 Found 332 hits Enz. Inhib. hit(s) with Target = '1-deoxy-D-xylulose 5-phosphate reductoisomerase'
Found 332 hits Enz. Inhib. hit(s) with Target = '1-deoxy-D-xylulose 5-phosphate reductoisomerase' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50028309
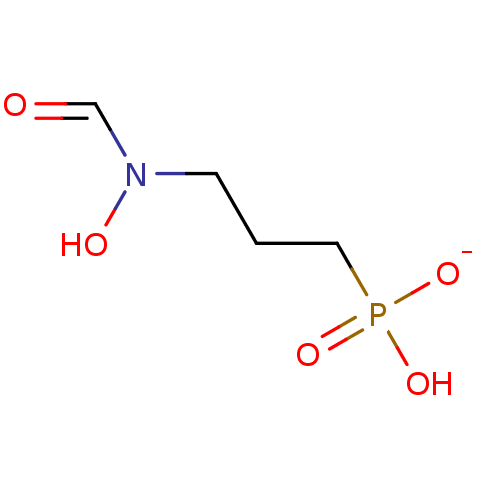
(Fosmidomycin Sodium)Show InChI InChI=1S/C4H10NO5P.Na/c6-4-5(7)2-1-3-11(8,9)10;/h4,7H,1-3H2,(H2,8,9,10);/q;+1/p-1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against recombinant Trypanosoma cruzi (Trypanosoma cruzi) Trypanothione reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Mycobacterium tuberculosis) | BDBM50441210
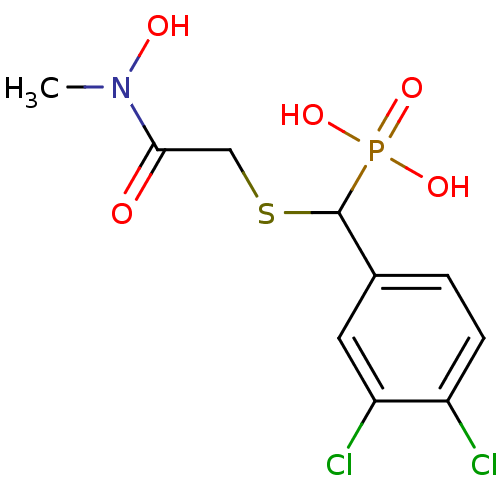
(CHEMBL2431008)Show InChI InChI=1S/C10H12Cl2NO5PS/c1-13(15)9(14)5-20-10(19(16,17)18)6-2-3-7(11)8(12)4-6/h2-4,10,15H,5H2,1H3,(H2,16,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehrstuhl für Biochemie
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis IspC by photometric assay |
J Med Chem 56: 8151-62 (2013)
Article DOI: 10.1021/jm4012559
BindingDB Entry DOI: 10.7270/Q2JH3NN8 |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase, apicoplastic
(Plasmodium falciparum (isolate 3D7)) | BDBM50528602
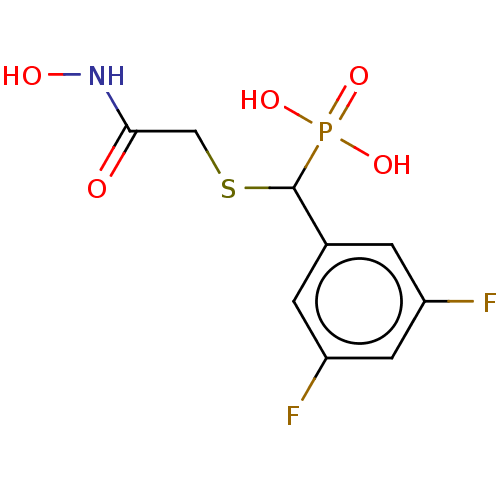
(CHEMBL4567904)Show InChI InChI=1S/C9H10F2NO5PS/c10-6-1-5(2-7(11)3-6)9(18(15,16)17)19-4-8(13)12-14/h1-3,9,14H,4H2,(H,12,13)(H2,15,16,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Plasmodium falciparum IspC expressed in Escherichia coli using 1-deoxy-D-xylulose 5-phosphate as substrate by photometric a... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.058
BindingDB Entry DOI: 10.7270/Q2WW7N4Z |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50528602

(CHEMBL4567904)Show InChI InChI=1S/C9H10F2NO5PS/c10-6-1-5(2-7(11)3-6)9(18(15,16)17)19-4-8(13)12-14/h1-3,9,14H,4H2,(H,12,13)(H2,15,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Escherichia coli IspC expressed in Escherichia coli M15 using 1-deoxy-D-xylulose 5-phosphate as substrate by photometric as... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.058
BindingDB Entry DOI: 10.7270/Q2WW7N4Z |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase, apicoplastic
(Plasmodium falciparum (isolate 3D7)) | BDBM50528593

(CHEMBL4590343)Show InChI InChI=1S/C10H12F2NO5PS/c1-13(15)9(14)5-20-10(19(16,17)18)6-2-7(11)4-8(12)3-6/h2-4,10,15H,5H2,1H3,(H2,16,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Plasmodium falciparum IspC expressed in Escherichia coli using 1-deoxy-D-xylulose 5-phosphate as substrate by photometric a... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.058
BindingDB Entry DOI: 10.7270/Q2WW7N4Z |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50528593

(CHEMBL4590343)Show InChI InChI=1S/C10H12F2NO5PS/c1-13(15)9(14)5-20-10(19(16,17)18)6-2-7(11)4-8(12)3-6/h2-4,10,15H,5H2,1H3,(H2,16,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Escherichia coli IspC expressed in Escherichia coli M15 using 1-deoxy-D-xylulose 5-phosphate as substrate by photometric as... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.058
BindingDB Entry DOI: 10.7270/Q2WW7N4Z |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase, apicoplastic
(Plasmodium falciparum (isolate 3D7)) | BDBM50528597
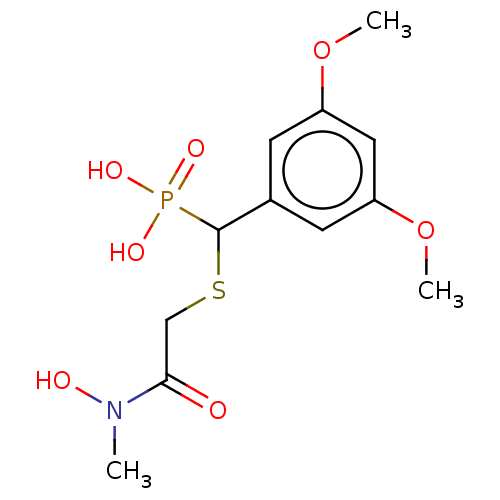
(CHEMBL4592986)Show InChI InChI=1S/C12H18NO7PS/c1-13(15)11(14)7-22-12(21(16,17)18)8-4-9(19-2)6-10(5-8)20-3/h4-6,12,15H,7H2,1-3H3,(H2,16,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Plasmodium falciparum IspC expressed in Escherichia coli using 1-deoxy-D-xylulose 5-phosphate as substrate by photometric a... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.058
BindingDB Entry DOI: 10.7270/Q2WW7N4Z |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50153713
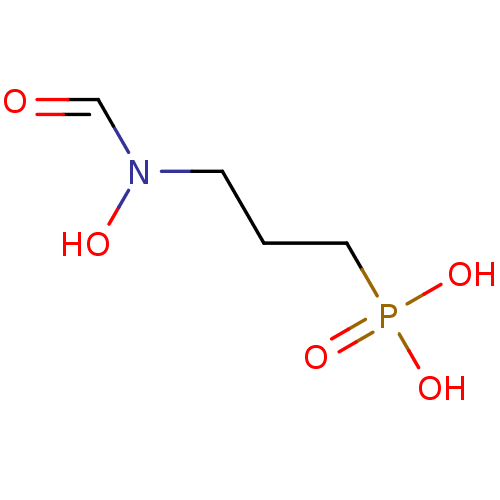
(3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...)Show InChI InChI=1S/C4H10NO5P/c6-4-5(7)2-1-3-11(8,9)10/h4,7H,1-3H2,(H2,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against DOXP reductoisomerase |
J Med Chem 48: 3547-63 (2005)
Article DOI: 10.1021/jm0491501
BindingDB Entry DOI: 10.7270/Q2VH5Q21 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50528598
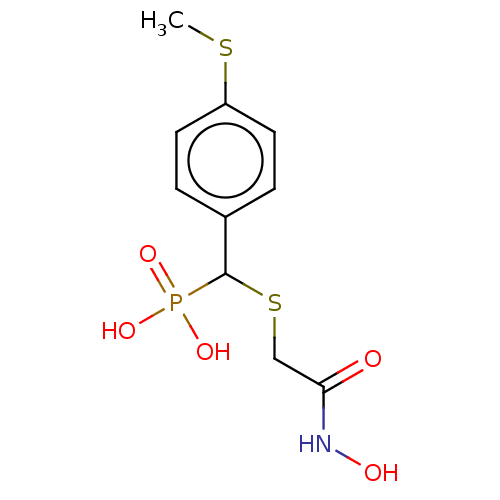
(CHEMBL4474216)Show InChI InChI=1S/C10H14NO5PS2/c1-18-8-4-2-7(3-5-8)10(17(14,15)16)19-6-9(12)11-13/h2-5,10,13H,6H2,1H3,(H,11,12)(H2,14,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Escherichia coli IspC expressed in Escherichia coli M15 using 1-deoxy-D-xylulose 5-phosphate as substrate by photometric as... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.058
BindingDB Entry DOI: 10.7270/Q2WW7N4Z |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase, apicoplastic
(Plasmodium falciparum (isolate 3D7)) | BDBM50459311
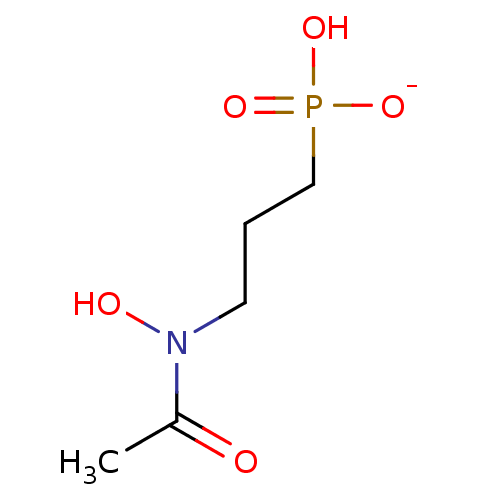
(CHEMBL1922604 | FR900098)Show SMILES [Na;v0+].[#6]-[#6](=O)-[#7](-[#8])-[#6]-[#6]-[#6]P([#8])([#8-])=O Show InChI InChI=1S/C5H12NO5P/c1-5(7)6(8)3-2-4-12(9,10)11/h8H,2-4H2,1H3,(H2,9,10,11)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
George Washington University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum DXR assessed as reduction in NADPH oxidation in presence of DOXP by UV-visible spectrophotometry |
J Med Chem 61: 8847-8858 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01026
BindingDB Entry DOI: 10.7270/Q2S75JZ7 |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase, apicoplastic
(Plasmodium falciparum (isolate 3D7)) | BDBM50528603
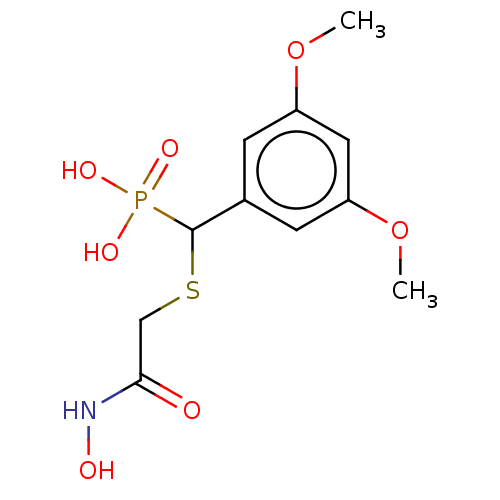
(CHEMBL4544750)Show InChI InChI=1S/C11H16NO7PS/c1-18-8-3-7(4-9(5-8)19-2)11(20(15,16)17)21-6-10(13)12-14/h3-5,11,14H,6H2,1-2H3,(H,12,13)(H2,15,16,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Plasmodium falciparum IspC expressed in Escherichia coli using 1-deoxy-D-xylulose 5-phosphate as substrate by photometric a... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.058
BindingDB Entry DOI: 10.7270/Q2WW7N4Z |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase, apicoplastic
(Plasmodium falciparum (isolate 3D7)) | BDBM50459311

(CHEMBL1922604 | FR900098)Show SMILES [Na;v0+].[#6]-[#6](=O)-[#7](-[#8])-[#6]-[#6]-[#6]P([#8])([#8-])=O Show InChI InChI=1S/C5H12NO5P/c1-5(7)6(8)3-2-4-12(9,10)11/h8H,2-4H2,1H3,(H2,9,10,11)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
George Washington University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum DXR |
J Med Chem 61: 8847-8858 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01026
BindingDB Entry DOI: 10.7270/Q2S75JZ7 |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50181153
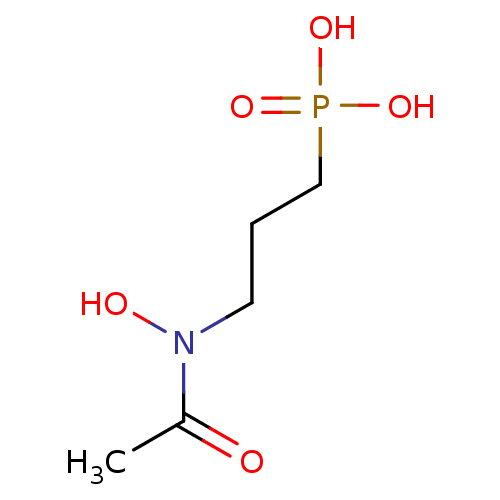
(3-(N-hydroxyacetamido)propylphosphonic acid | 3-(N...)Show InChI InChI=1S/C5H12NO5P/c1-5(7)6(8)3-2-4-12(9,10)11/h8H,2-4H2,1H3,(H2,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against DOXP reductoisomerase |
J Med Chem 48: 3547-63 (2005)
Article DOI: 10.1021/jm0491501
BindingDB Entry DOI: 10.7270/Q2VH5Q21 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
1-deoxy-D-xylulose 5-phosphate reductoisomerase, apicoplastic
(Plasmodium falciparum (isolate 3D7)) | BDBM50528598

(CHEMBL4474216)Show InChI InChI=1S/C10H14NO5PS2/c1-18-8-4-2-7(3-5-8)10(17(14,15)16)19-6-9(12)11-13/h2-5,10,13H,6H2,1H3,(H,11,12)(H2,14,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Plasmodium falciparum IspC expressed in Escherichia coli using 1-deoxy-D-xylulose 5-phosphate as substrate by photometric a... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.058
BindingDB Entry DOI: 10.7270/Q2WW7N4Z |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50181153

(3-(N-hydroxyacetamido)propylphosphonic acid | 3-(N...)Show InChI InChI=1S/C5H12NO5P/c1-5(7)6(8)3-2-4-12(9,10)11/h8H,2-4H2,1H3,(H2,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against recombinant Trypanosoma cruzi (Trypanosoma cruzi) Trypanothione reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50153713

(3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...)Show InChI InChI=1S/C4H10NO5P/c6-4-5(7)2-1-3-11(8,9)10/h4,7H,1-3H2,(H2,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli DXR |
ACS Med Chem Lett 2: 165-170 (2011)
Article DOI: 10.1021/ml100243r
BindingDB Entry DOI: 10.7270/Q2XD12NV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50181153

(3-(N-hydroxyacetamido)propylphosphonic acid | 3-(N...)Show InChI InChI=1S/C5H12NO5P/c1-5(7)6(8)3-2-4-12(9,10)11/h8H,2-4H2,1H3,(H2,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli DXR |
ACS Med Chem Lett 2: 165-170 (2011)
Article DOI: 10.1021/ml100243r
BindingDB Entry DOI: 10.7270/Q2XD12NV |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50181153

(3-(N-hydroxyacetamido)propylphosphonic acid | 3-(N...)Show InChI InChI=1S/C5H12NO5P/c1-5(7)6(8)3-2-4-12(9,10)11/h8H,2-4H2,1H3,(H2,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Escherichia coli DXR |
Bioorg Med Chem Lett 16: 1888-91 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.082
BindingDB Entry DOI: 10.7270/Q2FJ2GB6 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50153713

(3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...)Show InChI InChI=1S/C4H10NO5P/c6-4-5(7)2-1-3-11(8,9)10/h4,7H,1-3H2,(H2,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Escherichia coli DXR |
Bioorg Med Chem Lett 16: 1888-91 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.082
BindingDB Entry DOI: 10.7270/Q2FJ2GB6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50153713

(3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...)Show InChI InChI=1S/C4H10NO5P/c6-4-5(7)2-1-3-11(8,9)10/h4,7H,1-3H2,(H2,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Dxr |
J Med Chem 57: 9740-63 (2014)
Article DOI: 10.1021/jm5010978
BindingDB Entry DOI: 10.7270/Q29K4CVJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50153713

(3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...)Show InChI InChI=1S/C4H10NO5P/c6-4-5(7)2-1-3-11(8,9)10/h4,7H,1-3H2,(H2,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged Escherichia coli DXR pre-incubated for 2 mins before reaction initiation in presence of 160 uM NADPH in absence of 0.01% Tri... |
Bioorg Med Chem 22: 3713-9 (2014)
Article DOI: 10.1016/j.bmc.2014.05.004
BindingDB Entry DOI: 10.7270/Q2057HKT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
1-deoxy-D-xylulose 5-phosphate reductoisomerase, apicoplastic
(Plasmodium falciparum (isolate 3D7)) | BDBM50528590
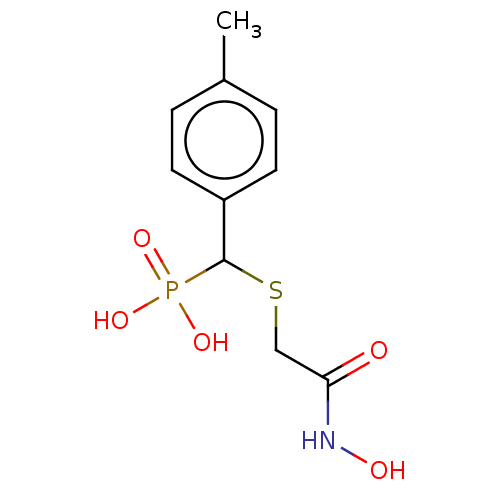
(CHEMBL4447571)Show InChI InChI=1S/C10H14NO5PS/c1-7-2-4-8(5-3-7)10(17(14,15)16)18-6-9(12)11-13/h2-5,10,13H,6H2,1H3,(H,11,12)(H2,14,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Plasmodium falciparum IspC expressed in Escherichia coli using 1-deoxy-D-xylulose 5-phosphate as substrate by photometric a... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.058
BindingDB Entry DOI: 10.7270/Q2WW7N4Z |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase, apicoplastic
(Plasmodium falciparum (isolate 3D7)) | BDBM50028309

(Fosmidomycin Sodium)Show InChI InChI=1S/C4H10NO5P.Na/c6-4-5(7)2-1-3-11(8,9)10;/h4,7H,1-3H2,(H2,8,9,10);/q;+1/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
George Washington University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum DXR |
J Med Chem 61: 8847-8858 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01026
BindingDB Entry DOI: 10.7270/Q2S75JZ7 |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50153713

(3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...)Show InChI InChI=1S/C4H10NO5P/c6-4-5(7)2-1-3-11(8,9)10/h4,7H,1-3H2,(H2,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universite de Strasbourg
| Assay Description
Assays were performed in 50 mM Tris/HCl buffer, pH 7.5, containing 3 mM MgCl2 and 2 mM DTT. The concentrations of NADPH and DXP were 0.15 and 0.5 mM ... |
Bioorg Chem 59: 140-4 (2015)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q2XS5T4J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Mycobacterium tuberculosis) | BDBM50441211

(CHEMBL2431007)Show InChI InChI=1S/C10H12F2NO5PS/c1-13(15)9(14)5-20-10(19(16,17)18)6-2-3-7(11)8(12)4-6/h2-4,10,15H,5H2,1H3,(H2,16,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehrstuhl für Biochemie
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis IspC by photometric assay |
J Med Chem 56: 8151-62 (2013)
Article DOI: 10.1021/jm4012559
BindingDB Entry DOI: 10.7270/Q2JH3NN8 |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50153713

(3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...)Show InChI InChI=1S/C4H10NO5P/c6-4-5(7)2-1-3-11(8,9)10/h4,7H,1-3H2,(H2,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Escherichia coli DXR |
J Med Chem 49: 2656-60 (2006)
Article DOI: 10.1021/jm051177c
BindingDB Entry DOI: 10.7270/Q22F7N24 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50380000
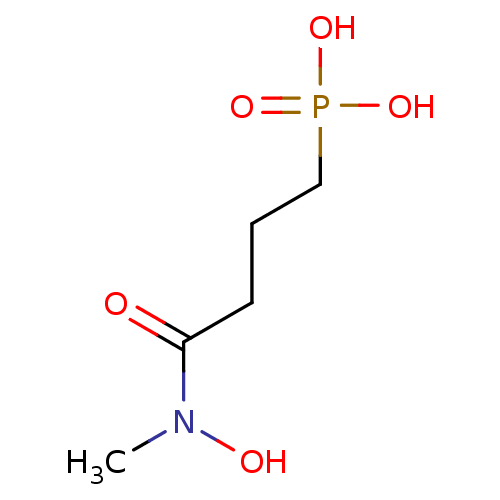
(CHEMBL258981)Show InChI InChI=1S/C5H12NO5P/c1-6(8)5(7)3-2-4-12(9,10)11/h8H,2-4H2,1H3,(H2,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonism of norepinephrine induced contraction of rat isolated thoracic aorta. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50028309

(Fosmidomycin Sodium)Show InChI InChI=1S/C4H10NO5P.Na/c6-4-5(7)2-1-3-11(8,9)10;/h4,7H,1-3H2,(H2,8,9,10);/q;+1/p-1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonism of norepinephrine induced contraction of rat isolated thoracic aorta. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50185361
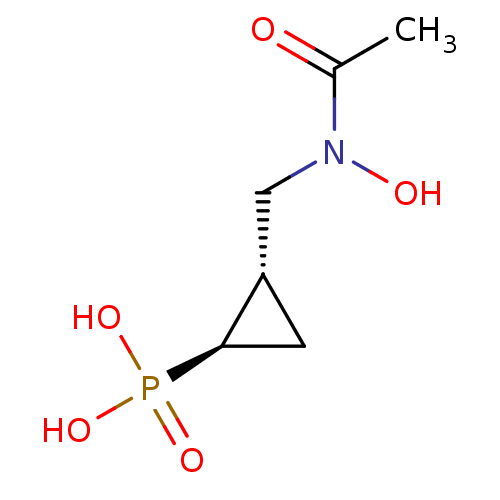
((1R,2S)-2-[N-(acetyl),N-(hydroxy)aminomethyl]-cycl...)Show InChI InChI=1S/C6H12NO5P/c1-4(8)7(9)3-5-2-6(5)13(10,11)12/h5-6,9H,2-3H2,1H3,(H2,10,11,12)/t5-,6+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Escherichia coli DXR |
J Med Chem 49: 2656-60 (2006)
Article DOI: 10.1021/jm051177c
BindingDB Entry DOI: 10.7270/Q22F7N24 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50422669
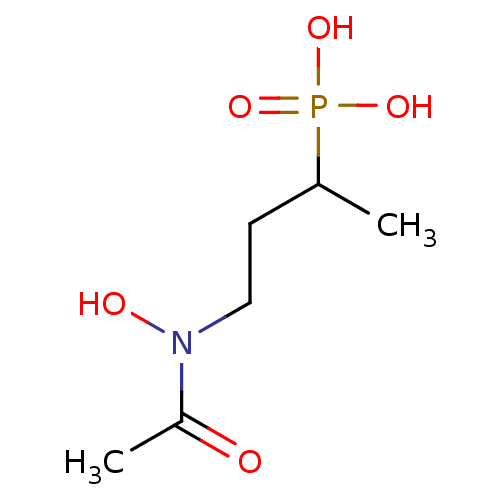
(CHEMBL607359 | UK-513)Show InChI InChI=1S/C6H14NO5P/c1-5(13(10,11)12)3-4-7(9)6(2)8/h5,9H,3-4H2,1-2H3,(H2,10,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against DOXP reductoisomerase |
J Med Chem 48: 3547-63 (2005)
Article DOI: 10.1021/jm0491501
BindingDB Entry DOI: 10.7270/Q2VH5Q21 |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50181153

(3-(N-hydroxyacetamido)propylphosphonic acid | 3-(N...)Show InChI InChI=1S/C5H12NO5P/c1-5(7)6(8)3-2-4-12(9,10)11/h8H,2-4H2,1H3,(H2,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Escherichia coli DXR |
J Med Chem 49: 2656-60 (2006)
Article DOI: 10.1021/jm051177c
BindingDB Entry DOI: 10.7270/Q22F7N24 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50181154
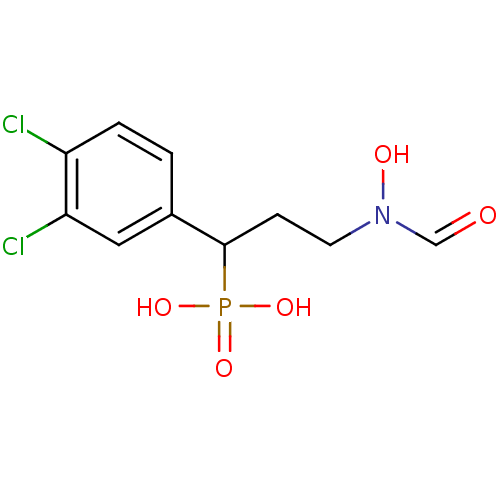
(1-(3,4-dichlorophenyl)-3-(N-hydroxyformamido)propy...)Show InChI InChI=1S/C10H12Cl2NO5P/c11-8-2-1-7(5-9(8)12)10(19(16,17)18)3-4-13(15)6-14/h1-2,5-6,10,15H,3-4H2,(H2,16,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Escherichia coli DXR |
Bioorg Med Chem Lett 16: 1888-91 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.082
BindingDB Entry DOI: 10.7270/Q2FJ2GB6 |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50181154

(1-(3,4-dichlorophenyl)-3-(N-hydroxyformamido)propy...)Show InChI InChI=1S/C10H12Cl2NO5P/c11-8-2-1-7(5-9(8)12)10(19(16,17)18)3-4-13(15)6-14/h1-2,5-6,10,15H,3-4H2,(H2,16,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli DXR |
ACS Med Chem Lett 2: 165-170 (2011)
Article DOI: 10.1021/ml100243r
BindingDB Entry DOI: 10.7270/Q2XD12NV |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase, apicoplastic
(Plasmodium falciparum (isolate 3D7)) | BDBM50028309

(Fosmidomycin Sodium)Show InChI InChI=1S/C4H10NO5P.Na/c6-4-5(7)2-1-3-11(8,9)10;/h4,7H,1-3H2,(H2,8,9,10);/q;+1/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
George Washington University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum DXR assessed as reduction in NADPH oxidation in presence of DOXP by UV-visible spectrophotometry |
J Med Chem 61: 8847-8858 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01026
BindingDB Entry DOI: 10.7270/Q2S75JZ7 |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50422650
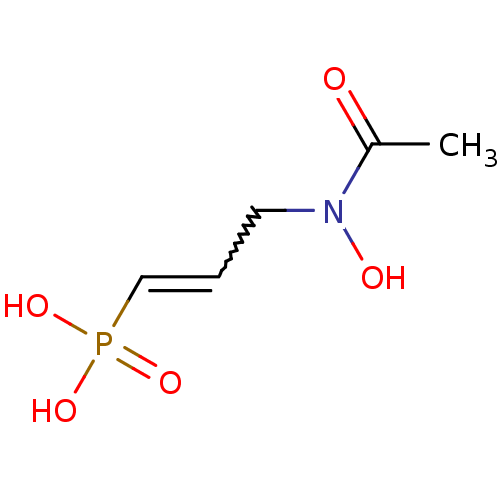
(CHEMBL606521)Show InChI InChI=1S/C5H10NO5P/c1-5(7)6(8)3-2-4-12(9,10)11/h2,4,8H,3H2,1H3,(H2,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against DOXP reductoisomerase |
J Med Chem 48: 3547-63 (2005)
Article DOI: 10.1021/jm0491501
BindingDB Entry DOI: 10.7270/Q2VH5Q21 |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50028362
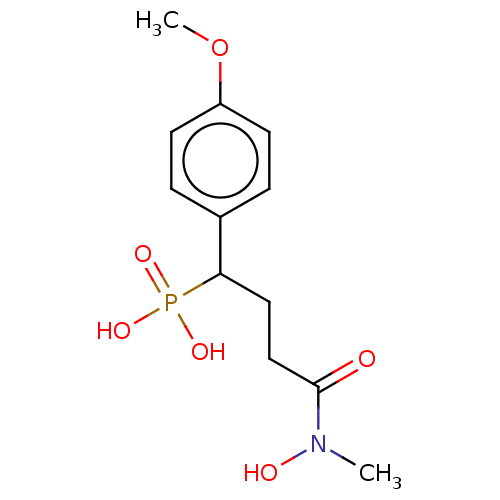
(CHEMBL3341763)Show InChI InChI=1S/C12H18NO6P/c1-13(15)12(14)8-7-11(20(16,17)18)9-3-5-10(19-2)6-4-9/h3-6,11,15H,7-8H2,1-2H3,(H2,16,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli M15 recombinant IspC using 1-deoxy-D-xylulose 5-phosphate as substrate by photometric analysis |
J Med Chem 57: 8827-38 (2014)
Article DOI: 10.1021/jm500850y
BindingDB Entry DOI: 10.7270/Q2KP83RX |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Mycobacterium tuberculosis) | BDBM50153713

(3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...)Show InChI InChI=1S/C4H10NO5P/c6-4-5(7)2-1-3-11(8,9)10/h4,7H,1-3H2,(H2,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and Mn2+ as cofactor preincu... |
J Med Chem 54: 4721-34 (2011)
Article DOI: 10.1021/jm200363d
BindingDB Entry DOI: 10.7270/Q2NK3FCT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Mycobacterium tuberculosis) | BDBM50153713

(3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...)Show InChI InChI=1S/C4H10NO5P/c6-4-5(7)2-1-3-11(8,9)10/h4,7H,1-3H2,(H2,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis DXR assessed as reduction of 1-deoxy-D-xylulose 5-phosphate into 2-C-methyl-D-erythritol-4-phosphate measure... |
Bioorg Med Chem Lett 21: 5403-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.005
BindingDB Entry DOI: 10.7270/Q290246H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Mycobacterium tuberculosis) | BDBM50153713

(3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...)Show InChI InChI=1S/C4H10NO5P/c6-4-5(7)2-1-3-11(8,9)10/h4,7H,1-3H2,(H2,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis DXR assessed as NADPH-dependent conversion of 1-deoxy-D-xylulose 5-phosphate to 2-C-methyl-D-erythritol 4-ph... |
J Biol Chem 282: 19905-16 (2007)
Article DOI: 10.1074/jbc.M701935200
BindingDB Entry DOI: 10.7270/Q28G8MK1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Mycobacterium smegmatis (strain ATCC 700084 / mc(2...) | BDBM50153713

(3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...)Show InChI InChI=1S/C4H10NO5P/c6-4-5(7)2-1-3-11(8,9)10/h4,7H,1-3H2,(H2,8,9,10) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium smegmatis DXR |
Eur J Med Chem 51: 277-85 (2012)
Article DOI: 10.1016/j.ejmech.2012.02.031
BindingDB Entry DOI: 10.7270/Q2474BWK |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Mycobacterium tuberculosis) | BDBM50153713

(3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...)Show InChI InChI=1S/C4H10NO5P/c6-4-5(7)2-1-3-11(8,9)10/h4,7H,1-3H2,(H2,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis DXR using DXP as substrate assessed as formation of MEP measured every 5 secs for 180 secs by spectrophotome... |
J Med Chem 56: 6190-9 (2013)
Article DOI: 10.1021/jm4006498
BindingDB Entry DOI: 10.7270/Q2S183W1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Mycobacterium tuberculosis) | BDBM50028309

(Fosmidomycin Sodium)Show InChI InChI=1S/C4H10NO5P.Na/c6-4-5(7)2-1-3-11(8,9)10;/h4,7H,1-3H2,(H2,8,9,10);/q;+1/p-1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against recombinant Trypanosoma cruzi (Trypanosoma cruzi) Trypanothione reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase, apicoplastic
(Plasmodium falciparum (isolate 3D7)) | BDBM50459310
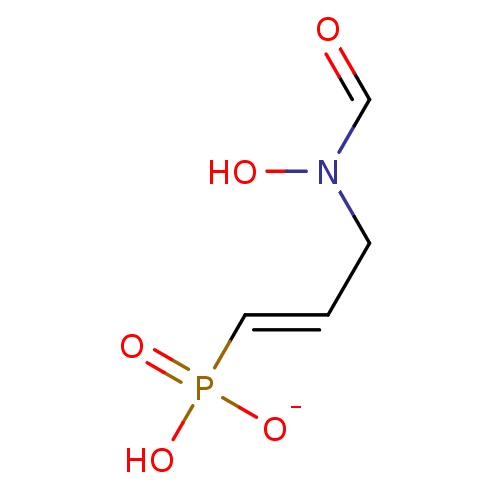
(CHEMBL4207168)Show SMILES [Na;v0+].[#8]-[#7](-[#6]\[#6]=[#6]\P([#8])([#8-])=O)-[#6]=O Show InChI InChI=1S/C4H8NO5P/c6-4-5(7)2-1-3-11(8,9)10/h1,3-4,7H,2H2,(H2,8,9,10)/p-1/b3-1+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
George Washington University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum DXR assessed as reduction in NADPH oxidation in presence of DOXP by UV-visible spectrophotometry |
J Med Chem 61: 8847-8858 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01026
BindingDB Entry DOI: 10.7270/Q2S75JZ7 |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50335484
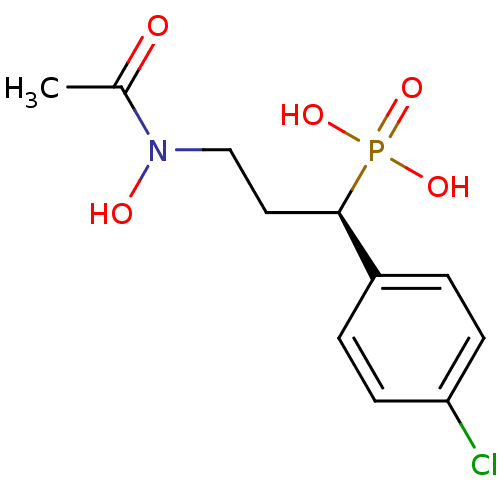
((R)-1-(4-chlorophenyl)-3-(N-hydroxyacetamido)propy...)Show SMILES CC(=O)N(O)CC[C@H](c1ccc(Cl)cc1)P(O)(O)=O |r| Show InChI InChI=1S/C11H15ClNO5P/c1-8(14)13(15)7-6-11(19(16,17)18)9-2-4-10(12)5-3-9/h2-5,11,15H,6-7H2,1H3,(H2,16,17,18)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli DXR |
ACS Med Chem Lett 2: 165-170 (2011)
Article DOI: 10.1021/ml100243r
BindingDB Entry DOI: 10.7270/Q2XD12NV |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50181152
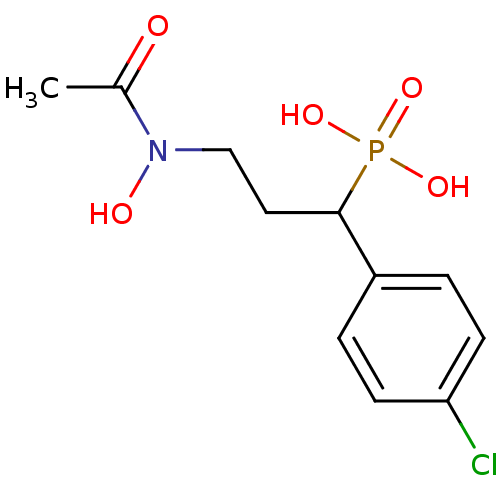
(1-(4-chlorophenyl)-3-(N-hydroxyacetamido)propylpho...)Show InChI InChI=1S/C11H15ClNO5P/c1-8(14)13(15)7-6-11(19(16,17)18)9-2-4-10(12)5-3-9/h2-5,11,15H,6-7H2,1H3,(H2,16,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Escherichia coli DXR |
Bioorg Med Chem Lett 16: 1888-91 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.082
BindingDB Entry DOI: 10.7270/Q2FJ2GB6 |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Mycobacterium tuberculosis) | BDBM50528598

(CHEMBL4474216)Show InChI InChI=1S/C10H14NO5PS2/c1-18-8-4-2-7(3-5-8)10(17(14,15)16)19-6-9(12)11-13/h2-5,10,13H,6H2,1H3,(H,11,12)(H2,14,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Mycobacterium tuberculosis IspC expressed in Escherichia coli M15 using 1-deoxy-D-xylulose 5-phosphate as substrate by phot... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.058
BindingDB Entry DOI: 10.7270/Q2WW7N4Z |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50422666
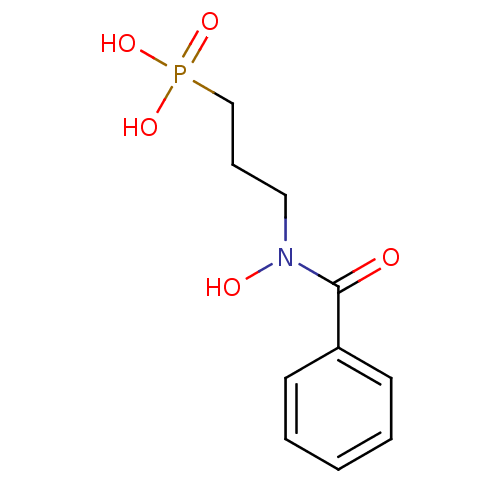
(CHEMBL607492)Show InChI InChI=1S/C10H14NO5P/c12-10(9-5-2-1-3-6-9)11(13)7-4-8-17(14,15)16/h1-3,5-6,13H,4,7-8H2,(H2,14,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against DOXP reductoisomerase |
J Med Chem 48: 3547-63 (2005)
Article DOI: 10.1021/jm0491501
BindingDB Entry DOI: 10.7270/Q2VH5Q21 |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase, chloroplastic
(Arabidopsis thaliana) | BDBM50153713

(3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...)Show InChI InChI=1S/C4H10NO5P/c6-4-5(7)2-1-3-11(8,9)10/h4,7H,1-3H2,(H2,8,9,10) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF-SE
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana IspC expressed in Escherichia coli after 40 min by spectrophotometric analysis |
Pest Manag Sci 69: 559-63 (2013)
Article DOI: 10.1002/ps.3479
BindingDB Entry DOI: 10.7270/Q2DZ0C67 |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Mycobacterium tuberculosis) | BDBM50441208
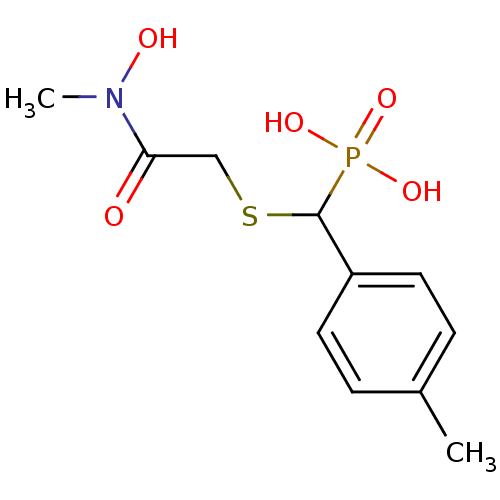
(CHEMBL2431010)Show InChI InChI=1S/C11H16NO5PS/c1-8-3-5-9(6-4-8)11(18(15,16)17)19-7-10(13)12(2)14/h3-6,11,14H,7H2,1-2H3,(H2,15,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehrstuhl für Biochemie
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis IspC by photometric assay |
J Med Chem 56: 8151-62 (2013)
Article DOI: 10.1021/jm4012559
BindingDB Entry DOI: 10.7270/Q2JH3NN8 |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Mycobacterium tuberculosis) | BDBM50528593

(CHEMBL4590343)Show InChI InChI=1S/C10H12F2NO5PS/c1-13(15)9(14)5-20-10(19(16,17)18)6-2-7(11)4-8(12)3-6/h2-4,10,15H,5H2,1H3,(H2,16,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Mycobacterium tuberculosis IspC expressed in Escherichia coli M15 using 1-deoxy-D-xylulose 5-phosphate as substrate by phot... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.058
BindingDB Entry DOI: 10.7270/Q2WW7N4Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data