 Found 116 hits of Enzyme Inhibition Constant Data
Found 116 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM14059
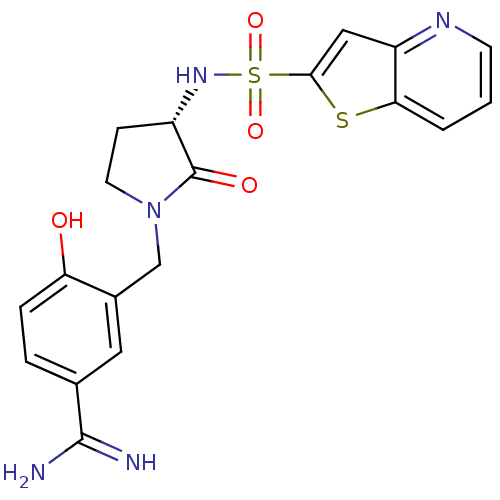
(4-hydroxy-3-[[(3S)-2-oxo-3-(9-thia-5-azabicyclo[4....)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ncccc4s3)C2=O)c1 |r| Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)11-3-4-15(25)12(8-11)10-24-7-5-13(19(24)26)23-30(27,28)17-9-14-16(29-17)2-1-6-22-14/h1-4,6,8-9,13,23,25H,5,7,10H2,(H3,20,21)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081505
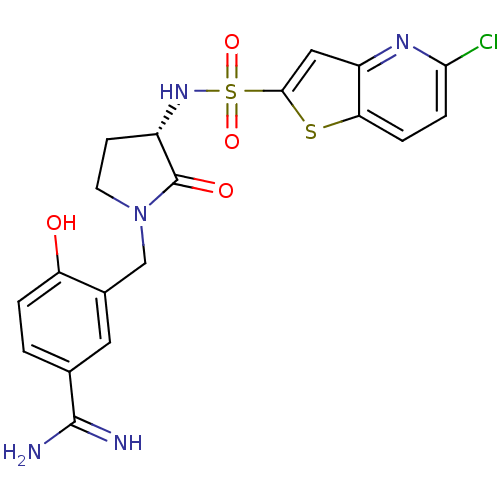
(3-[(S)-3-(5-Chloro-thieno[3,2-b]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-16-4-3-15-13(23-16)8-17(30-15)31(28,29)24-12-5-6-25(19(12)27)9-11-7-10(18(21)22)1-2-14(11)26/h1-4,7-8,12,24,26H,5-6,9H2,(H3,21,22)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081512
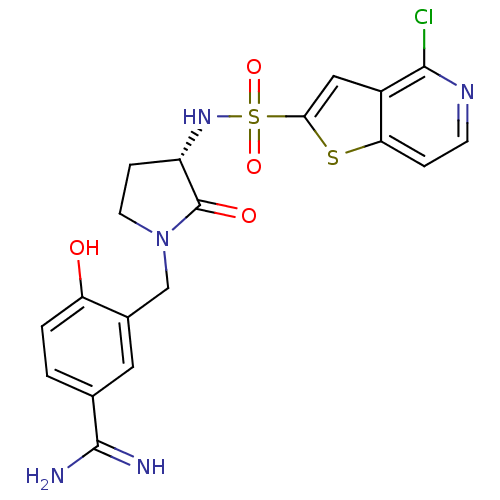
(3-[(S)-3-(4-Chloro-thieno[3,2-c]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4c(Cl)nccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-17-12-8-16(30-15(12)3-5-23-17)31(28,29)24-13-4-6-25(19(13)27)9-11-7-10(18(21)22)1-2-14(11)26/h1-3,5,7-8,13,24,26H,4,6,9H2,(H3,21,22)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081499
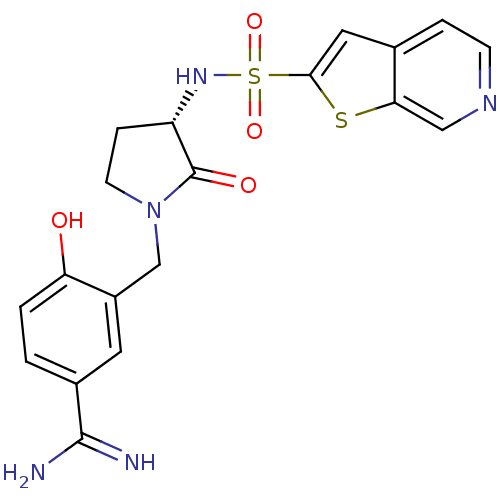
(4-Hydroxy-3-[(S)-2-oxo-3-(thieno[2,3-c]pyridine-2-...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ccncc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)12-1-2-15(25)13(7-12)10-24-6-4-14(19(24)26)23-30(27,28)17-8-11-3-5-22-9-16(11)29-17/h1-3,5,7-9,14,23,25H,4,6,10H2,(H3,20,21)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease Coagulation factor X |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM13286
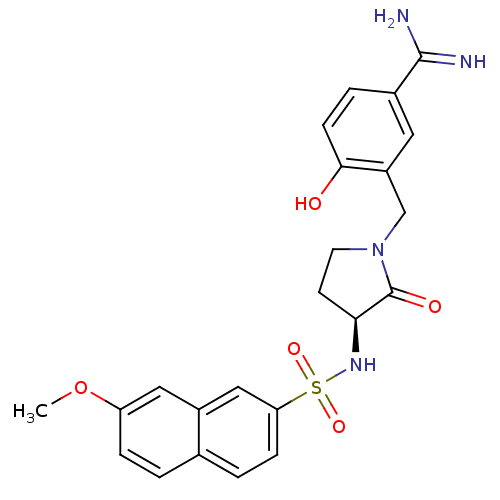
(4-Hydroxy-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfon...)Show SMILES COc1ccc2ccc(cc2c1)S(=O)(=O)N[C@H]1CCN(Cc2cc(ccc2O)C(N)=N)C1=O |r| Show InChI InChI=1S/C23H24N4O5S/c1-32-18-5-2-14-3-6-19(12-16(14)11-18)33(30,31)26-20-8-9-27(23(20)29)13-17-10-15(22(24)25)4-7-21(17)28/h2-7,10-12,20,26,28H,8-9,13H2,1H3,(H3,24,25)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease Coagulation factor X |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081510
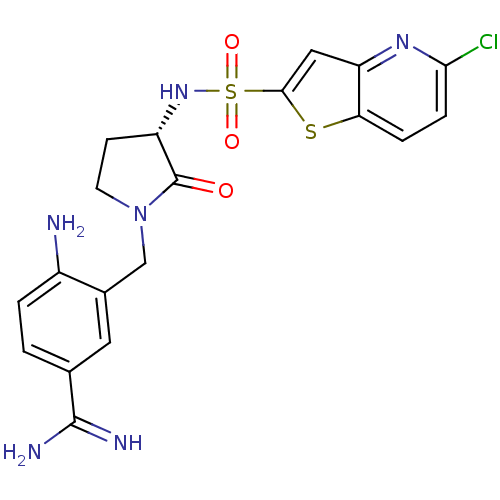
(4-Amino-3-[(S)-3-(5-chloro-thieno[3,2-b]pyridine-2...)Show SMILES NC(=N)c1ccc(N)c(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19ClN6O3S2/c20-16-4-3-15-14(24-16)8-17(30-15)31(28,29)25-13-5-6-26(19(13)27)9-11-7-10(18(22)23)1-2-12(11)21/h1-4,7-8,13,25H,5-6,9,21H2,(H3,22,23)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081517
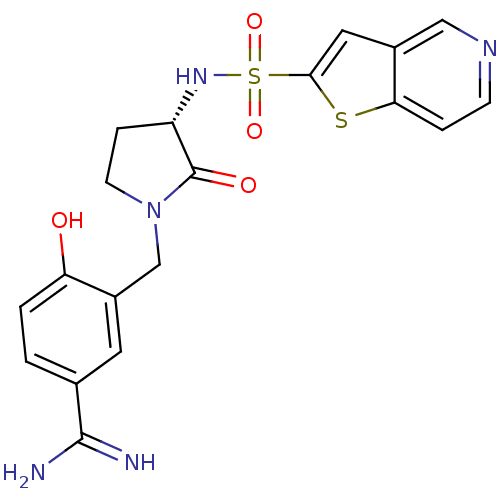
(4-Hydroxy-3-[(S)-2-oxo-3-(thieno[3,2-c]pyridine-2-...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4cnccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)11-1-2-15(25)13(7-11)10-24-6-4-14(19(24)26)23-30(27,28)17-8-12-9-22-5-3-16(12)29-17/h1-3,5,7-9,14,23,25H,4,6,10H2,(H3,20,21)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081515
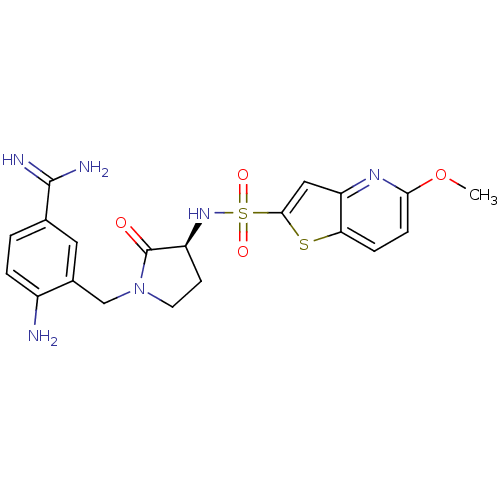
(4-Amino-3-[(S)-3-(5-methoxy-thieno[3,2-b]pyridine-...)Show SMILES COc1ccc2sc(cc2n1)S(=O)(=O)N[C@H]1CCN(Cc2cc(ccc2N)C(N)=N)C1=O Show InChI InChI=1S/C20H22N6O4S2/c1-30-17-5-4-16-15(24-17)9-18(31-16)32(28,29)25-14-6-7-26(20(14)27)10-12-8-11(19(22)23)2-3-13(12)21/h2-5,8-9,14,25H,6-7,10,21H2,1H3,(H3,22,23)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081506
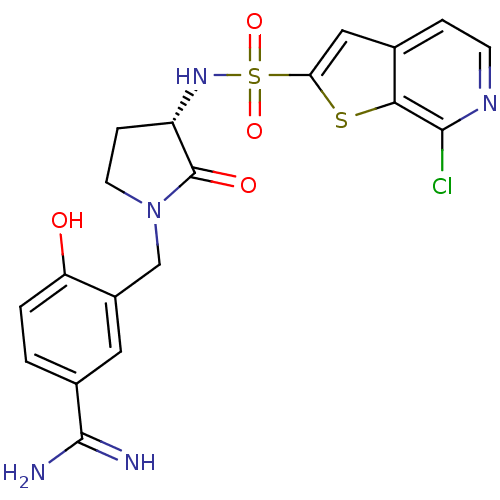
(3-[(S)-3-(7-Chloro-thieno[2,3-c]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ccnc(Cl)c4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-17-16-10(3-5-23-17)8-15(30-16)31(28,29)24-13-4-6-25(19(13)27)9-12-7-11(18(21)22)1-2-14(12)26/h1-3,5,7-8,13,24,26H,4,6,9H2,(H3,21,22)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081011
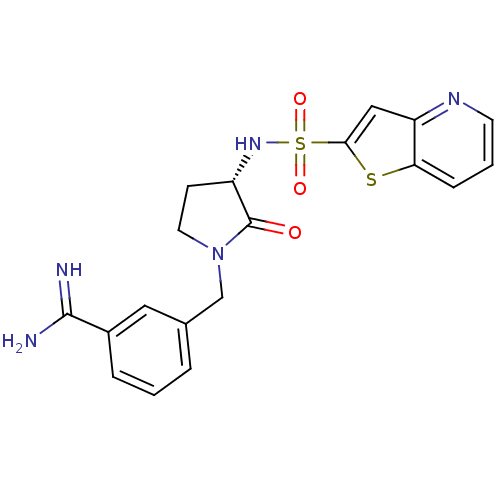
(3-[(S)-2-Oxo-3-(thieno[3,2-b]pyridine-2-sulfonylam...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4ncccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O3S2/c20-18(21)13-4-1-3-12(9-13)11-24-8-6-14(19(24)25)23-29(26,27)17-10-15-16(28-17)5-2-7-22-15/h1-5,7,9-10,14,23H,6,8,11H2,(H3,20,21)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081520
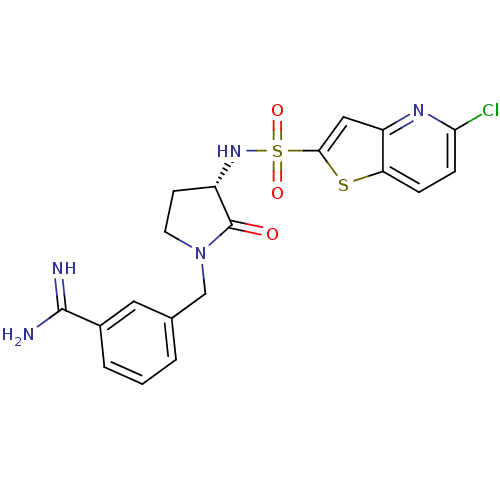
(3-[(S)-3-(5-Chloro-thieno[3,2-b]pyridine-2-sulfony...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O3S2/c20-16-5-4-15-14(23-16)9-17(29-15)30(27,28)24-13-6-7-25(19(13)26)10-11-2-1-3-12(8-11)18(21)22/h1-5,8-9,13,24H,6-7,10H2,(H3,21,22)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081513
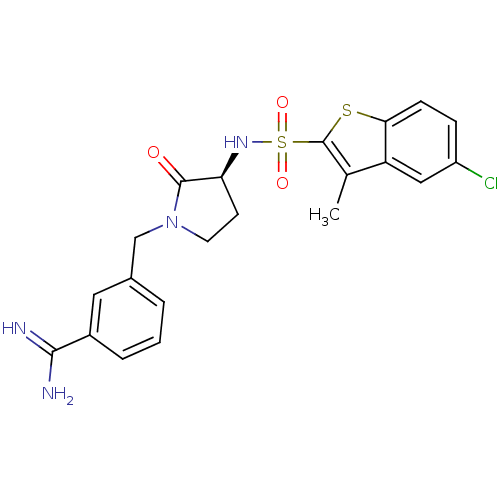
(3-[(S)-3-(5-Chloro-3-methyl-benzo[b]thiophene-2-su...)Show SMILES Cc1c(sc2ccc(Cl)cc12)S(=O)(=O)N[C@H]1CCN(Cc2cccc(c2)C(N)=N)C1=O Show InChI InChI=1S/C21H21ClN4O3S2/c1-12-16-10-15(22)5-6-18(16)30-21(12)31(28,29)25-17-7-8-26(20(17)27)11-13-3-2-4-14(9-13)19(23)24/h2-6,9-10,17,25H,7-8,11H2,1H3,(H3,23,24)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease Coagulation factor X |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081509
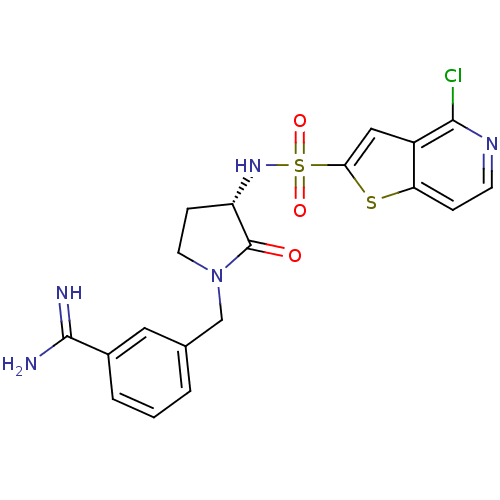
(3-[(S)-3-(4-Chloro-thieno[3,2-c]pyridine-2-sulfony...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4c(Cl)nccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O3S2/c20-17-13-9-16(29-15(13)4-6-23-17)30(27,28)24-14-5-7-25(19(14)26)10-11-2-1-3-12(8-11)18(21)22/h1-4,6,8-9,14,24H,5,7,10H2,(H3,21,22)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM13280
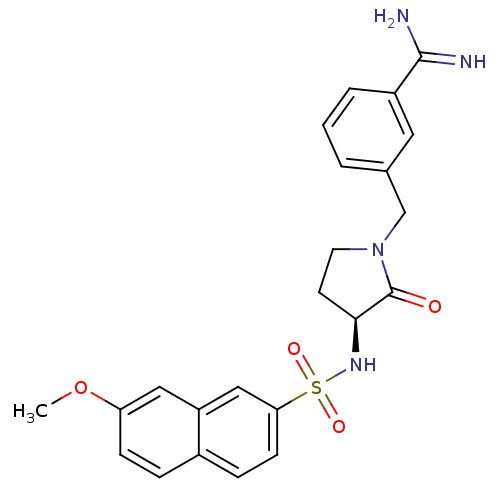
(3-{[(3S)-3-[(7-methoxynaphthalene-2-)sulfonamido]-...)Show SMILES COc1ccc2ccc(cc2c1)S(=O)(=O)N[C@H]1CCN(Cc2cccc(c2)C(N)=N)C1=O |r| Show InChI InChI=1S/C23H24N4O4S/c1-31-19-7-5-16-6-8-20(13-18(16)12-19)32(29,30)26-21-9-10-27(23(21)28)14-15-3-2-4-17(11-15)22(24)25/h2-8,11-13,21,26H,9-10,14H2,1H3,(H3,24,25)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease Coagulation factor X |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081507
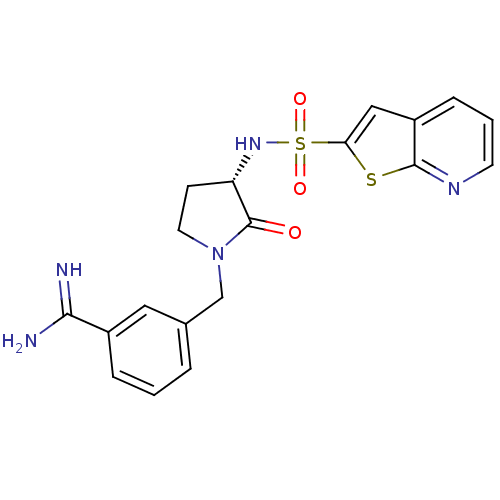
(3-[(S)-2-Oxo-3-(thieno[2,3-b]pyridine-2-sulfonylam...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4cccnc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O3S2/c20-17(21)13-4-1-3-12(9-13)11-24-8-6-15(19(24)25)23-29(26,27)16-10-14-5-2-7-22-18(14)28-16/h1-5,7,9-10,15,23H,6,8,11H2,(H3,20,21)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081522
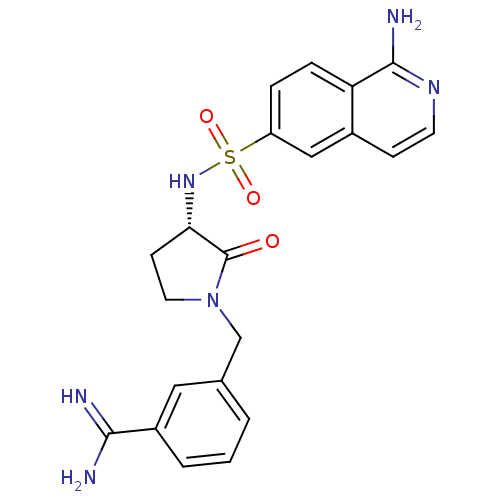
(3-[(S)-3-(1-Amino-isoquinoline-6-sulfonylamino)-2-...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4c(N)nccc4c3)C2=O)c1 Show InChI InChI=1S/C21H22N6O3S/c22-19(23)15-3-1-2-13(10-15)12-27-9-7-18(21(27)28)26-31(29,30)16-4-5-17-14(11-16)6-8-25-20(17)24/h1-6,8,10-11,18,26H,7,9,12H2,(H3,22,23)(H2,24,25)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease Coagulation factor X |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081518
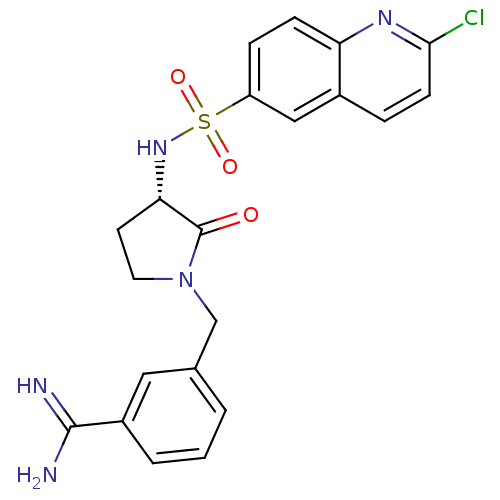
(3-[(S)-3-(2-Chloro-quinoline-6-sulfonylamino)-2-ox...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4nc(Cl)ccc4c3)C2=O)c1 Show InChI InChI=1S/C21H20ClN5O3S/c22-19-7-4-14-11-16(5-6-17(14)25-19)31(29,30)26-18-8-9-27(21(18)28)12-13-2-1-3-15(10-13)20(23)24/h1-7,10-11,18,26H,8-9,12H2,(H3,23,24)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50080499

(3-[(S)-3-(7-Amino-naphthalene-2-sulfonylamino)-2-o...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4ccc(N)cc4c3)C2=O)c1 Show InChI InChI=1S/C22H23N5O3S/c23-18-6-4-15-5-7-19(12-17(15)11-18)31(29,30)26-20-8-9-27(22(20)28)13-14-2-1-3-16(10-14)21(24)25/h1-7,10-12,20,26H,8-9,13,23H2,(H3,24,25)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease Coagulation factor X |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081502

(3-[(S)-3-(6-Chloro-thieno[2,3-b]pyridine-2-sulfony...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4ccc(Cl)nc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O3S2/c20-15-5-4-13-9-16(29-18(13)23-15)30(27,28)24-14-6-7-25(19(14)26)10-11-2-1-3-12(8-11)17(21)22/h1-5,8-9,14,24H,6-7,10H2,(H3,21,22)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease Coagulation factor X |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081500
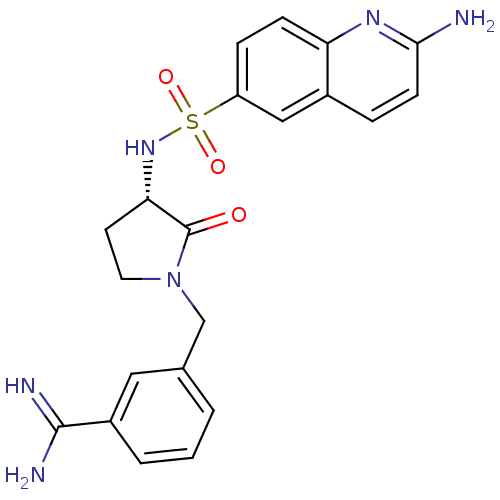
(3-[(S)-3-(2-Amino-quinoline-6-sulfonylamino)-2-oxo...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4nc(N)ccc4c3)C2=O)c1 Show InChI InChI=1S/C21H22N6O3S/c22-19-7-4-14-11-16(5-6-17(14)25-19)31(29,30)26-18-8-9-27(21(18)28)12-13-2-1-3-15(10-13)20(23)24/h1-7,10-11,18,26H,8-9,12H2,(H2,22,25)(H3,23,24)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081503
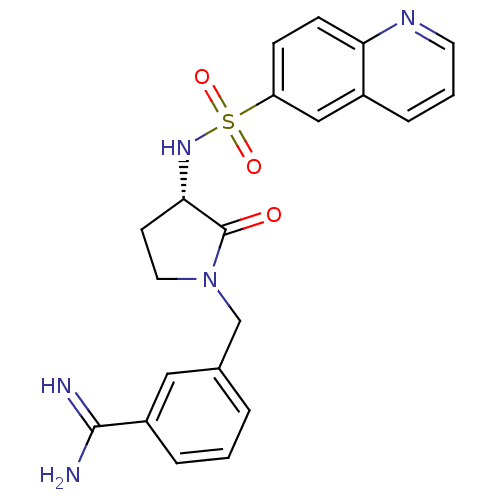
(3-[(S)-2-Oxo-3-(quinoline-6-sulfonylamino)-pyrroli...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4ncccc4c3)C2=O)c1 Show InChI InChI=1S/C21H21N5O3S/c22-20(23)16-4-1-3-14(11-16)13-26-10-8-19(21(26)27)25-30(28,29)17-6-7-18-15(12-17)5-2-9-24-18/h1-7,9,11-12,19,25H,8,10,13H2,(H3,22,23)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM13286

(4-Hydroxy-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfon...)Show SMILES COc1ccc2ccc(cc2c1)S(=O)(=O)N[C@H]1CCN(Cc2cc(ccc2O)C(N)=N)C1=O |r| Show InChI InChI=1S/C23H24N4O5S/c1-32-18-5-2-14-3-6-19(12-16(14)11-18)33(30,31)26-20-8-9-27(23(20)29)13-17-10-15(22(24)25)4-7-21(17)28/h2-7,10-12,20,26,28H,8-9,13H2,1H3,(H3,24,25)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 206 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081515

(4-Amino-3-[(S)-3-(5-methoxy-thieno[3,2-b]pyridine-...)Show SMILES COc1ccc2sc(cc2n1)S(=O)(=O)N[C@H]1CCN(Cc2cc(ccc2N)C(N)=N)C1=O Show InChI InChI=1S/C20H22N6O4S2/c1-30-17-5-4-16-15(24-17)9-18(31-16)32(28,29)25-14-6-7-26(20(14)27)10-12-8-11(19(22)23)2-3-13(12)21/h2-5,8-9,14,25H,6-7,10,21H2,1H3,(H3,22,23)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50080477

(3-[(S)-3-(Naphthalene-2-sulfonylamino)-2-oxo-pyrro...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4ccccc4c3)C2=O)c1 Show InChI InChI=1S/C22H22N4O3S/c23-21(24)18-7-3-4-15(12-18)14-26-11-10-20(22(26)27)25-30(28,29)19-9-8-16-5-1-2-6-17(16)13-19/h1-9,12-13,20,25H,10-11,14H2,(H3,23,24)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease Coagulation factor X |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081519
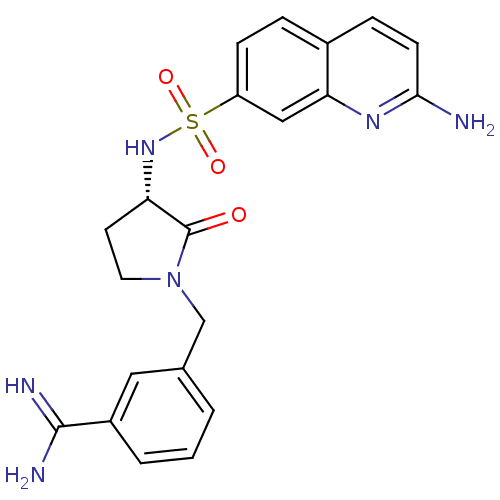
(3-[(S)-3-(2-Amino-quinoline-7-sulfonylamino)-2-oxo...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4ccc(N)nc4c3)C2=O)c1 Show InChI InChI=1S/C21H22N6O3S/c22-19-7-5-14-4-6-16(11-18(14)25-19)31(29,30)26-17-8-9-27(21(17)28)12-13-2-1-3-15(10-13)20(23)24/h1-7,10-11,17,26H,8-9,12H2,(H2,22,25)(H3,23,24)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease Coagulation factor X |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM13286

(4-Hydroxy-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfon...)Show SMILES COc1ccc2ccc(cc2c1)S(=O)(=O)N[C@H]1CCN(Cc2cc(ccc2O)C(N)=N)C1=O |r| Show InChI InChI=1S/C23H24N4O5S/c1-32-18-5-2-14-3-6-19(12-16(14)11-18)33(30,31)26-20-8-9-27(23(20)29)13-17-10-15(22(24)25)4-7-21(17)28/h2-7,10-12,20,26,28H,8-9,13H2,1H3,(H3,24,25)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 305 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM14059

(4-hydroxy-3-[[(3S)-2-oxo-3-(9-thia-5-azabicyclo[4....)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ncccc4s3)C2=O)c1 |r| Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)11-3-4-15(25)12(8-11)10-24-7-5-13(19(24)26)23-30(27,28)17-9-14-16(29-17)2-1-6-22-14/h1-4,6,8-9,13,23,25H,5,7,10H2,(H3,20,21)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against tissue plasminogen activator (t-Pa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081501
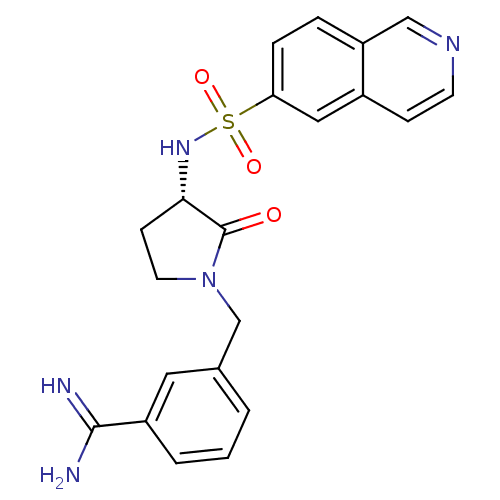
(3-[(S)-3-(Isoquinoline-6-sulfonylamino)-2-oxo-pyrr...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4cnccc4c3)C2=O)c1 Show InChI InChI=1S/C21H21N5O3S/c22-20(23)16-3-1-2-14(10-16)13-26-9-7-19(21(26)27)25-30(28,29)18-5-4-17-12-24-8-6-15(17)11-18/h1-6,8,10-12,19,25H,7,9,13H2,(H3,22,23)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease Coagulation factor X |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081516
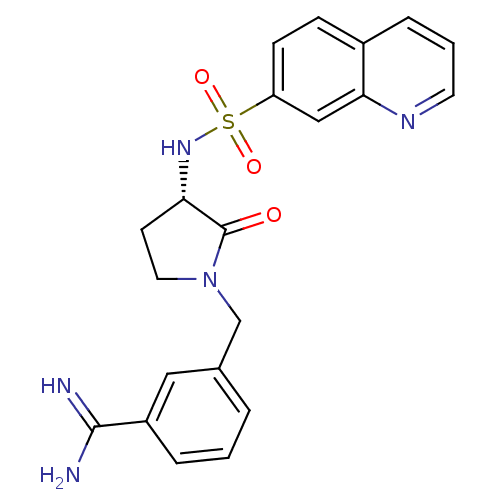
(3-[(S)-2-Oxo-3-(quinoline-7-sulfonylamino)-pyrroli...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4cccnc4c3)C2=O)c1 Show InChI InChI=1S/C21H21N5O3S/c22-20(23)16-4-1-3-14(11-16)13-26-10-8-18(21(26)27)25-30(28,29)17-7-6-15-5-2-9-24-19(15)12-17/h1-7,9,11-12,18,25H,8,10,13H2,(H3,22,23)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease Coagulation factor X |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081514

(3-[(S)-3-(2-Methoxy-quinoline-7-sulfonylamino)-2-o...)Show SMILES COc1ccc2ccc(cc2n1)S(=O)(=O)N[C@H]1CCN(Cc2cccc(c2)C(N)=N)C1=O Show InChI InChI=1S/C22H23N5O4S/c1-31-20-8-6-15-5-7-17(12-19(15)25-20)32(29,30)26-18-9-10-27(22(18)28)13-14-3-2-4-16(11-14)21(23)24/h2-8,11-12,18,26H,9-10,13H2,1H3,(H3,23,24)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease Coagulation factor X |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081511
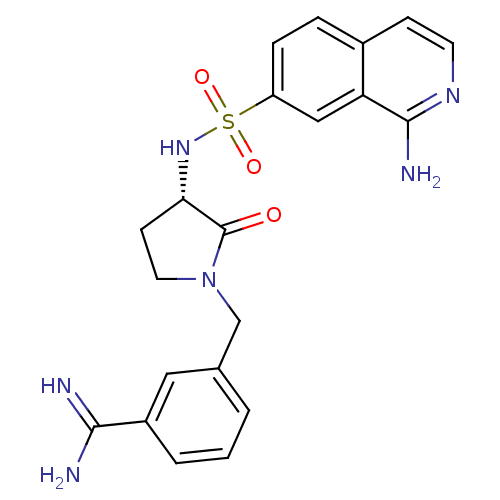
(3-[(S)-3-(1-Amino-isoquinoline-7-sulfonylamino)-2-...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4ccnc(N)c4c3)C2=O)c1 Show InChI InChI=1S/C21H22N6O3S/c22-19(23)15-3-1-2-13(10-15)12-27-9-7-18(21(27)28)26-31(29,30)16-5-4-14-6-8-25-20(24)17(14)11-16/h1-6,8,10-11,18,26H,7,9,12H2,(H3,22,23)(H2,24,25)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease Coagulation factor X |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081504

(3-[(S)-3-(Isoquinoline-7-sulfonylamino)-2-oxo-pyrr...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4ccncc4c3)C2=O)c1 Show InChI InChI=1S/C21H21N5O3S/c22-20(23)16-3-1-2-14(10-16)13-26-9-7-19(21(26)27)25-30(28,29)18-5-4-15-6-8-24-12-17(15)11-18/h1-6,8,10-12,19,25H,7,9,13H2,(H3,22,23)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease Coagulation factor X |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081521

(3-[(S)-3-(2-Chloro-quinoline-7-sulfonylamino)-2-ox...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4ccc(Cl)nc4c3)C2=O)c1 Show InChI InChI=1S/C21H20ClN5O3S/c22-19-7-5-14-4-6-16(11-18(14)25-19)31(29,30)26-17-8-9-27(21(17)28)12-13-2-1-3-15(10-13)20(23)24/h1-7,10-11,17,26H,8-9,12H2,(H3,23,24)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease Coagulation factor X |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081515

(4-Amino-3-[(S)-3-(5-methoxy-thieno[3,2-b]pyridine-...)Show SMILES COc1ccc2sc(cc2n1)S(=O)(=O)N[C@H]1CCN(Cc2cc(ccc2N)C(N)=N)C1=O Show InChI InChI=1S/C20H22N6O4S2/c1-30-17-5-4-16-15(24-17)9-18(31-16)32(28,29)25-14-6-7-26(20(14)27)10-12-8-11(19(22)23)2-3-13(12)21/h2-5,8-9,14,25H,6-7,10,21H2,1H3,(H3,22,23)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081505

(3-[(S)-3-(5-Chloro-thieno[3,2-b]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-16-4-3-15-13(23-16)8-17(30-15)31(28,29)24-12-5-6-25(19(12)27)9-11-7-10(18(21)22)1-2-14(11)26/h1-4,7-8,12,24,26H,5-6,9H2,(H3,21,22)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM14059

(4-hydroxy-3-[[(3S)-2-oxo-3-(9-thia-5-azabicyclo[4....)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ncccc4s3)C2=O)c1 |r| Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)11-3-4-15(25)12(8-11)10-24-7-5-13(19(24)26)23-30(27,28)17-9-14-16(29-17)2-1-6-22-14/h1-4,6,8-9,13,23,25H,5,7,10H2,(H3,20,21)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM13286

(4-Hydroxy-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfon...)Show SMILES COc1ccc2ccc(cc2c1)S(=O)(=O)N[C@H]1CCN(Cc2cc(ccc2O)C(N)=N)C1=O |r| Show InChI InChI=1S/C23H24N4O5S/c1-32-18-5-2-14-3-6-19(12-16(14)11-18)33(30,31)26-20-8-9-27(23(20)29)13-17-10-15(22(24)25)4-7-21(17)28/h2-7,10-12,20,26,28H,8-9,13H2,1H3,(H3,24,25)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against tissue plasminogen activator (t-Pa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081510

(4-Amino-3-[(S)-3-(5-chloro-thieno[3,2-b]pyridine-2...)Show SMILES NC(=N)c1ccc(N)c(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19ClN6O3S2/c20-16-4-3-15-14(24-16)8-17(30-15)31(28,29)25-13-5-6-26(19(13)27)9-11-7-10(18(22)23)1-2-12(11)21/h1-4,7-8,13,25H,5-6,9,21H2,(H3,22,23)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081508

(3-[(S)-3-(2-Ethoxy-quinoline-6-sulfonylamino)-2-ox...)Show SMILES CCOc1ccc2cc(ccc2n1)S(=O)(=O)N[C@H]1CCN(Cc2cccc(c2)C(N)=N)C1=O Show InChI InChI=1S/C23H25N5O4S/c1-2-32-21-9-6-16-13-18(7-8-19(16)26-21)33(30,31)27-20-10-11-28(23(20)29)14-15-4-3-5-17(12-15)22(24)25/h3-9,12-13,20,27H,2,10-11,14H2,1H3,(H3,24,25)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50081510

(4-Amino-3-[(S)-3-(5-chloro-thieno[3,2-b]pyridine-2...)Show SMILES NC(=N)c1ccc(N)c(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19ClN6O3S2/c20-16-4-3-15-14(24-16)8-17(30-15)31(28,29)25-13-5-6-26(19(13)27)9-11-7-10(18(22)23)1-2-12(11)21/h1-4,7-8,13,25H,5-6,9,21H2,(H3,22,23)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against plasmin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081505

(3-[(S)-3-(5-Chloro-thieno[3,2-b]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-16-4-3-15-13(23-16)8-17(30-15)31(28,29)24-12-5-6-25(19(12)27)9-11-7-10(18(21)22)1-2-14(11)26/h1-4,7-8,12,24,26H,5-6,9H2,(H3,21,22)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081510

(4-Amino-3-[(S)-3-(5-chloro-thieno[3,2-b]pyridine-2...)Show SMILES NC(=N)c1ccc(N)c(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19ClN6O3S2/c20-16-4-3-15-14(24-16)8-17(30-15)31(28,29)25-13-5-6-26(19(13)27)9-11-7-10(18(22)23)1-2-12(11)21/h1-4,7-8,13,25H,5-6,9,21H2,(H3,22,23)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM13286

(4-Hydroxy-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfon...)Show SMILES COc1ccc2ccc(cc2c1)S(=O)(=O)N[C@H]1CCN(Cc2cc(ccc2O)C(N)=N)C1=O |r| Show InChI InChI=1S/C23H24N4O5S/c1-32-18-5-2-14-3-6-19(12-16(14)11-18)33(30,31)26-20-8-9-27(23(20)29)13-17-10-15(22(24)25)4-7-21(17)28/h2-7,10-12,20,26,28H,8-9,13H2,1H3,(H3,24,25)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against activated protein C (APC) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081499

(4-Hydroxy-3-[(S)-2-oxo-3-(thieno[2,3-c]pyridine-2-...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ccncc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)12-1-2-15(25)13(7-12)10-24-6-4-14(19(24)26)23-30(27,28)17-8-11-3-5-22-9-16(11)29-17/h1-3,5,7-9,14,23,25H,4,6,10H2,(H3,20,21)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM14059

(4-hydroxy-3-[[(3S)-2-oxo-3-(9-thia-5-azabicyclo[4....)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ncccc4s3)C2=O)c1 |r| Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)11-3-4-15(25)12(8-11)10-24-7-5-13(19(24)26)23-30(27,28)17-9-14-16(29-17)2-1-6-22-14/h1-4,6,8-9,13,23,25H,5,7,10H2,(H3,20,21)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against plasmin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM13286

(4-Hydroxy-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfon...)Show SMILES COc1ccc2ccc(cc2c1)S(=O)(=O)N[C@H]1CCN(Cc2cc(ccc2O)C(N)=N)C1=O |r| Show InChI InChI=1S/C23H24N4O5S/c1-32-18-5-2-14-3-6-19(12-16(14)11-18)33(30,31)26-20-8-9-27(23(20)29)13-17-10-15(22(24)25)4-7-21(17)28/h2-7,10-12,20,26,28H,8-9,13H2,1H3,(H3,24,25)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against plasmin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081506

(3-[(S)-3-(7-Chloro-thieno[2,3-c]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ccnc(Cl)c4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-17-16-10(3-5-23-17)8-15(30-16)31(28,29)24-13-4-6-25(19(13)27)9-12-7-11(18(21)22)1-2-14(12)26/h1-3,5,7-8,13,24,26H,4,6,9H2,(H3,21,22)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081517

(4-Hydroxy-3-[(S)-2-oxo-3-(thieno[3,2-c]pyridine-2-...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4cnccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)11-1-2-15(25)13(7-11)10-24-6-4-14(19(24)26)23-30(27,28)17-8-12-9-22-5-3-16(12)29-17/h1-3,5,7-9,14,23,25H,4,6,10H2,(H3,20,21)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50081505

(3-[(S)-3-(5-Chloro-thieno[3,2-b]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-16-4-3-15-13(23-16)8-17(30-15)31(28,29)24-12-5-6-25(19(12)27)9-11-7-10(18(21)22)1-2-14(11)26/h1-4,7-8,12,24,26H,5-6,9H2,(H3,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against tissue plasminogen activator (t-Pa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081502

(3-[(S)-3-(6-Chloro-thieno[2,3-b]pyridine-2-sulfony...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4ccc(Cl)nc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O3S2/c20-15-5-4-13-9-16(29-18(13)23-15)30(27,28)24-14-6-7-25(19(14)26)10-11-2-1-3-12(8-11)17(21)22/h1-5,8-9,14,24H,6-7,10H2,(H3,21,22)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081503

(3-[(S)-2-Oxo-3-(quinoline-6-sulfonylamino)-pyrroli...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4ncccc4c3)C2=O)c1 Show InChI InChI=1S/C21H21N5O3S/c22-20(23)16-4-1-3-14(11-16)13-26-10-8-19(21(26)27)25-30(28,29)17-6-7-18-15(12-17)5-2-9-24-18/h1-7,9,11-12,19,25H,8,10,13H2,(H3,22,23)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081511

(3-[(S)-3-(1-Amino-isoquinoline-7-sulfonylamino)-2-...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4ccnc(N)c4c3)C2=O)c1 Show InChI InChI=1S/C21H22N6O3S/c22-19(23)15-3-1-2-13(10-15)12-27-9-7-18(21(27)28)26-31(29,30)16-5-4-14-6-8-25-20(24)17(14)11-16/h1-6,8,10-11,18,26H,7,9,12H2,(H3,22,23)(H2,24,25)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081508

(3-[(S)-3-(2-Ethoxy-quinoline-6-sulfonylamino)-2-ox...)Show SMILES CCOc1ccc2cc(ccc2n1)S(=O)(=O)N[C@H]1CCN(Cc2cccc(c2)C(N)=N)C1=O Show InChI InChI=1S/C23H25N5O4S/c1-2-32-21-9-6-16-13-18(7-8-19(16)26-21)33(30,31)27-20-10-11-28(23(20)29)14-15-4-3-5-17(12-15)22(24)25/h3-9,12-13,20,27H,2,10-11,14H2,1H3,(H3,24,25)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081514

(3-[(S)-3-(2-Methoxy-quinoline-7-sulfonylamino)-2-o...)Show SMILES COc1ccc2ccc(cc2n1)S(=O)(=O)N[C@H]1CCN(Cc2cccc(c2)C(N)=N)C1=O Show InChI InChI=1S/C22H23N5O4S/c1-31-20-8-6-15-5-7-17(12-19(15)25-20)32(29,30)26-18-9-10-27(22(18)28)13-14-3-2-4-16(11-14)21(23)24/h2-8,11-12,18,26H,9-10,13H2,1H3,(H3,23,24)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081519

(3-[(S)-3-(2-Amino-quinoline-7-sulfonylamino)-2-oxo...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4ccc(N)nc4c3)C2=O)c1 Show InChI InChI=1S/C21H22N6O3S/c22-19-7-5-14-4-6-16(11-18(14)25-19)31(29,30)26-17-8-9-27(21(17)28)12-13-2-1-3-15(10-13)20(23)24/h1-7,10-11,17,26H,8-9,12H2,(H2,22,25)(H3,23,24)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081509

(3-[(S)-3-(4-Chloro-thieno[3,2-c]pyridine-2-sulfony...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4c(Cl)nccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O3S2/c20-17-13-9-16(29-15(13)4-6-23-17)30(27,28)24-14-5-7-25(19(14)26)10-11-2-1-3-12(8-11)18(21)22/h1-4,6,8-9,14,24H,5,7,10H2,(H3,21,22)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081011

(3-[(S)-2-Oxo-3-(thieno[3,2-b]pyridine-2-sulfonylam...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4ncccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O3S2/c20-18(21)13-4-1-3-12(9-13)11-24-8-6-14(19(24)25)23-29(26,27)17-10-15-16(28-17)5-2-7-22-15/h1-5,7,9-10,14,23H,6,8,11H2,(H3,20,21)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081500

(3-[(S)-3-(2-Amino-quinoline-6-sulfonylamino)-2-oxo...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4nc(N)ccc4c3)C2=O)c1 Show InChI InChI=1S/C21H22N6O3S/c22-19-7-4-14-11-16(5-6-17(14)25-19)31(29,30)26-18-8-9-27(21(18)28)12-13-2-1-3-15(10-13)20(23)24/h1-7,10-11,18,26H,8-9,12H2,(H2,22,25)(H3,23,24)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081516

(3-[(S)-2-Oxo-3-(quinoline-7-sulfonylamino)-pyrroli...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4cccnc4c3)C2=O)c1 Show InChI InChI=1S/C21H21N5O3S/c22-20(23)16-4-1-3-14(11-16)13-26-10-8-18(21(26)27)25-30(28,29)17-7-6-15-5-2-9-24-19(15)12-17/h1-7,9,11-12,18,25H,8,10,13H2,(H3,22,23)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081506

(3-[(S)-3-(7-Chloro-thieno[2,3-c]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ccnc(Cl)c4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-17-16-10(3-5-23-17)8-15(30-16)31(28,29)24-13-4-6-25(19(13)27)9-12-7-11(18(21)22)1-2-14(12)26/h1-3,5,7-8,13,24,26H,4,6,9H2,(H3,21,22)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081499

(4-Hydroxy-3-[(S)-2-oxo-3-(thieno[2,3-c]pyridine-2-...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ccncc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)12-1-2-15(25)13(7-12)10-24-6-4-14(19(24)26)23-30(27,28)17-8-11-3-5-22-9-16(11)29-17/h1-3,5,7-9,14,23,25H,4,6,10H2,(H3,20,21)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081501

(3-[(S)-3-(Isoquinoline-6-sulfonylamino)-2-oxo-pyrr...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4cnccc4c3)C2=O)c1 Show InChI InChI=1S/C21H21N5O3S/c22-20(23)16-3-1-2-14(10-16)13-26-9-7-19(21(26)27)25-30(28,29)18-5-4-17-12-24-8-6-15(17)11-18/h1-6,8,10-12,19,25H,7,9,13H2,(H3,22,23)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081504

(3-[(S)-3-(Isoquinoline-7-sulfonylamino)-2-oxo-pyrr...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4ccncc4c3)C2=O)c1 Show InChI InChI=1S/C21H21N5O3S/c22-20(23)16-3-1-2-14(10-16)13-26-9-7-19(21(26)27)25-30(28,29)18-5-4-15-6-8-24-12-17(15)11-18/h1-6,8,10-12,19,25H,7,9,13H2,(H3,22,23)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081521

(3-[(S)-3-(2-Chloro-quinoline-7-sulfonylamino)-2-ox...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4ccc(Cl)nc4c3)C2=O)c1 Show InChI InChI=1S/C21H20ClN5O3S/c22-19-7-5-14-4-6-16(11-18(14)25-19)31(29,30)26-17-8-9-27(21(17)28)12-13-2-1-3-15(10-13)20(23)24/h1-7,10-11,17,26H,8-9,12H2,(H3,23,24)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081518

(3-[(S)-3-(2-Chloro-quinoline-6-sulfonylamino)-2-ox...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4nc(Cl)ccc4c3)C2=O)c1 Show InChI InChI=1S/C21H20ClN5O3S/c22-19-7-4-14-11-16(5-6-17(14)25-19)31(29,30)26-18-8-9-27(21(18)28)12-13-2-1-3-15(10-13)20(23)24/h1-7,10-11,18,26H,8-9,12H2,(H3,23,24)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081517

(4-Hydroxy-3-[(S)-2-oxo-3-(thieno[3,2-c]pyridine-2-...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4cnccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)11-1-2-15(25)13(7-11)10-24-6-4-14(19(24)26)23-30(27,28)17-8-12-9-22-5-3-16(12)29-17/h1-3,5,7-9,14,23,25H,4,6,10H2,(H3,20,21)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081512

(3-[(S)-3-(4-Chloro-thieno[3,2-c]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4c(Cl)nccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-17-12-8-16(30-15(12)3-5-23-17)31(28,29)24-13-4-6-25(19(13)27)9-11-7-10(18(21)22)1-2-14(11)26/h1-3,5,7-8,13,24,26H,4,6,9H2,(H3,21,22)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081507

(3-[(S)-2-Oxo-3-(thieno[2,3-b]pyridine-2-sulfonylam...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4cccnc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O3S2/c20-17(21)13-4-1-3-12(9-13)11-24-8-6-15(19(24)25)23-29(26,27)16-10-14-5-2-7-22-18(14)28-16/h1-5,7,9-10,15,23H,6,8,11H2,(H3,20,21)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081520

(3-[(S)-3-(5-Chloro-thieno[3,2-b]pyridine-2-sulfony...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O3S2/c20-16-5-4-15-14(23-16)9-17(29-15)30(27,28)24-13-6-7-25(19(13)26)10-11-2-1-3-12(8-11)18(21)22/h1-5,8-9,13,24H,6-7,10H2,(H3,21,22)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081522

(3-[(S)-3-(1-Amino-isoquinoline-6-sulfonylamino)-2-...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4c(N)nccc4c3)C2=O)c1 Show InChI InChI=1S/C21H22N6O3S/c22-19(23)15-3-1-2-13(10-15)12-27-9-7-18(21(27)28)26-31(29,30)16-4-5-17-14(11-16)6-8-25-20(17)24/h1-6,8,10-11,18,26H,7,9,12H2,(H3,22,23)(H2,24,25)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against trypsin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081520

(3-[(S)-3-(5-Chloro-thieno[3,2-b]pyridine-2-sulfony...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O3S2/c20-16-5-4-15-14(23-16)9-17(29-15)30(27,28)24-13-6-7-25(19(13)26)10-11-2-1-3-12(8-11)18(21)22/h1-5,8-9,13,24H,6-7,10H2,(H3,21,22)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50081505

(3-[(S)-3-(5-Chloro-thieno[3,2-b]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-16-4-3-15-13(23-16)8-17(30-15)31(28,29)24-12-5-6-25(19(12)27)9-11-7-10(18(21)22)1-2-14(11)26/h1-4,7-8,12,24,26H,5-6,9H2,(H3,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against plasmin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081502

(3-[(S)-3-(6-Chloro-thieno[2,3-b]pyridine-2-sulfony...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4ccc(Cl)nc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O3S2/c20-15-5-4-13-9-16(29-18(13)23-15)30(27,28)24-14-6-7-25(19(14)26)10-11-2-1-3-12(8-11)17(21)22/h1-5,8-9,14,24H,6-7,10H2,(H3,21,22)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081518

(3-[(S)-3-(2-Chloro-quinoline-6-sulfonylamino)-2-ox...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4nc(Cl)ccc4c3)C2=O)c1 Show InChI InChI=1S/C21H20ClN5O3S/c22-19-7-4-14-11-16(5-6-17(14)25-19)31(29,30)26-18-8-9-27(21(18)28)12-13-2-1-3-15(10-13)20(23)24/h1-7,10-11,18,26H,8-9,12H2,(H3,23,24)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081508

(3-[(S)-3-(2-Ethoxy-quinoline-6-sulfonylamino)-2-ox...)Show SMILES CCOc1ccc2cc(ccc2n1)S(=O)(=O)N[C@H]1CCN(Cc2cccc(c2)C(N)=N)C1=O Show InChI InChI=1S/C23H25N5O4S/c1-2-32-21-9-6-16-13-18(7-8-19(16)26-21)33(30,31)27-20-10-11-28(23(20)29)14-15-4-3-5-17(12-15)22(24)25/h3-9,12-13,20,27H,2,10-11,14H2,1H3,(H3,24,25)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081507

(3-[(S)-2-Oxo-3-(thieno[2,3-b]pyridine-2-sulfonylam...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4cccnc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O3S2/c20-17(21)13-4-1-3-12(9-13)11-24-8-6-15(19(24)25)23-29(26,27)16-10-14-5-2-7-22-18(14)28-16/h1-5,7,9-10,15,23H,6,8,11H2,(H3,20,21)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081512

(3-[(S)-3-(4-Chloro-thieno[3,2-c]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4c(Cl)nccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-17-12-8-16(30-15(12)3-5-23-17)31(28,29)24-13-4-6-25(19(13)27)9-11-7-10(18(21)22)1-2-14(11)26/h1-3,5,7-8,13,24,26H,4,6,9H2,(H3,21,22)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081500

(3-[(S)-3-(2-Amino-quinoline-6-sulfonylamino)-2-oxo...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4nc(N)ccc4c3)C2=O)c1 Show InChI InChI=1S/C21H22N6O3S/c22-19-7-4-14-11-16(5-6-17(14)25-19)31(29,30)26-18-8-9-27(21(18)28)12-13-2-1-3-15(10-13)20(23)24/h1-7,10-11,18,26H,8-9,12H2,(H2,22,25)(H3,23,24)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081522

(3-[(S)-3-(1-Amino-isoquinoline-6-sulfonylamino)-2-...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4c(N)nccc4c3)C2=O)c1 Show InChI InChI=1S/C21H22N6O3S/c22-19(23)15-3-1-2-13(10-15)12-27-9-7-18(21(27)28)26-31(29,30)16-4-5-17-14(11-16)6-8-25-20(17)24/h1-6,8,10-11,18,26H,7,9,12H2,(H3,22,23)(H2,24,25)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081501

(3-[(S)-3-(Isoquinoline-6-sulfonylamino)-2-oxo-pyrr...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4cnccc4c3)C2=O)c1 Show InChI InChI=1S/C21H21N5O3S/c22-20(23)16-3-1-2-14(10-16)13-26-9-7-19(21(26)27)25-30(28,29)18-5-4-17-12-24-8-6-15(17)11-18/h1-6,8,10-12,19,25H,7,9,13H2,(H3,22,23)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081011

(3-[(S)-2-Oxo-3-(thieno[3,2-b]pyridine-2-sulfonylam...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4ncccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O3S2/c20-18(21)13-4-1-3-12(9-13)11-24-8-6-14(19(24)25)23-29(26,27)17-10-15-16(28-17)5-2-7-22-15/h1-5,7,9-10,14,23H,6,8,11H2,(H3,20,21)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081514

(3-[(S)-3-(2-Methoxy-quinoline-7-sulfonylamino)-2-o...)Show SMILES COc1ccc2ccc(cc2n1)S(=O)(=O)N[C@H]1CCN(Cc2cccc(c2)C(N)=N)C1=O Show InChI InChI=1S/C22H23N5O4S/c1-31-20-8-6-15-5-7-17(12-19(15)25-20)32(29,30)26-18-9-10-27(22(18)28)13-14-3-2-4-16(11-14)21(23)24/h2-8,11-12,18,26H,9-10,13H2,1H3,(H3,23,24)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081503

(3-[(S)-2-Oxo-3-(quinoline-6-sulfonylamino)-pyrroli...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4ncccc4c3)C2=O)c1 Show InChI InChI=1S/C21H21N5O3S/c22-20(23)16-4-1-3-14(11-16)13-26-10-8-19(21(26)27)25-30(28,29)17-6-7-18-15(12-17)5-2-9-24-18/h1-7,9,11-12,19,25H,8,10,13H2,(H3,22,23)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081511

(3-[(S)-3-(1-Amino-isoquinoline-7-sulfonylamino)-2-...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4ccnc(N)c4c3)C2=O)c1 Show InChI InChI=1S/C21H22N6O3S/c22-19(23)15-3-1-2-13(10-15)12-27-9-7-18(21(27)28)26-31(29,30)16-5-4-14-6-8-25-20(24)17(14)11-16/h1-6,8,10-11,18,26H,7,9,12H2,(H3,22,23)(H2,24,25)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081504

(3-[(S)-3-(Isoquinoline-7-sulfonylamino)-2-oxo-pyrr...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4ccncc4c3)C2=O)c1 Show InChI InChI=1S/C21H21N5O3S/c22-20(23)16-3-1-2-14(10-16)13-26-9-7-19(21(26)27)25-30(28,29)18-5-4-15-6-8-24-12-17(15)11-18/h1-6,8,10-12,19,25H,7,9,13H2,(H3,22,23)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081521

(3-[(S)-3-(2-Chloro-quinoline-7-sulfonylamino)-2-ox...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4ccc(Cl)nc4c3)C2=O)c1 Show InChI InChI=1S/C21H20ClN5O3S/c22-19-7-5-14-4-6-16(11-18(14)25-19)31(29,30)26-17-8-9-27(21(17)28)12-13-2-1-3-15(10-13)20(23)24/h1-7,10-11,17,26H,8-9,12H2,(H3,23,24)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081519

(3-[(S)-3-(2-Amino-quinoline-7-sulfonylamino)-2-oxo...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4ccc(N)nc4c3)C2=O)c1 Show InChI InChI=1S/C21H22N6O3S/c22-19-7-5-14-4-6-16(11-18(14)25-19)31(29,30)26-17-8-9-27(21(17)28)12-13-2-1-3-15(10-13)20(23)24/h1-7,10-11,17,26H,8-9,12H2,(H2,22,25)(H3,23,24)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081509

(3-[(S)-3-(4-Chloro-thieno[3,2-c]pyridine-2-sulfony...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4c(Cl)nccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O3S2/c20-17-13-9-16(29-15(13)4-6-23-17)30(27,28)24-14-5-7-25(19(14)26)10-11-2-1-3-12(8-11)18(21)22/h1-4,6,8-9,14,24H,5,7,10H2,(H3,21,22)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081516

(3-[(S)-2-Oxo-3-(quinoline-7-sulfonylamino)-pyrroli...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3ccc4cccnc4c3)C2=O)c1 Show InChI InChI=1S/C21H21N5O3S/c22-20(23)16-4-1-3-14(11-16)13-26-10-8-18(21(26)27)25-30(28,29)17-7-6-15-5-2-9-24-19(15)12-17/h1-7,9,11-12,18,25H,8,10,13H2,(H3,22,23)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM14059

(4-hydroxy-3-[[(3S)-2-oxo-3-(9-thia-5-azabicyclo[4....)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ncccc4s3)C2=O)c1 |r| Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)11-3-4-15(25)12(8-11)10-24-7-5-13(19(24)26)23-30(27,28)17-9-14-16(29-17)2-1-6-22-14/h1-4,6,8-9,13,23,25H,5,7,10H2,(H3,20,21)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50081510

(4-Amino-3-[(S)-3-(5-chloro-thieno[3,2-b]pyridine-2...)Show SMILES NC(=N)c1ccc(N)c(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19ClN6O3S2/c20-16-4-3-15-14(24-16)8-17(30-15)31(28,29)25-13-5-6-26(19(13)27)9-11-7-10(18(22)23)1-2-12(11)21/h1-4,7-8,13,25H,5-6,9,21H2,(H3,22,23)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against activated protein C (APC) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50081512

(3-[(S)-3-(4-Chloro-thieno[3,2-c]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4c(Cl)nccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-17-12-8-16(30-15(12)3-5-23-17)31(28,29)24-13-4-6-25(19(13)27)9-11-7-10(18(21)22)1-2-14(11)26/h1-3,5,7-8,13,24,26H,4,6,9H2,(H3,21,22)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against plasmin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM14059

(4-hydroxy-3-[[(3S)-2-oxo-3-(9-thia-5-azabicyclo[4....)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ncccc4s3)C2=O)c1 |r| Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)11-3-4-15(25)12(8-11)10-24-7-5-13(19(24)26)23-30(27,28)17-9-14-16(29-17)2-1-6-22-14/h1-4,6,8-9,13,23,25H,5,7,10H2,(H3,20,21)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against activated protein C (APC) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50081506

(3-[(S)-3-(7-Chloro-thieno[2,3-c]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ccnc(Cl)c4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-17-16-10(3-5-23-17)8-15(30-16)31(28,29)24-13-4-6-25(19(13)27)9-12-7-11(18(21)22)1-2-14(12)26/h1-3,5,7-8,13,24,26H,4,6,9H2,(H3,21,22)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against activated protein C (APC) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50081520

(3-[(S)-3-(5-Chloro-thieno[3,2-b]pyridine-2-sulfony...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O3S2/c20-16-5-4-15-14(23-16)9-17(29-15)30(27,28)24-13-6-7-25(19(13)26)10-11-2-1-3-12(8-11)18(21)22/h1-5,8-9,13,24H,6-7,10H2,(H3,21,22)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against plasmin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50081499

(4-Hydroxy-3-[(S)-2-oxo-3-(thieno[2,3-c]pyridine-2-...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ccncc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)12-1-2-15(25)13(7-12)10-24-6-4-14(19(24)26)23-30(27,28)17-8-11-3-5-22-9-16(11)29-17/h1-3,5,7-9,14,23,25H,4,6,10H2,(H3,20,21)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against plasmin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50081011

(3-[(S)-2-Oxo-3-(thieno[3,2-b]pyridine-2-sulfonylam...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4ncccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O3S2/c20-18(21)13-4-1-3-12(9-13)11-24-8-6-14(19(24)25)23-29(26,27)17-10-15-16(28-17)5-2-7-22-15/h1-5,7,9-10,14,23H,6,8,11H2,(H3,20,21)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against plasmin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50081506

(3-[(S)-3-(7-Chloro-thieno[2,3-c]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ccnc(Cl)c4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-17-16-10(3-5-23-17)8-15(30-16)31(28,29)24-13-4-6-25(19(13)27)9-12-7-11(18(21)22)1-2-14(12)26/h1-3,5,7-8,13,24,26H,4,6,9H2,(H3,21,22)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against plasmin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50081515

(4-Amino-3-[(S)-3-(5-methoxy-thieno[3,2-b]pyridine-...)Show SMILES COc1ccc2sc(cc2n1)S(=O)(=O)N[C@H]1CCN(Cc2cc(ccc2N)C(N)=N)C1=O Show InChI InChI=1S/C20H22N6O4S2/c1-30-17-5-4-16-15(24-17)9-18(31-16)32(28,29)25-14-6-7-26(20(14)27)10-12-8-11(19(22)23)2-3-13(12)21/h2-5,8-9,14,25H,6-7,10,21H2,1H3,(H3,22,23)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against plasmin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50081517

(4-Hydroxy-3-[(S)-2-oxo-3-(thieno[3,2-c]pyridine-2-...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4cnccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)11-1-2-15(25)13(7-11)10-24-6-4-14(19(24)26)23-30(27,28)17-8-12-9-22-5-3-16(12)29-17/h1-3,5,7-9,14,23,25H,4,6,10H2,(H3,20,21)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against plasmin |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50081506

(3-[(S)-3-(7-Chloro-thieno[2,3-c]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ccnc(Cl)c4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-17-16-10(3-5-23-17)8-15(30-16)31(28,29)24-13-4-6-25(19(13)27)9-12-7-11(18(21)22)1-2-14(12)26/h1-3,5,7-8,13,24,26H,4,6,9H2,(H3,21,22)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against tissue plasminogen activator (t-Pa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50081512

(3-[(S)-3-(4-Chloro-thieno[3,2-c]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4c(Cl)nccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-17-12-8-16(30-15(12)3-5-23-17)31(28,29)24-13-4-6-25(19(13)27)9-11-7-10(18(21)22)1-2-14(11)26/h1-3,5,7-8,13,24,26H,4,6,9H2,(H3,21,22)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against activated protein C (APC) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50081512

(3-[(S)-3-(4-Chloro-thieno[3,2-c]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4c(Cl)nccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-17-12-8-16(30-15(12)3-5-23-17)31(28,29)24-13-4-6-25(19(13)27)9-11-7-10(18(21)22)1-2-14(11)26/h1-3,5,7-8,13,24,26H,4,6,9H2,(H3,21,22)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against tissue plasminogen activator (t-Pa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50081499

(4-Hydroxy-3-[(S)-2-oxo-3-(thieno[2,3-c]pyridine-2-...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ccncc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)12-1-2-15(25)13(7-12)10-24-6-4-14(19(24)26)23-30(27,28)17-8-11-3-5-22-9-16(11)29-17/h1-3,5,7-9,14,23,25H,4,6,10H2,(H3,20,21)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against tissue plasminogen activator (t-Pa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50081515

(4-Amino-3-[(S)-3-(5-methoxy-thieno[3,2-b]pyridine-...)Show SMILES COc1ccc2sc(cc2n1)S(=O)(=O)N[C@H]1CCN(Cc2cc(ccc2N)C(N)=N)C1=O Show InChI InChI=1S/C20H22N6O4S2/c1-30-17-5-4-16-15(24-17)9-18(31-16)32(28,29)25-14-6-7-26(20(14)27)10-12-8-11(19(22)23)2-3-13(12)21/h2-5,8-9,14,25H,6-7,10,21H2,1H3,(H3,22,23)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against tissue plasminogen activator (t-Pa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50081510

(4-Amino-3-[(S)-3-(5-chloro-thieno[3,2-b]pyridine-2...)Show SMILES NC(=N)c1ccc(N)c(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19ClN6O3S2/c20-16-4-3-15-14(24-16)8-17(30-15)31(28,29)25-13-5-6-26(19(13)27)9-11-7-10(18(22)23)1-2-12(11)21/h1-4,7-8,13,25H,5-6,9,21H2,(H3,22,23)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against tissue plasminogen activator (t-Pa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50081011

(3-[(S)-2-Oxo-3-(thieno[3,2-b]pyridine-2-sulfonylam...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4ncccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O3S2/c20-18(21)13-4-1-3-12(9-13)11-24-8-6-14(19(24)25)23-29(26,27)17-10-15-16(28-17)5-2-7-22-15/h1-5,7,9-10,14,23H,6,8,11H2,(H3,20,21)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against tissue plasminogen activator (t-Pa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50081520

(3-[(S)-3-(5-Chloro-thieno[3,2-b]pyridine-2-sulfony...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O3S2/c20-16-5-4-15-14(23-16)9-17(29-15)30(27,28)24-13-6-7-25(19(13)26)10-11-2-1-3-12(8-11)18(21)22/h1-5,8-9,13,24H,6-7,10H2,(H3,21,22)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against tissue plasminogen activator (t-Pa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50081517

(4-Hydroxy-3-[(S)-2-oxo-3-(thieno[3,2-c]pyridine-2-...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4cnccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)11-1-2-15(25)13(7-11)10-24-6-4-14(19(24)26)23-30(27,28)17-8-12-9-22-5-3-16(12)29-17/h1-3,5,7-9,14,23,25H,4,6,10H2,(H3,20,21)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against tissue plasminogen activator (t-Pa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50081515

(4-Amino-3-[(S)-3-(5-methoxy-thieno[3,2-b]pyridine-...)Show SMILES COc1ccc2sc(cc2n1)S(=O)(=O)N[C@H]1CCN(Cc2cc(ccc2N)C(N)=N)C1=O Show InChI InChI=1S/C20H22N6O4S2/c1-30-17-5-4-16-15(24-17)9-18(31-16)32(28,29)25-14-6-7-26(20(14)27)10-12-8-11(19(22)23)2-3-13(12)21/h2-5,8-9,14,25H,6-7,10,21H2,1H3,(H3,22,23)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against activated protein C (APC) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50081505

(3-[(S)-3-(5-Chloro-thieno[3,2-b]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-16-4-3-15-13(23-16)8-17(30-15)31(28,29)24-12-5-6-25(19(12)27)9-11-7-10(18(21)22)1-2-14(11)26/h1-4,7-8,12,24,26H,5-6,9H2,(H3,21,22)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against activated protein C (APC) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50081011

(3-[(S)-2-Oxo-3-(thieno[3,2-b]pyridine-2-sulfonylam...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4ncccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O3S2/c20-18(21)13-4-1-3-12(9-13)11-24-8-6-14(19(24)25)23-29(26,27)17-10-15-16(28-17)5-2-7-22-15/h1-5,7,9-10,14,23H,6,8,11H2,(H3,20,21)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against activated protein C (APC) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50081499

(4-Hydroxy-3-[(S)-2-oxo-3-(thieno[2,3-c]pyridine-2-...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ccncc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)12-1-2-15(25)13(7-12)10-24-6-4-14(19(24)26)23-30(27,28)17-8-11-3-5-22-9-16(11)29-17/h1-3,5,7-9,14,23,25H,4,6,10H2,(H3,20,21)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against activated protein C (APC) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50081517

(4-Hydroxy-3-[(S)-2-oxo-3-(thieno[3,2-c]pyridine-2-...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4cnccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)11-1-2-15(25)13(7-11)10-24-6-4-14(19(24)26)23-30(27,28)17-8-12-9-22-5-3-16(12)29-17/h1-3,5,7-9,14,23,25H,4,6,10H2,(H3,20,21)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against activated protein C (APC) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50081520

(3-[(S)-3-(5-Chloro-thieno[3,2-b]pyridine-2-sulfony...)Show SMILES NC(=N)c1cccc(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O3S2/c20-16-5-4-15-14(23-16)9-17(29-15)30(27,28)24-13-6-7-25(19(13)26)10-11-2-1-3-12(8-11)18(21)22/h1-5,8-9,13,24H,6-7,10H2,(H3,21,22)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against activated protein C (APC) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Rattus norvegicus) | BDBM50081499

(4-Hydroxy-3-[(S)-2-oxo-3-(thieno[2,3-c]pyridine-2-...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ccncc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)12-1-2-15(25)13(7-12)10-24-6-4-14(19(24)26)23-30(27,28)17-8-11-3-5-22-9-16(11)29-17/h1-3,5,7-9,14,23,25H,4,6,10H2,(H3,20,21)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against plasmin in rat |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data