

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306131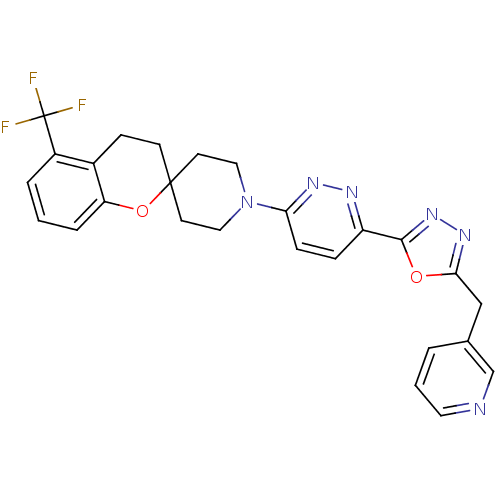 (1'-(6-(5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SCD1 | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306131 (1'-(6-(5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of SCD1 in HEK293A cell microsomes assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50296309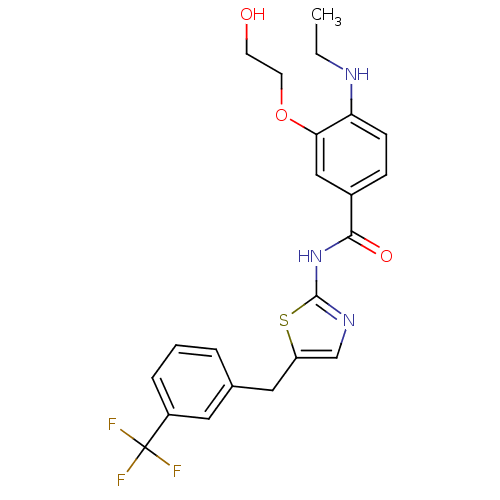 (4-(ethylamino)-3-(2-hydroxyethoxy)-N-(5-(3-(triflu...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of stearoyl-CoA desaturase 1 in human microsome assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 19: 4159-66 (2009) Article DOI: 10.1016/j.bmcl.2009.05.123 BindingDB Entry DOI: 10.7270/Q2PZ58VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50257967 (CHEMBL494748 | N-(2-(7-(4-chloro-3-(trifluoromethy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114085 BindingDB Entry DOI: 10.7270/Q2XW4PS2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50257967 (CHEMBL494748 | N-(2-(7-(4-chloro-3-(trifluoromethy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of Delta9-desaturase in human HepG2 cells | Bioorg Med Chem Lett 19: 2048-52 (2009) Article DOI: 10.1016/j.bmcl.2009.02.019 BindingDB Entry DOI: 10.7270/Q2XG9R1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50257967 (CHEMBL494748 | N-(2-(7-(4-chloro-3-(trifluoromethy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of stearoyl-CoA delta9 desaturase in human HepG2 cells | Bioorg Med Chem Lett 19: 4070-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.017 BindingDB Entry DOI: 10.7270/Q2959HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50330378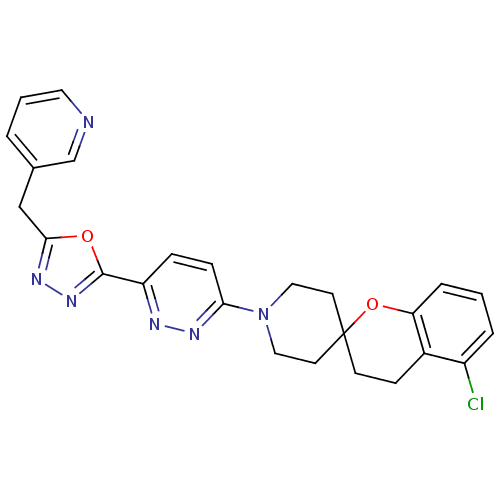 (5-Chloro-1'-{6-[5-(pyridin-3-ylmethyl)-1,3,4-oxadi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of SCD1 in HEK293A cell microsomes assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306131 (1'-(6-(5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate after 60 mins in presence of S9 microsomal fraction | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306116 (CHEMBL594289 | N-(2-hydroxy-2-phenylethyl)-6-(spir...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50330379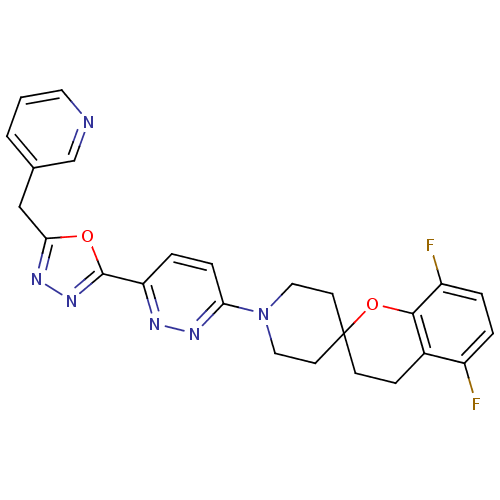 (5,8-Difluoro-1'-{6-[5-(pyridin-3-ylmethyl)-1,3,4-o...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of SCD1 in HEK293A cell microsomes assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50296295 (CHEMBL552173 | N-(5-(3,5-bis(trifluoromethyl)benzy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50330380 (5-Methyl-1'-{6-[5-(pyridin-3-ylmethyl)-1,3,4-oxadi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of SCD1 in HEK293A cell microsomes assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50330381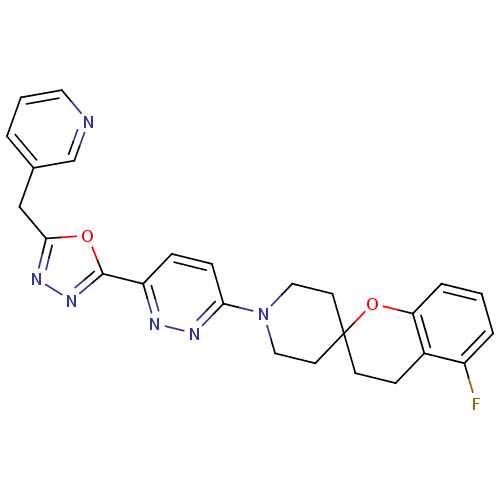 (5-Fluoro-1'-{6-[5-(pyridin-3-ylmethyl)-1,3,4-oxadi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of SCD1 in HEK293A cell microsomes assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50296295 (CHEMBL552173 | N-(5-(3,5-bis(trifluoromethyl)benzy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of stearoyl-CoA desaturase 1 in human microsome assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 19: 4159-66 (2009) Article DOI: 10.1016/j.bmcl.2009.05.123 BindingDB Entry DOI: 10.7270/Q2PZ58VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306126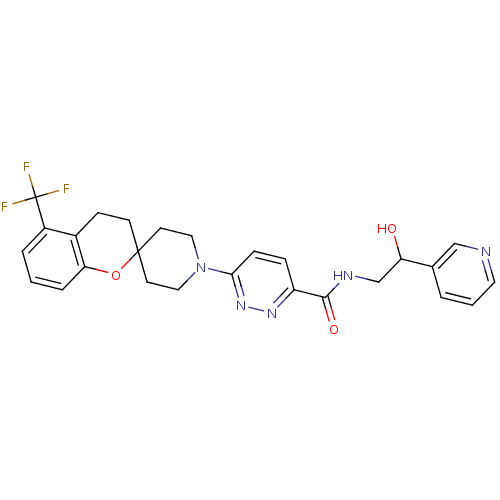 (CHEMBL605412 | N-(2-hydroxy-2-(pyridin-3-yl)ethyl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate after 60 mins in presence of S9 microsomal fraction | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306112 (CHEMBL595247 | N-(2-hydroxy-2-phenylethyl)-6-(4-hy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50298907 (CHEMBL572876 | N-(2-(6-(3,4-dichlorobenzylamino)-3...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of stearoyl-CoA delta9 desaturase in human HepG2 cells | Bioorg Med Chem Lett 19: 4070-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.017 BindingDB Entry DOI: 10.7270/Q2959HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50448653 (CHEMBL3127535) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, LLC Curated by ChEMBL | Assay Description Inhibition of SCD1 in human A431 cells assessed as [13C]-palmitic acid conversion to [13C]-palmitoleic acid after 4 hrs by LC/MS analysis | Bioorg Med Chem Lett 24: 1437-41 (2014) Article DOI: 10.1016/j.bmcl.2013.12.075 BindingDB Entry DOI: 10.7270/Q26M38BM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306114 (CHEMBL594084 | N-(2-hydroxy-2-phenylethyl)-6-(3-hy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50296528 (CHEMBL557445 | N-(5-(4-fluoro-3-(trifluoromethyl)b...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50296305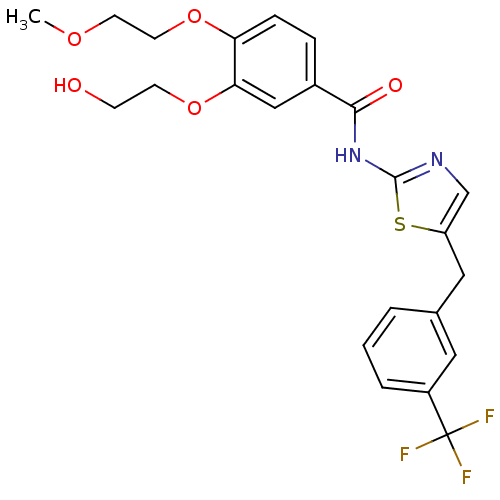 (3-(2-hydroxyethoxy)-4-(2-methoxyethoxy)-N-(5-(3-(t...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of stearoyl-CoA desaturase 1 in human microsome assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 19: 4159-66 (2009) Article DOI: 10.1016/j.bmcl.2009.05.123 BindingDB Entry DOI: 10.7270/Q2PZ58VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306110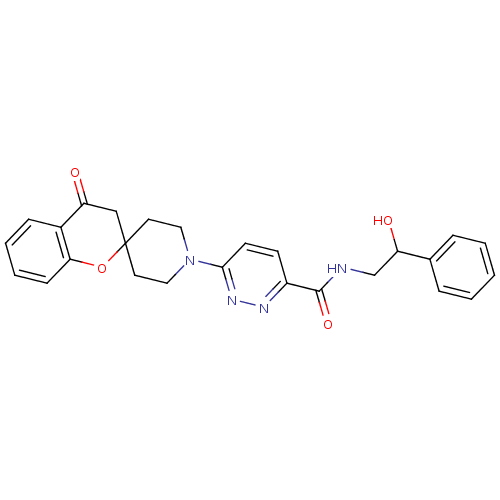 (CHEMBL607314 | N-(2-hydroxy-2-phenylethyl)-6-(4-ox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50330380 (5-Methyl-1'-{6-[5-(pyridin-3-ylmethyl)-1,3,4-oxadi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in HEK293A cells assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50330381 (5-Fluoro-1'-{6-[5-(pyridin-3-ylmethyl)-1,3,4-oxadi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in HEK293A cells assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306131 (1'-(6-(5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in HEK293A cells assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306131 (1'-(6-(5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306124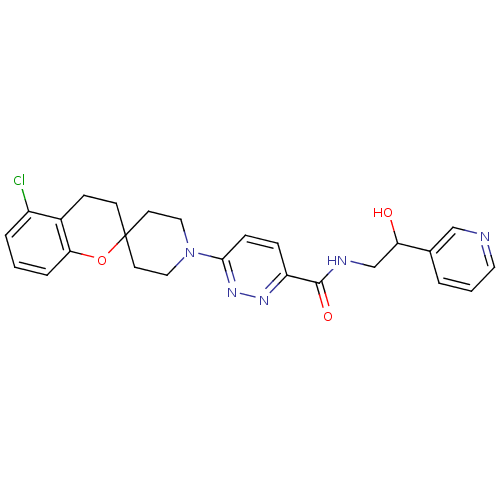 (6-(5-chlorospiro[chroman-2,4'-piperidine]-1'-yl)-N...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate after 60 mins in presence of S9 microsomal fraction | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM186603 (US9168248, 76) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Canada Inc. US Patent | Assay Description The potency of compounds of formula I against the stearoyl-CoA desaturase was determined by measuring the conversion of radiolabeled stearoyl-CoA to ... | US Patent US9168248 (2015) BindingDB Entry DOI: 10.7270/Q2SF2TZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306125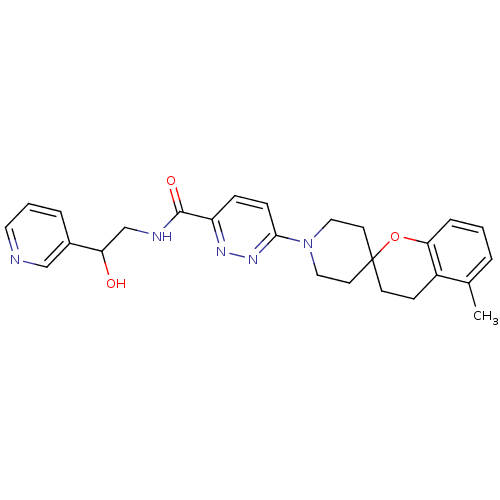 (CHEMBL595255 | N-(2-hydroxy-2-(pyridin-3-yl)ethyl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate after 60 mins in presence of S9 microsomal fraction | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306129 (1'-(6-(3-(pyridin-3-ylmethyl)-1H-1,2,4-triazol-5-y...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate after 60 mins in presence of S9 microsomal fraction | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50312702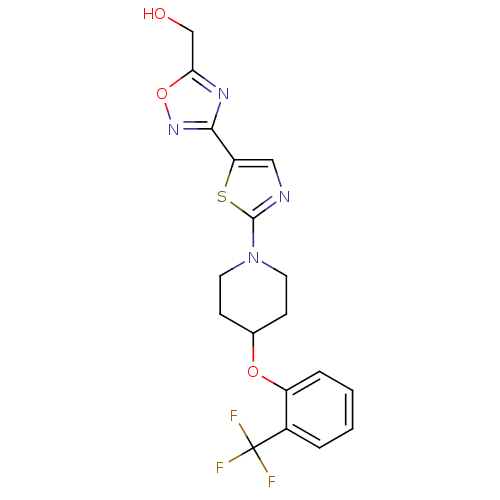 ((3-(2-(4-(2-(trifluoromethyl)phenoxy)piperidin-1-y...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD1 in human HepG2 cells assessed as [14C]stearic acid to [14C]oleic acid conversion pretreated 15 mins before [14C]stearic acid addit... | Bioorg Med Chem Lett 20: 1593-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.083 BindingDB Entry DOI: 10.7270/Q2DJ5FR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50330382 (1'-{6-[5-(Pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of SCD1 in HEK293A cell microsomes assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50330378 (5-Chloro-1'-{6-[5-(pyridin-3-ylmethyl)-1,3,4-oxadi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in HEK293A cells assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50330379 (5,8-Difluoro-1'-{6-[5-(pyridin-3-ylmethyl)-1,3,4-o...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in HEK293A cells assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50296526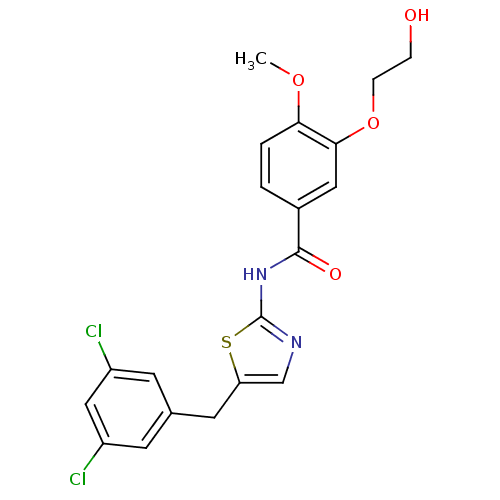 (CHEMBL552126 | N-(5-(3,5-dichlorobenzyl)thiazol-2-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50296531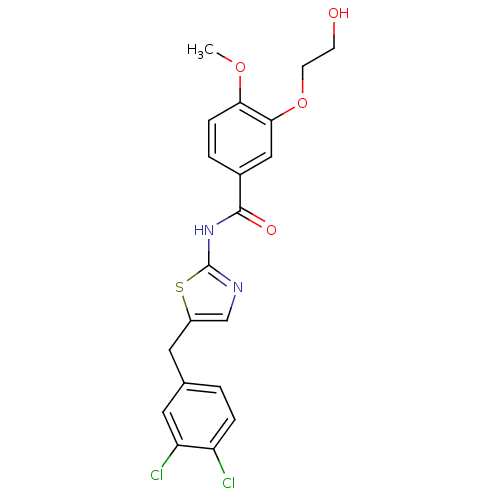 (CHEMBL550800 | N-(5-(3,4-dichlorobenzyl)thiazol-2-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50362592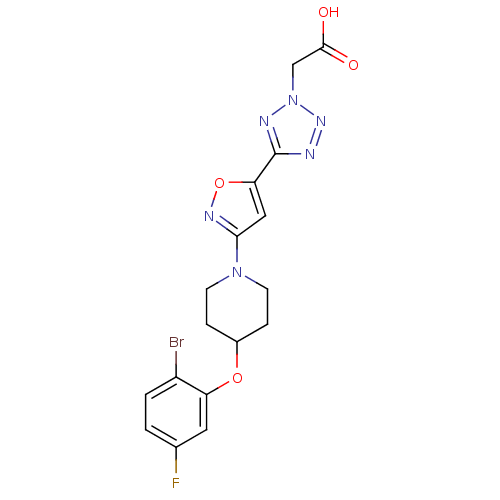 (CHEMBL1938870) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human SCD-1 | J Med Chem 54: 5082-96 (2011) Article DOI: 10.1021/jm200319u BindingDB Entry DOI: 10.7270/Q2348MGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM186592 (US9168248, 16) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Canada Inc. US Patent | Assay Description The potency of compounds of formula I against the stearoyl-CoA desaturase was determined by measuring the conversion of radiolabeled stearoyl-CoA to ... | US Patent US9168248 (2015) BindingDB Entry DOI: 10.7270/Q2SF2TZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM186593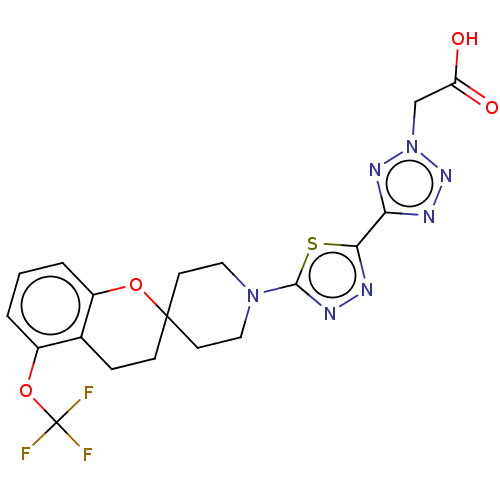 (US9168248, 17) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Canada Inc. US Patent | Assay Description The potency of compounds of formula I against the stearoyl-CoA desaturase was determined by measuring the conversion of radiolabeled stearoyl-CoA to ... | US Patent US9168248 (2015) BindingDB Entry DOI: 10.7270/Q2SF2TZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM186594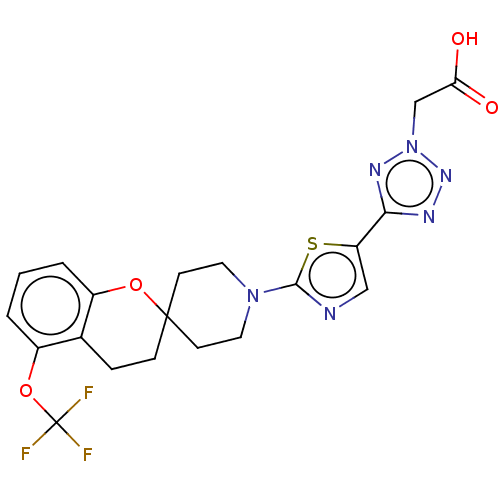 (US9168248, 18) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Canada Inc. US Patent | Assay Description The potency of compounds of formula I against the stearoyl-CoA desaturase was determined by measuring the conversion of radiolabeled stearoyl-CoA to ... | US Patent US9168248 (2015) BindingDB Entry DOI: 10.7270/Q2SF2TZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM186595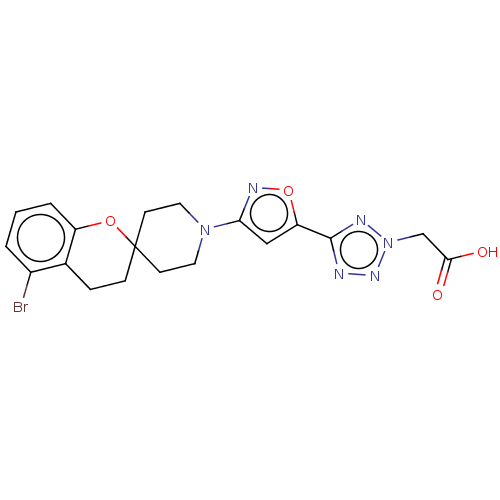 (US9168248, 22) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Canada Inc. US Patent | Assay Description The potency of compounds of formula I against the stearoyl-CoA desaturase was determined by measuring the conversion of radiolabeled stearoyl-CoA to ... | US Patent US9168248 (2015) BindingDB Entry DOI: 10.7270/Q2SF2TZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM186596 (US9168248, 28) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Canada Inc. US Patent | Assay Description The potency of compounds of formula I against the stearoyl-CoA desaturase was determined by measuring the conversion of radiolabeled stearoyl-CoA to ... | US Patent US9168248 (2015) BindingDB Entry DOI: 10.7270/Q2SF2TZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM186597 (US9168248, 42) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Canada Inc. US Patent | Assay Description The potency of compounds of formula I against the stearoyl-CoA desaturase was determined by measuring the conversion of radiolabeled stearoyl-CoA to ... | US Patent US9168248 (2015) BindingDB Entry DOI: 10.7270/Q2SF2TZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM186602 (US9168248, 75) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Canada Inc. US Patent | Assay Description The potency of compounds of formula I against the stearoyl-CoA desaturase was determined by measuring the conversion of radiolabeled stearoyl-CoA to ... | US Patent US9168248 (2015) BindingDB Entry DOI: 10.7270/Q2SF2TZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50267928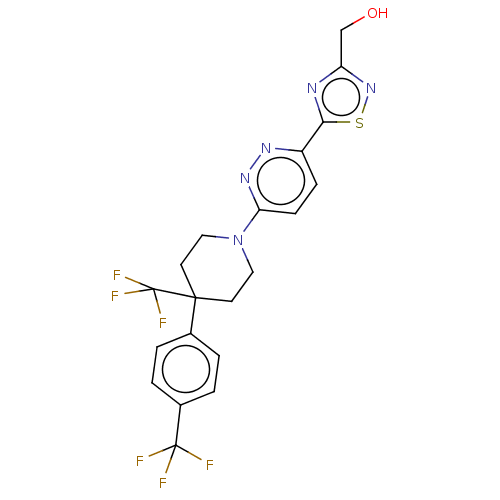 (CHEMBL4066506) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Ltd., 26-1, Muraokahigashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: keisuke.imamura@takeda.com. Curated by ChEMBL | Assay Description Displacement of (5-(6-(10H-spiro(1-benzofuran-3,30-pyrrolidin)-10-yl)pyridazin-3-yl)-1,2,4-oxadiazol-3-yl)[3H2]methanol from SCD1 in human liver micr... | Bioorg Med Chem 25: 3768-3779 (2017) Article DOI: 10.1016/j.bmc.2017.05.016 BindingDB Entry DOI: 10.7270/Q2Z60RJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306127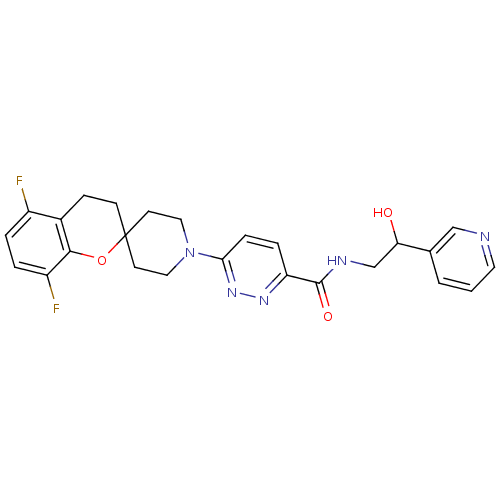 (6-(5,8-difluorospiro[chroman-2,4'-piperidine]-1'-y...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate after 60 mins in presence of S9 microsomal fraction | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50296529 (CHEMBL552270 | N-(5-(3-chloro-4-fluorobenzyl)thiaz...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50296304 (4-ethoxy-3-(2-hydroxyethoxy)-N-(5-(3-(trifluoromet...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of stearoyl-CoA desaturase 1 in human microsome assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 19: 4159-66 (2009) Article DOI: 10.1016/j.bmcl.2009.05.123 BindingDB Entry DOI: 10.7270/Q2PZ58VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306124 (6-(5-chlorospiro[chroman-2,4'-piperidine]-1'-yl)-N...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50448659 (CHEMBL3127654) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, LLC Curated by ChEMBL | Assay Description Inhibition of SCD1 in human A431 cells assessed as [13C]-palmitic acid conversion to [13C]-palmitoleic acid after 4 hrs by LC/MS analysis | Bioorg Med Chem Lett 24: 1437-41 (2014) Article DOI: 10.1016/j.bmcl.2013.12.075 BindingDB Entry DOI: 10.7270/Q26M38BM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1200 total ) | Next | Last >> |