 Found 170 hits Enz. Inhib. hit(s) with all data for entry = 50040833
Found 170 hits Enz. Inhib. hit(s) with all data for entry = 50040833 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50095523
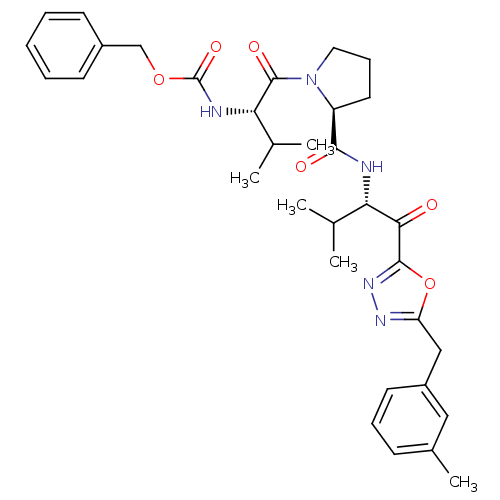
(CHEMBL285231 | [(S)-2-methyl-1-((S)-2-{(S)-2-methy...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)c1nnc(Cc2cccc(C)c2)o1 Show InChI InChI=1S/C33H41N5O6/c1-20(2)27(29(39)31-37-36-26(44-31)18-24-14-9-11-22(5)17-24)34-30(40)25-15-10-16-38(25)32(41)28(21(3)4)35-33(42)43-19-23-12-7-6-8-13-23/h6-9,11-14,17,20-21,25,27-28H,10,15-16,18-19H2,1-5H3,(H,34,40)(H,35,42)/t25-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50204512
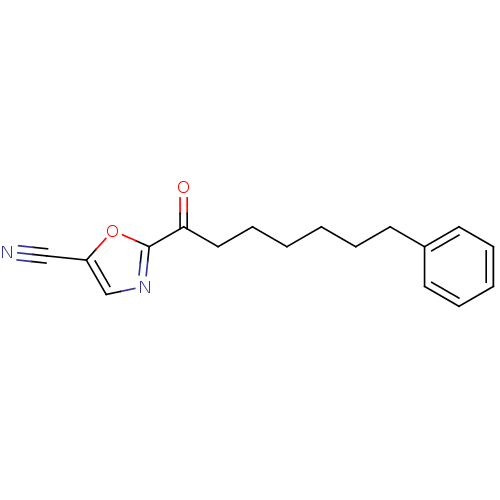
(2-(7-phenylheptanoyl)oxazole-5-carbonitrile | CHEM...)Show InChI InChI=1S/C17H18N2O2/c18-12-15-13-19-17(21-15)16(20)11-7-2-1-4-8-14-9-5-3-6-10-14/h3,5-6,9-10,13H,1-2,4,7-8,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50095526
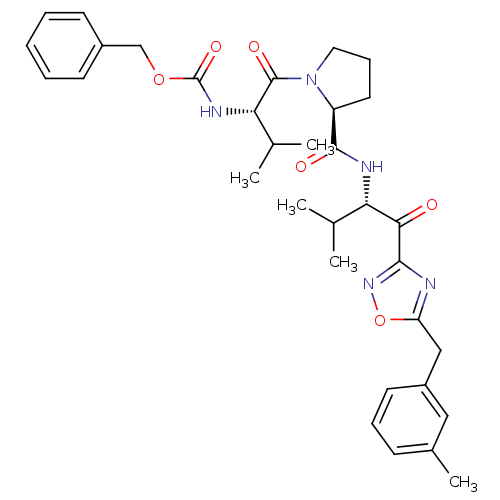
(CHEMBL24058 | [(S)-2-methyl-1-((S)-2-{(S)-2-methyl...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)c1noc(Cc2cccc(C)c2)n1 Show InChI InChI=1S/C33H41N5O6/c1-20(2)27(29(39)30-34-26(44-37-30)18-24-14-9-11-22(5)17-24)35-31(40)25-15-10-16-38(25)32(41)28(21(3)4)36-33(42)43-19-23-12-7-6-8-13-23/h6-9,11-14,17,20-21,25,27-28H,10,15-16,18-19H2,1-5H3,(H,35,40)(H,36,42)/t25-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50400823

(CHEMBL2204343)Show SMILES O[Si]1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H23ClFNO2Si/c21-17-5-9-19(10-6-17)26(25)14-12-23(13-15-26)11-1-2-20(24)16-3-7-18(22)8-4-16/h3-10,25H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D2 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50400837
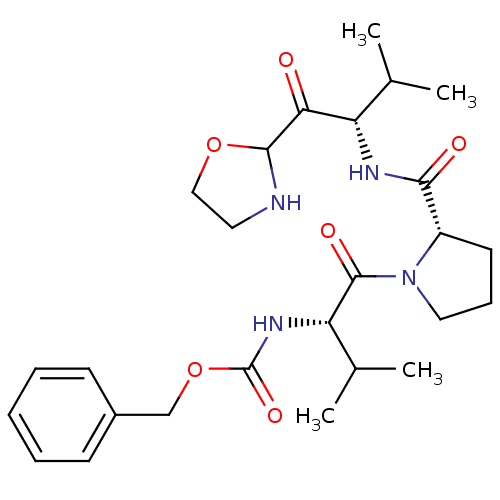
(CHEMBL2205678)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C)C(=O)C1NCCO1 |r| Show InChI InChI=1S/C26H38N4O6/c1-16(2)20(22(31)24-27-12-14-35-24)28-23(32)19-11-8-13-30(19)25(33)21(17(3)4)29-26(34)36-15-18-9-6-5-7-10-18/h5-7,9-10,16-17,19-21,24,27H,8,11-15H2,1-4H3,(H,28,32)(H,29,34)/t19-,20-,21-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50204483
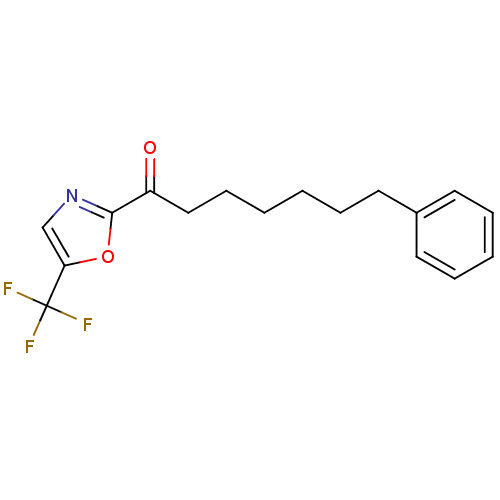
(7-phenyl-1-(5-(trifluoromethyl)oxazol-2-yl)heptan-...)Show InChI InChI=1S/C17H18F3NO2/c18-17(19,20)15-12-21-16(23-15)14(22)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50204490
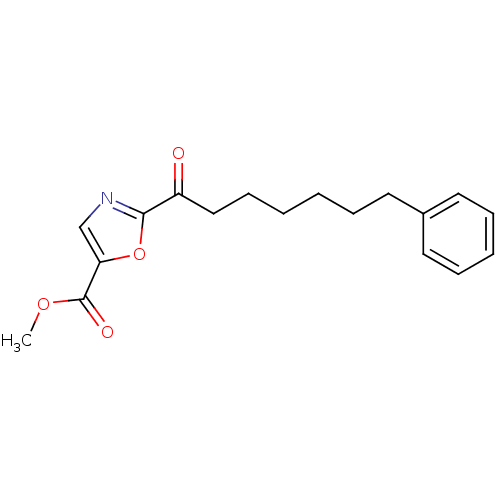
(CHEMBL220784 | methyl 2-(7-phenylheptanoyl)oxazole...)Show InChI InChI=1S/C18H21NO4/c1-22-18(21)16-13-19-17(23-16)15(20)12-8-3-2-5-9-14-10-6-4-7-11-14/h4,6-7,10-11,13H,2-3,5,8-9,12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193921
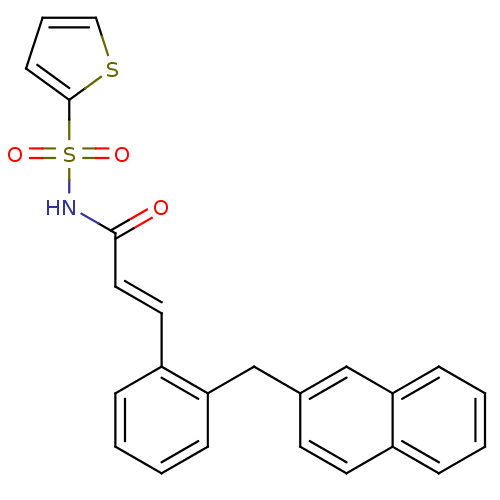
(3-(2-(naphthalen-2-ylmethyl)phenyl)-N-(thiophen-2-...)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1Cc1ccc2ccccc2c1 Show InChI InChI=1S/C24H19NO3S2/c26-23(25-30(27,28)24-10-5-15-29-24)14-13-20-7-2-4-9-22(20)17-18-11-12-19-6-1-3-8-21(19)16-18/h1-16H,17H2,(H,25,26)/b14-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of EP3 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036058
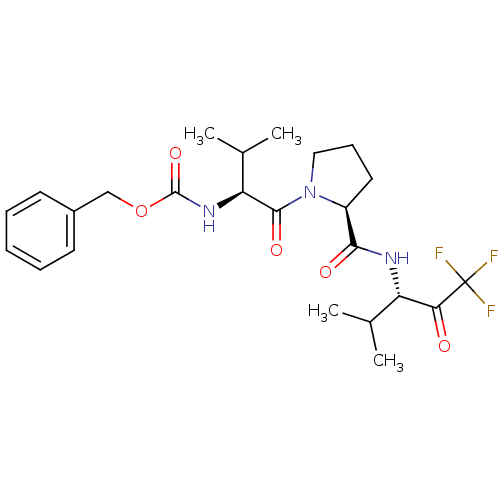
(CHEMBL354883 | benzyl (S)-1-((S)-2-(((S)-1,1,1-tri...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C24H32F3N3O5/c1-14(2)18(20(31)24(25,26)27)28-21(32)17-11-8-12-30(17)22(33)19(15(3)4)29-23(34)35-13-16-9-6-5-7-10-16/h5-7,9-10,14-15,17-19H,8,11-13H2,1-4H3,(H,28,32)(H,29,34)/t17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of sigma 1 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50400835
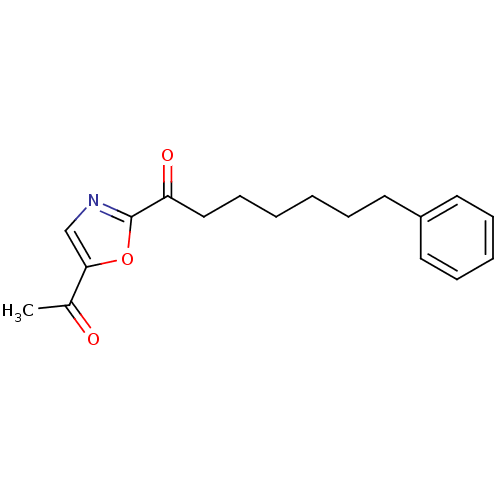
(CHEMBL219660)Show InChI InChI=1S/C18H21NO3/c1-14(20)17-13-19-18(22-17)16(21)12-8-3-2-5-9-15-10-6-4-7-11-15/h4,6-7,10-11,13H,2-3,5,8-9,12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50400836

(CHEMBL219659)Show InChI InChI=1S/C19H24N2O3/c1-21(2)19(23)17-14-20-18(24-17)16(22)13-9-4-3-6-10-15-11-7-5-8-12-15/h5,7-8,11-12,14H,3-4,6,9-10,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50400818

(CHEMBL2204339)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C23H17NO5S3/c25-22(24-32(28,29)23-10-5-15-30-23)14-12-18-7-3-4-9-21(18)31(26,27)20-13-11-17-6-1-2-8-19(17)16-20/h1-16H,(H,24,25)/b14-12+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of EP3 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D2 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50204475
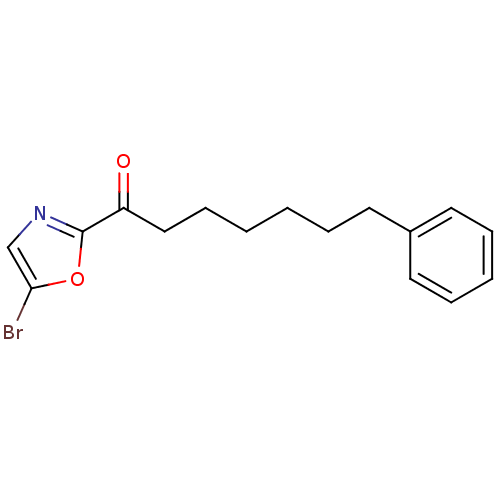
(1-(5-bromooxazol-2-yl)-7-phenylheptan-1-one | CHEM...)Show InChI InChI=1S/C16H18BrNO2/c17-15-12-18-16(20-15)14(19)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50400832
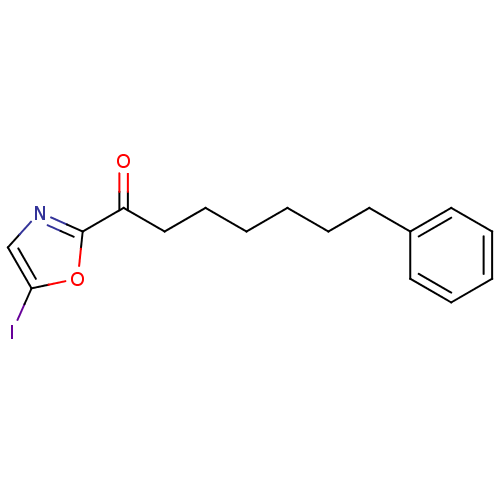
(CHEMBL220555)Show InChI InChI=1S/C16H18INO2/c17-15-12-18-16(20-15)14(19)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50031199
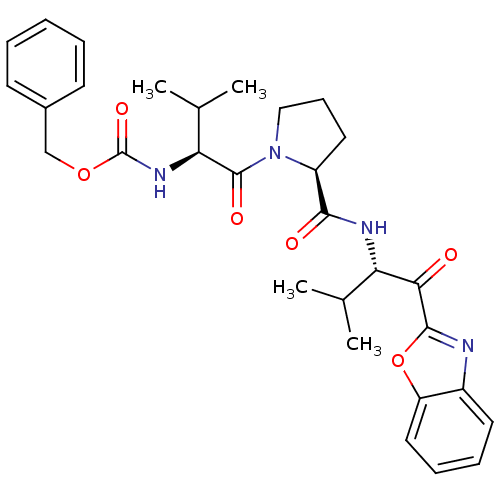
(((S)-1-{(S)-2-[(S)-1-(Benzooxazole-2-carbonyl)-2-m...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)c1nc2ccccc2o1 Show InChI InChI=1S/C30H36N4O6/c1-18(2)24(26(35)28-31-21-13-8-9-15-23(21)40-28)32-27(36)22-14-10-16-34(22)29(37)25(19(3)4)33-30(38)39-17-20-11-6-5-7-12-20/h5-9,11-13,15,18-19,22,24-25H,10,14,16-17H2,1-4H3,(H,32,36)(H,33,38)/t22-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50400823

(CHEMBL2204343)Show SMILES O[Si]1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H23ClFNO2Si/c21-17-5-9-19(10-6-17)26(25)14-12-23(13-15-26)11-1-2-20(24)16-3-7-18(22)8-4-16/h3-10,25H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of sigma 1 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50400833
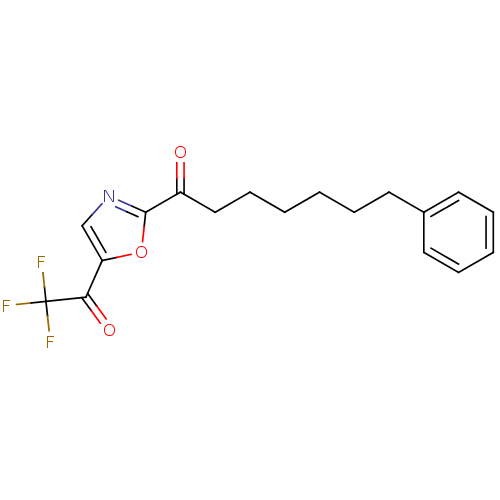
(CHEMBL220378)Show InChI InChI=1S/C18H18F3NO3/c19-18(20,21)16(24)15-12-22-17(25-15)14(23)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50400823

(CHEMBL2204343)Show SMILES O[Si]1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H23ClFNO2Si/c21-17-5-9-19(10-6-17)26(25)14-12-23(13-15-26)11-1-2-20(24)16-3-7-18(22)8-4-16/h3-10,25H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D3 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50204525
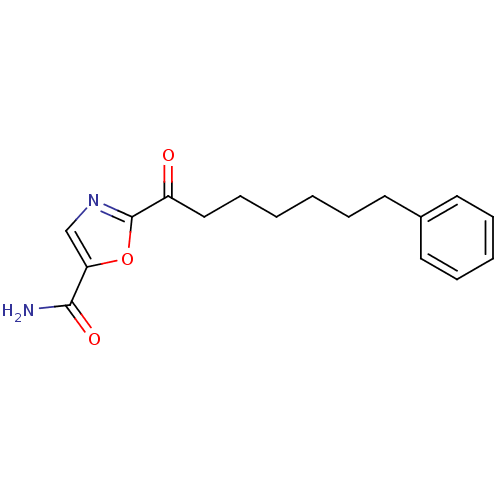
(2-(7-phenylheptanoyl)oxazole-5-carboxamide | CHEMB...)Show InChI InChI=1S/C17H20N2O3/c18-16(21)15-12-19-17(22-15)14(20)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2,(H2,18,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50400831
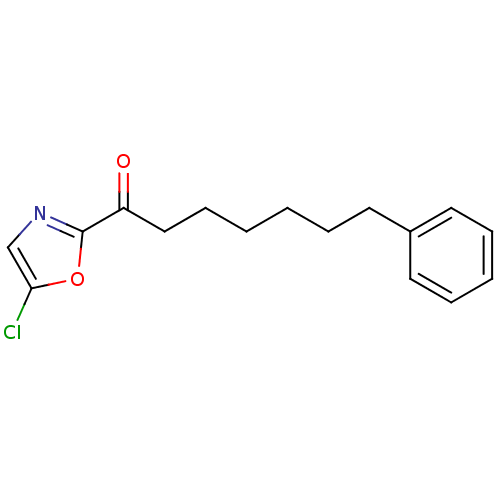
(CHEMBL220613)Show InChI InChI=1S/C16H18ClNO2/c17-15-12-18-16(20-15)14(19)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50400834
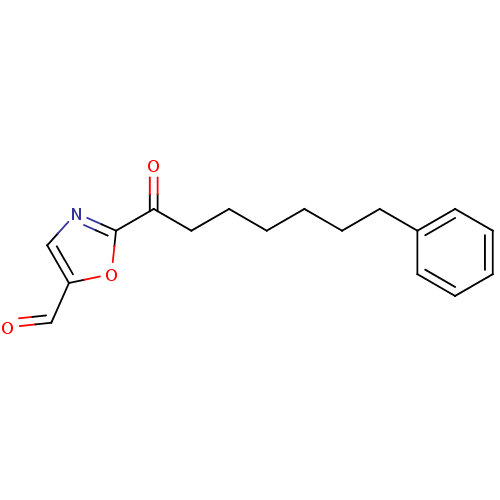
(CHEMBL220727)Show InChI InChI=1S/C17H19NO3/c19-13-15-12-18-17(21-15)16(20)11-7-2-1-4-8-14-9-5-3-6-10-14/h3,5-6,9-10,12-13H,1-2,4,7-8,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50154726
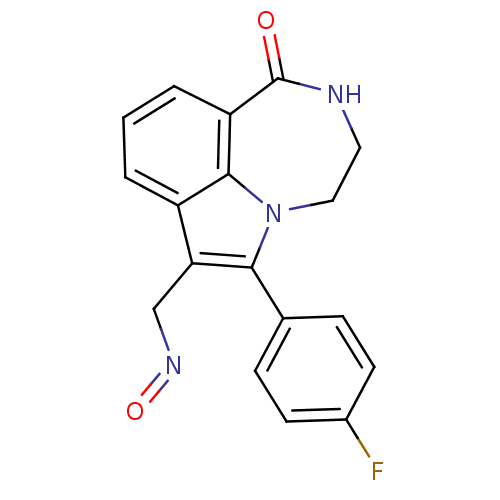
(6-(4-Fluoro-phenyl)-1-oxo-1,2,3,4-tetrahydro-[1,4]...)Show InChI InChI=1S/C18H14FN3O2/c19-12-6-4-11(5-7-12)16-15(10-21-24)13-2-1-3-14-17(13)22(16)9-8-20-18(14)23/h1-7H,8-10H2,(H,20,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PARP-1 |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50120724
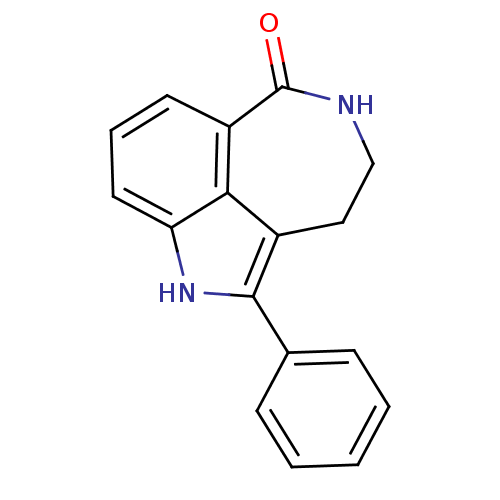
(2-Phenyl-1,3,4,5-tetrahydro-azepino[5,4,3-cd]indol...)Show InChI InChI=1S/C17H14N2O/c20-17-13-7-4-8-14-15(13)12(9-10-18-17)16(19-14)11-5-2-1-3-6-11/h1-8,19H,9-10H2,(H,18,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PARP-1 |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50400820
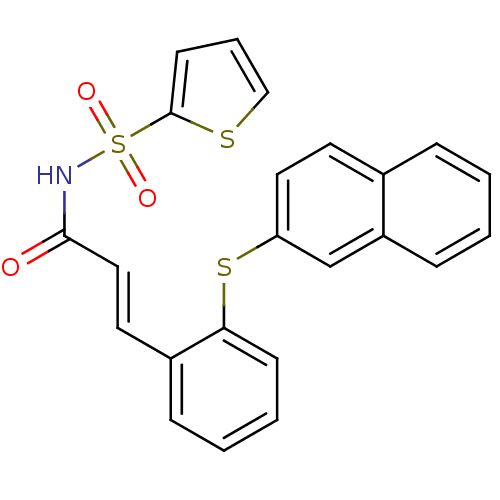
(CHEMBL2204337)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1Sc1ccc2ccccc2c1 Show InChI InChI=1S/C23H17NO3S3/c25-22(24-30(26,27)23-10-5-15-28-23)14-12-18-7-3-4-9-21(18)29-20-13-11-17-6-1-2-8-19(17)16-20/h1-16H,(H,24,25)/b14-12+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of EP3 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50154739
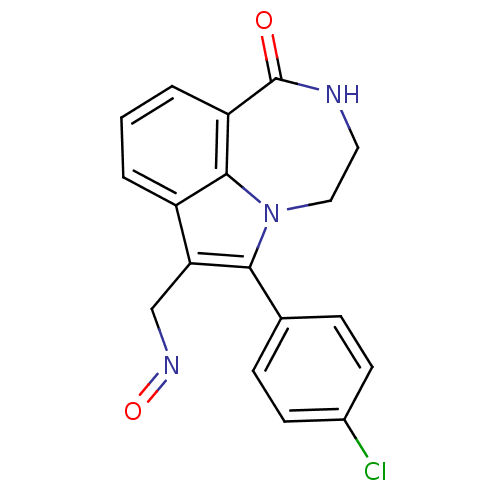
(6-(4-Chloro-phenyl)-1-oxo-1,2,3,4-tetrahydro-[1,4]...)Show InChI InChI=1S/C18H14ClN3O2/c19-12-6-4-11(5-7-12)16-15(10-21-24)13-2-1-3-14-17(13)22(16)9-8-20-18(14)23/h1-7H,8-10H2,(H,20,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PARP-1 |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50154759
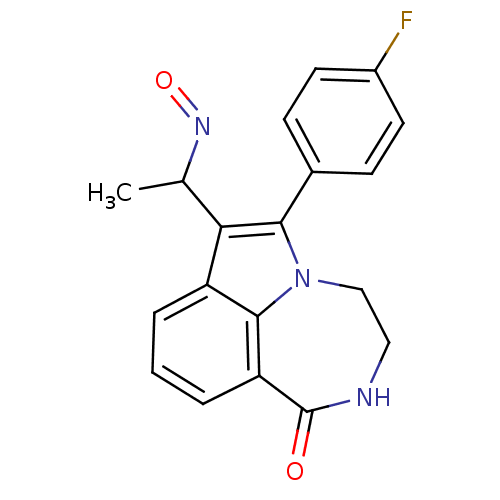
(6-(4-Fluoro-phenyl)-7-(1-hydroxyimino-ethyl)-3,4-d...)Show SMILES CC(N=O)c1c(-c2ccc(F)cc2)n2CCNC(=O)c3cccc1c23 Show InChI InChI=1S/C19H16FN3O2/c1-11(22-25)16-14-3-2-4-15-18(14)23(10-9-21-19(15)24)17(16)12-5-7-13(20)8-6-12/h2-8,11H,9-10H2,1H3,(H,21,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PARP-1 |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50154735
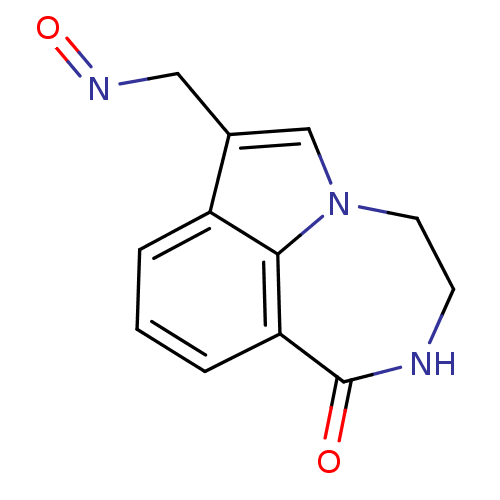
(1-Oxo-1,2,3,4-tetrahydro-[1,4]diazepino[6,7,1-hi]i...)Show InChI InChI=1S/C12H11N3O2/c16-12-10-3-1-2-9-8(6-14-17)7-15(11(9)10)5-4-13-12/h1-3,7H,4-6H2,(H,13,16) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PARP-1 |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50400817
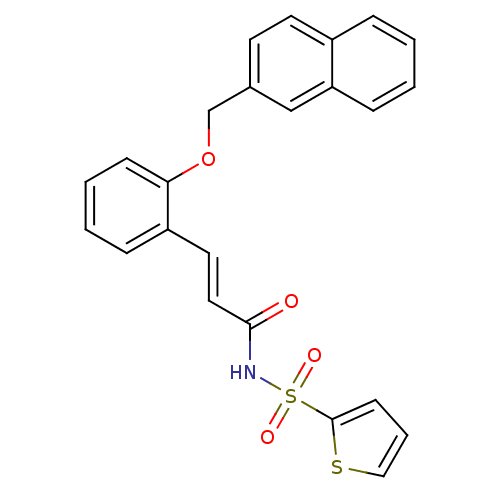
(CHEMBL2204340)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1OCc1ccc2ccccc2c1 Show InChI InChI=1S/C24H19NO4S2/c26-23(25-31(27,28)24-10-5-15-30-24)14-13-20-7-3-4-9-22(20)29-17-18-11-12-19-6-1-2-8-21(19)16-18/h1-16H,17H2,(H,25,26)/b14-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of EP3 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 10.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D3 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50154723

(6-(4-Fluoro-phenyl)-3,4-dihydro-2H-[1,4]diazepino[...)Show InChI InChI=1S/C17H13FN2O/c18-13-6-4-11(5-7-13)15-10-12-2-1-3-14-16(12)20(15)9-8-19-17(14)21/h1-7,10H,8-9H2,(H,19,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PARP-1 |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50165843
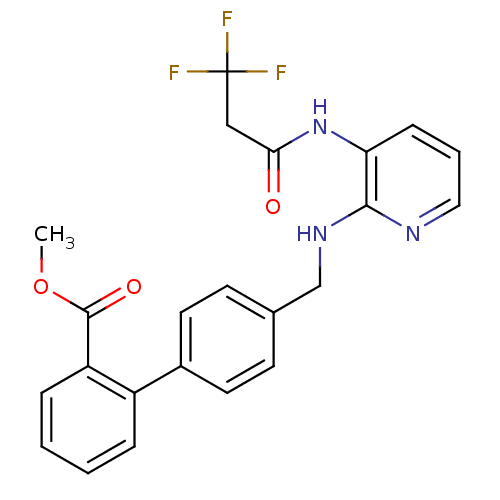
(4'-{[3-(3,3,3-Trifluoro-propionylamino)-pyridin-2-...)Show SMILES COC(=O)c1ccccc1-c1ccc(CNc2ncccc2NC(=O)CC(F)(F)F)cc1 Show InChI InChI=1S/C23H20F3N3O3/c1-32-22(31)18-6-3-2-5-17(18)16-10-8-15(9-11-16)14-28-21-19(7-4-12-27-21)29-20(30)13-23(24,25)26/h2-12H,13-14H2,1H3,(H,27,28)(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at Bradykinin B1 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50400823

(CHEMBL2204343)Show SMILES O[Si]1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H23ClFNO2Si/c21-17-5-9-19(10-6-17)26(25)14-12-23(13-15-26)11-1-2-20(24)16-3-7-18(22)8-4-16/h3-10,25H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 14.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM50400823

(CHEMBL2204343)Show SMILES O[Si]1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H23ClFNO2Si/c21-17-5-9-19(10-6-17)26(25)14-12-23(13-15-26)11-1-2-20(24)16-3-7-18(22)8-4-16/h3-10,25H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 16.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D5 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50400819

(CHEMBL2204338)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1S(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C23H17NO4S3/c25-22(24-31(27,28)23-10-5-15-29-23)14-12-18-7-3-4-9-21(18)30(26)20-13-11-17-6-1-2-8-19(17)16-20/h1-16H,(H,24,25)/b14-12+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of EP3 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50400862
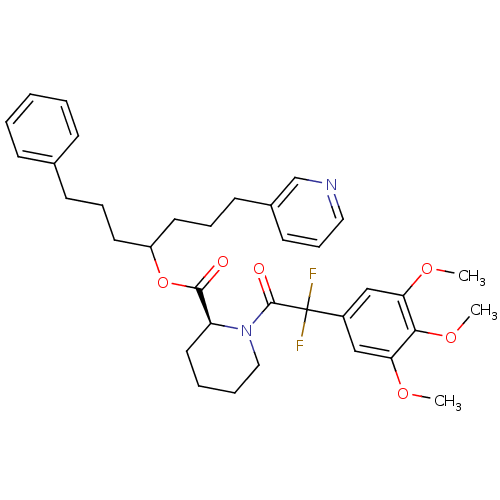
(CHEMBL2205015)Show SMILES COc1cc(cc(OC)c1OC)C(F)(F)C(=O)N1CCCC[C@H]1C(=O)OC(CCCc1ccccc1)CCCc1cccnc1 |r| Show InChI InChI=1S/C35H42F2N2O6/c1-42-30-22-27(23-31(43-2)32(30)44-3)35(36,37)34(41)39-21-8-7-19-29(39)33(40)45-28(17-9-14-25-12-5-4-6-13-25)18-10-15-26-16-11-20-38-24-26/h4-6,11-13,16,20,22-24,28-29H,7-10,14-15,17-19,21H2,1-3H3/t28?,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FKBP12 |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036051
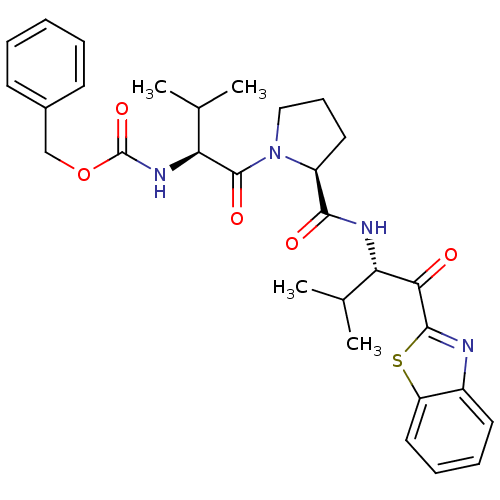
(((S)-1-{(S)-2-[(S)-1-(Benzothiazole-2-carbonyl)-2-...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C30H36N4O5S/c1-18(2)24(26(35)28-31-21-13-8-9-15-23(21)40-28)32-27(36)22-14-10-16-34(22)29(37)25(19(3)4)33-30(38)39-17-20-11-6-5-7-12-20/h5-9,11-13,15,18-19,22,24-25H,10,14,16-17H2,1-4H3,(H,32,36)(H,33,38)/t22-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50400830
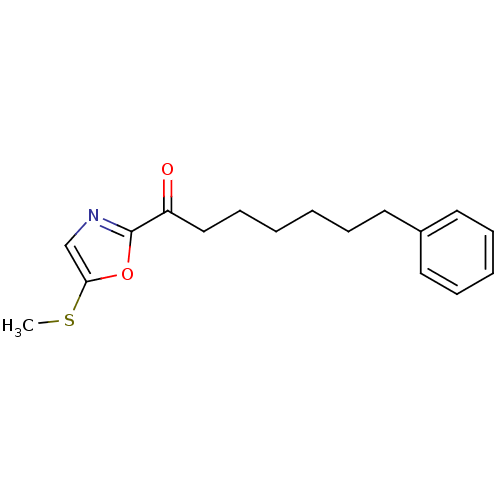
(CHEMBL219954)Show InChI InChI=1S/C17H21NO2S/c1-21-16-13-18-17(20-16)15(19)12-8-3-2-5-9-14-10-6-4-7-11-14/h4,6-7,10-11,13H,2-3,5,8-9,12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036040
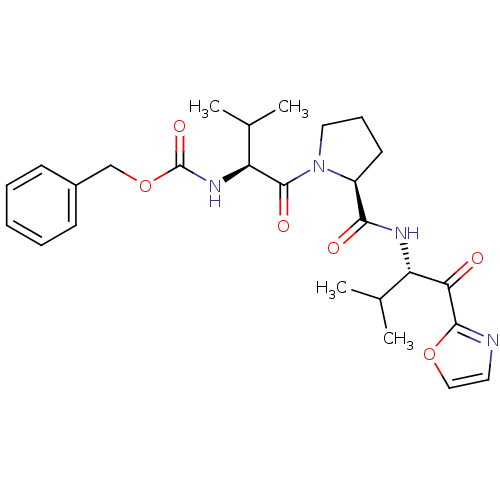
(((S)-2-Methyl-1-{(S)-2-[(S)-2-methyl-1-(oxazole-2-...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)c1ncco1 Show InChI InChI=1S/C26H34N4O6/c1-16(2)20(22(31)24-27-12-14-35-24)28-23(32)19-11-8-13-30(19)25(33)21(17(3)4)29-26(34)36-15-18-9-6-5-7-10-18/h5-7,9-10,12,14,16-17,19-21H,8,11,13,15H2,1-4H3,(H,28,32)(H,29,34)/t19-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50204506
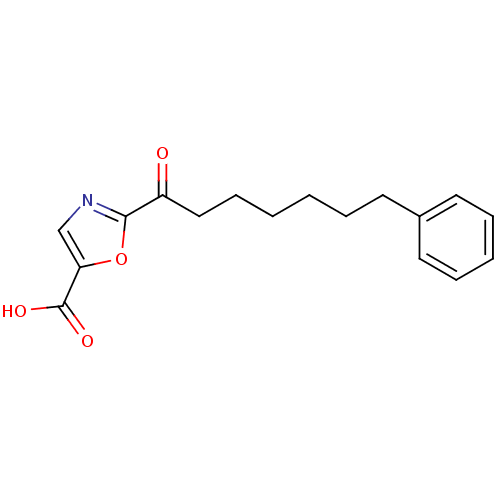
(2-(7-phenylheptanoyl)oxazole-5-carboxylic acid | C...)Show InChI InChI=1S/C17H19NO4/c19-14(16-18-12-15(22-16)17(20)21)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50374375
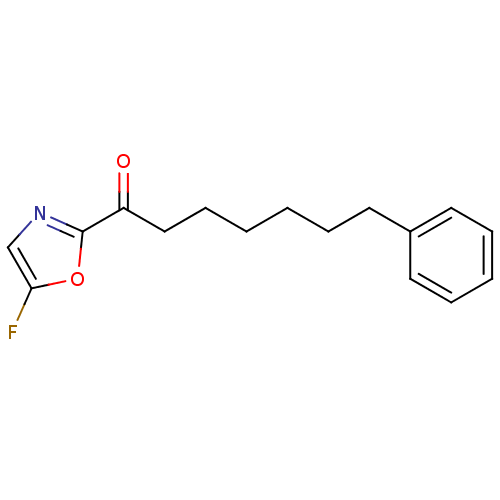
(CHEMBL373906)Show InChI InChI=1S/C16H18FNO2/c17-15-12-18-16(20-15)14(19)11-7-2-1-4-8-13-9-5-3-6-10-13/h3,5-6,9-10,12H,1-2,4,7-8,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D5 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50154755

(7-{1-[(E)-Hydroxyimino]-ethyl}-3,4-dihydro-2H-[1,4...)Show InChI InChI=1S/C13H13N3O2/c1-8(15-18)11-7-16-6-5-14-13(17)10-4-2-3-9(11)12(10)16/h2-4,7-8H,5-6H2,1H3,(H,14,17) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PARP-1 |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50400843
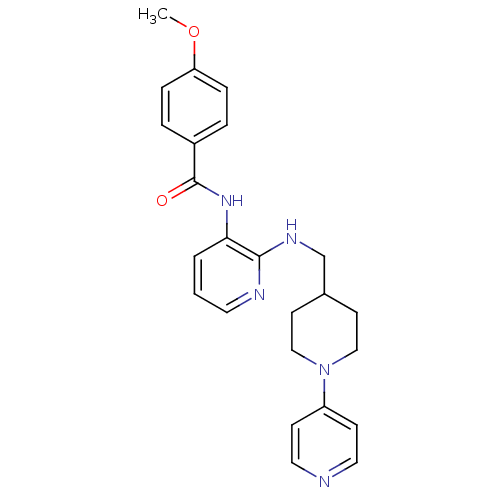
(CHEMBL2205679)Show SMILES COc1ccc(cc1)C(=O)Nc1cccnc1NCC1CCN(CC1)c1ccncc1 Show InChI InChI=1S/C24H27N5O2/c1-31-21-6-4-19(5-7-21)24(30)28-22-3-2-12-26-23(22)27-17-18-10-15-29(16-11-18)20-8-13-25-14-9-20/h2-9,12-14,18H,10-11,15-17H2,1H3,(H,26,27)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50400818

(CHEMBL2204339)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C23H17NO5S3/c25-22(24-32(28,29)23-10-5-15-30-23)14-12-18-7-3-4-9-21(18)31(26,27)20-13-11-17-6-1-2-8-19(17)16-20/h1-16H,(H,24,25)/b14-12+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of EP3 receptor in presence of 0.005 % HAS |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036057
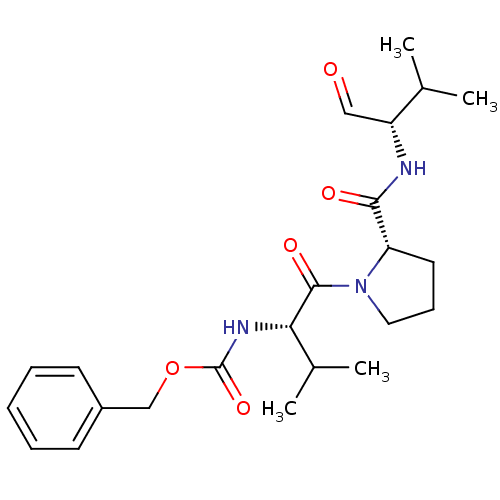
(CHEMBL165525 | benzyl (S)-1-((S)-2-(((S)-3-methyl-...)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C)C=O Show InChI InChI=1S/C23H33N3O5/c1-15(2)18(13-27)24-21(28)19-11-8-12-26(19)22(29)20(16(3)4)25-23(30)31-14-17-9-6-5-7-10-17/h5-7,9-10,13,15-16,18-20H,8,11-12,14H2,1-4H3,(H,24,28)(H,25,30)/t18-,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50204493
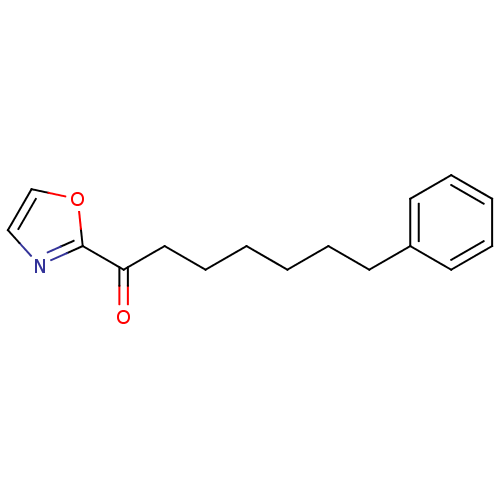
(1-(oxazol-2-yl)-7-phenylheptan-1-one | CHEMBL22012...)Show InChI InChI=1S/C16H19NO2/c18-15(16-17-12-13-19-16)11-7-2-1-4-8-14-9-5-3-6-10-14/h3,5-6,9-10,12-13H,1-2,4,7-8,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of FAAH |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50182456
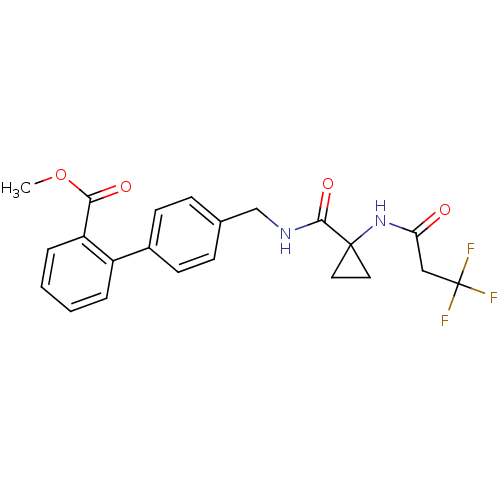
(CHEMBL202089 | methyl 4'-{[({1-[(3,3,3-trifluoropr...)Show SMILES COC(=O)c1ccccc1-c1ccc(CNC(=O)C2(CC2)NC(=O)CC(F)(F)F)cc1 Show InChI InChI=1S/C22H21F3N2O4/c1-31-19(29)17-5-3-2-4-16(17)15-8-6-14(7-9-15)13-26-20(30)21(10-11-21)27-18(28)12-22(23,24)25/h2-9H,10-13H2,1H3,(H,26,30)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at Bradykinin B1 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data