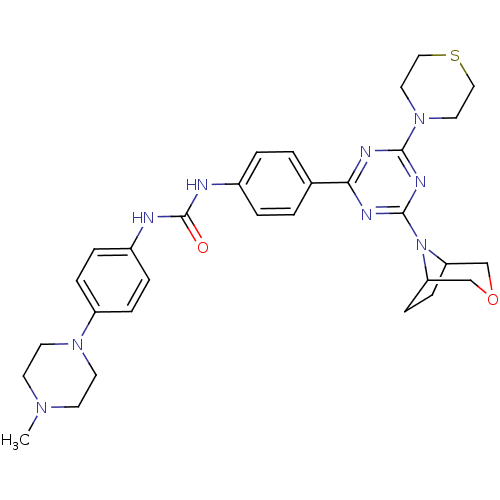
(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-th...)Show SMILES CN1CCN(CC1)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCSCC2)N2C3CCC2COC3)cc1 Show InChI InChI=1S/C31H39N9O2S/c1-37-12-14-38(15-13-37)25-8-6-24(7-9-25)33-31(41)32-23-4-2-22(3-5-23)28-34-29(39-16-18-43-19-17-39)36-30(35-28)40-26-10-11-27(40)21-42-20-26/h2-9,26-27H,10-21H2,1H3,(H2,32,33,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315080

(4-(3-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6...)Show SMILES C[C@@H]1COCCN1c1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)NCCN(C)C)cc1)N1C2CCC1COC2 |r| Show InChI InChI=1S/C32H41N9O4/c1-21-18-44-17-16-40(21)30-36-28(37-31(38-30)41-26-12-13-27(41)20-45-19-26)22-4-8-24(9-5-22)34-32(43)35-25-10-6-23(7-11-25)29(42)33-14-15-39(2)3/h4-11,21,26-27H,12-20H2,1-3H3,(H,33,42)(H2,34,35,43)/t21-,26?,27?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 to 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315062

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-(3...)Show SMILES CN1CCN(CC1)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)C2=CCOCC2)N2C3CCC2COC3)cc1 |t:30| Show InChI InChI=1S/C32H38N8O3/c1-38-14-16-39(17-15-38)26-8-6-25(7-9-26)34-32(41)33-24-4-2-22(3-5-24)29-35-30(23-12-18-42-19-13-23)37-31(36-29)40-27-10-11-28(40)21-43-20-27/h2-9,12,27-28H,10-11,13-21H2,1H3,(H2,33,34,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 to 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315079

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-((...)Show SMILES C[C@@H]1COCCN1c1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N2CCC(CC2)N(C)C)cc1)N1C2CCC1COC2 |r| Show InChI InChI=1S/C35H45N9O4/c1-23-20-47-19-18-43(23)33-38-31(39-34(40-33)44-29-12-13-30(44)22-48-21-29)24-4-8-26(9-5-24)36-35(46)37-27-10-6-25(7-11-27)32(45)42-16-14-28(15-17-42)41(2)3/h4-11,23,28-30H,12-22H2,1-3H3,(H2,36,37,46)/t23-,29?,30?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 to 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315071

(4-(3-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6...)Show SMILES CN(C)CCNC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)C2CCOCC2)cc1 Show InChI InChI=1S/C32H40N8O4/c1-39(2)16-15-33-30(41)23-5-9-25(10-6-23)35-32(42)34-24-7-3-21(4-8-24)28-36-29(22-13-17-43-18-14-22)38-31(37-28)40-26-11-12-27(40)20-44-19-26/h3-10,22,26-27H,11-20H2,1-2H3,(H,33,41)(H2,34,35,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 to 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315077

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-((...)Show SMILES C[C@@H]1COCCN1c1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N2CCN(C)CC2)cc1)N1C2CCC1COC2 |r| Show InChI InChI=1S/C33H41N9O4/c1-22-19-45-18-17-41(22)31-36-29(37-32(38-31)42-27-11-12-28(42)21-46-20-27)23-3-7-25(8-4-23)34-33(44)35-26-9-5-24(6-10-26)30(43)40-15-13-39(2)14-16-40/h3-10,22,27-28H,11-21H2,1-2H3,(H2,34,35,44)/t22-,27?,28?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 to 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315070

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-(t...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)C2CCOCC2)cc1 Show InChI InChI=1S/C35H44N8O4/c1-41(2)28-13-17-42(18-14-28)33(44)25-5-9-27(10-6-25)37-35(45)36-26-7-3-23(4-8-26)31-38-32(24-15-19-46-20-16-24)40-34(39-31)43-29-11-12-30(43)22-47-21-29/h3-10,24,28-30H,11-22H2,1-2H3,(H2,36,37,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 to 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315068

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-(t...)Show SMILES CN1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)C2CCOCC2)cc1 Show InChI InChI=1S/C33H40N8O4/c1-39-14-16-40(17-15-39)31(42)24-4-8-26(9-5-24)35-33(43)34-25-6-2-22(3-7-25)29-36-30(23-12-18-44-19-13-23)38-32(37-29)41-27-10-11-28(41)21-45-20-27/h2-9,23,27-28H,10-21H2,1H3,(H2,34,35,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 to 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315078

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-((...)Show SMILES CC(C)N1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOC[C@H]2C)N2C3CCC2COC3)cc1 |r| Show InChI InChI=1S/C35H45N9O4/c1-23(2)41-14-16-42(17-15-41)32(45)26-6-10-28(11-7-26)37-35(46)36-27-8-4-25(5-9-27)31-38-33(43-18-19-47-20-24(43)3)40-34(39-31)44-29-12-13-30(44)22-48-21-29/h4-11,23-24,29-30H,12-22H2,1-3H3,(H2,36,37,46)/t24-,29?,30?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 to 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315069

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-(t...)Show SMILES CC(C)N1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)C2CCOCC2)cc1 Show InChI InChI=1S/C35H44N8O4/c1-23(2)41-15-17-42(18-16-41)33(44)26-5-9-28(10-6-26)37-35(45)36-27-7-3-24(4-8-27)31-38-32(25-13-19-46-20-14-25)40-34(39-31)43-29-11-12-30(43)22-47-21-29/h3-10,23,25,29-30H,11-22H2,1-2H3,(H2,36,37,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 to 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315051

(1-(4-(4,6-di(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-...)Show SMILES CNCc1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)N2C3CCC2COC3)cc1 Show InChI InChI=1S/C30H36N8O3/c1-31-14-19-2-6-21(7-3-19)32-30(39)33-22-8-4-20(5-9-22)27-34-28(37-23-10-11-24(37)16-40-15-23)36-29(35-27)38-25-12-13-26(38)18-41-17-25/h2-9,23-26,31H,10-18H2,1H3,(H2,32,33,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 to 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315056

(1-(4-(4,6-di(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-...)Show SMILES OCc1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)N2C3CCC2COC3)cc1 Show InChI InChI=1S/C29H33N7O4/c37-13-18-1-5-20(6-2-18)30-29(38)31-21-7-3-19(4-8-21)26-32-27(35-22-9-10-23(35)15-39-14-22)34-28(33-26)36-24-11-12-25(36)17-40-16-24/h1-8,22-25,37H,9-17H2,(H2,30,31,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 to 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315073

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-((...)Show SMILES C[C@@H]1COCCN1c1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)N2CCNCC2)cc1)N1C2CCC1COC2 |r| Show InChI InChI=1S/C31H39N9O3/c1-21-18-42-17-16-39(21)29-35-28(36-30(37-29)40-26-10-11-27(40)20-43-19-26)22-2-4-23(5-3-22)33-31(41)34-24-6-8-25(9-7-24)38-14-12-32-13-15-38/h2-9,21,26-27,32H,10-20H2,1H3,(H2,33,34,41)/t21-,26?,27?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 286 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 to 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315064

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-(t...)Show SMILES O=C(Nc1ccc(cc1)N1CCNCC1)Nc1ccc(cc1)-c1nc(nc(n1)N1C2CCC1COC2)C1CCOCC1 Show InChI InChI=1S/C31H38N8O3/c40-31(34-24-5-7-25(8-6-24)38-15-13-32-14-16-38)33-23-3-1-21(2-4-23)28-35-29(22-11-17-41-18-12-22)37-30(36-28)39-26-9-10-27(39)20-42-19-26/h1-8,22,26-27,32H,9-20H2,(H2,33,34,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 294 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 to 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315067

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-(t...)Show SMILES CN(C)C1CCN(CC1)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)C2CCOCC2)cc1 Show InChI InChI=1S/C34H44N8O3/c1-40(2)27-13-17-41(18-14-27)28-9-7-26(8-10-28)36-34(43)35-25-5-3-23(4-6-25)31-37-32(24-15-19-44-20-16-24)39-33(38-31)42-29-11-12-30(42)22-45-21-29/h3-10,24,27,29-30H,11-22H2,1-2H3,(H2,35,36,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 382 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 to 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315054

(1-(4-(4,6-di(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-...)Show SMILES O=C(Nc1ccc(OCCN2CCCC2)cc1)Nc1ccc(cc1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C34H42N8O4/c43-34(36-25-7-13-30(14-8-25)46-18-17-40-15-1-2-16-40)35-24-5-3-23(4-6-24)31-37-32(41-26-9-10-27(41)20-44-19-26)39-33(38-31)42-28-11-12-29(42)22-45-21-28/h3-8,13-14,26-29H,1-2,9-12,15-22H2,(H2,35,36,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 389 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 to 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2648-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.031
BindingDB Entry DOI: 10.7270/Q2DZ08GK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data