

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079375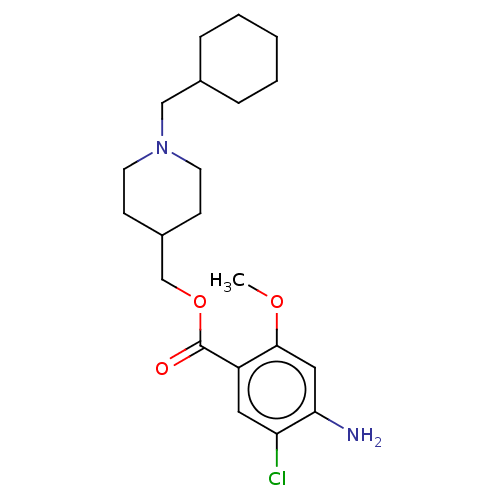 (CHEMBL3417008 | US9663465, 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327460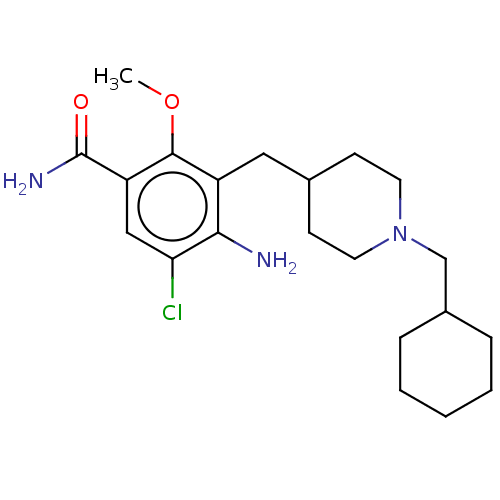 (4-amino-5-chloro-[[1-(cyclohexylmethyl)-4-piperidy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327466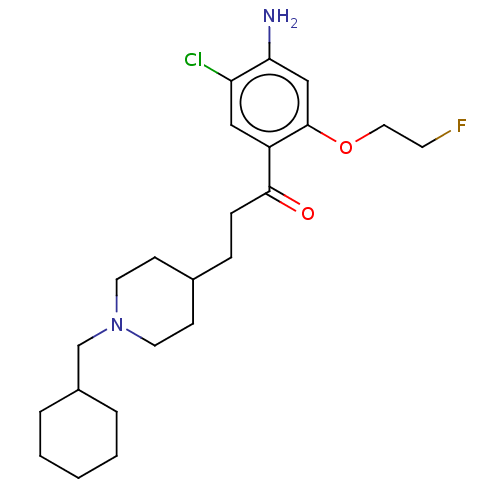 (1-[4-amino-5-chloro-2-(2-fluoroéthoxy)phenyl]-3-[1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327456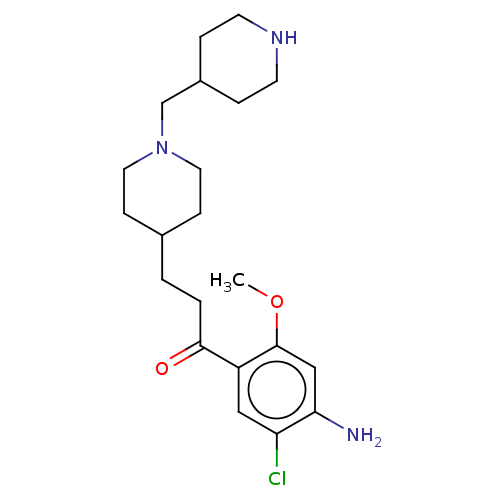 (1-(4-amino-5-chloro-2-methoxyphenyl)-3-[1-[(piperi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079365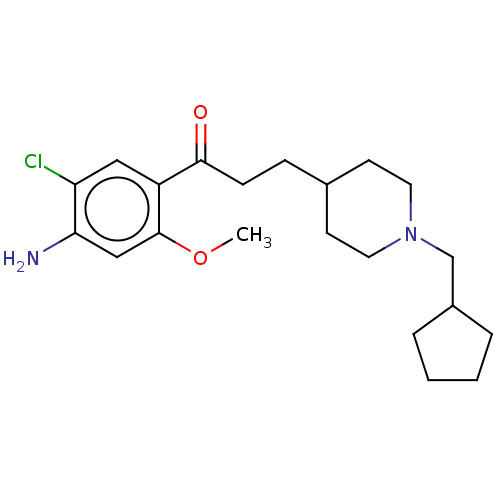 (CHEMBL3416996 | US9663465, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327448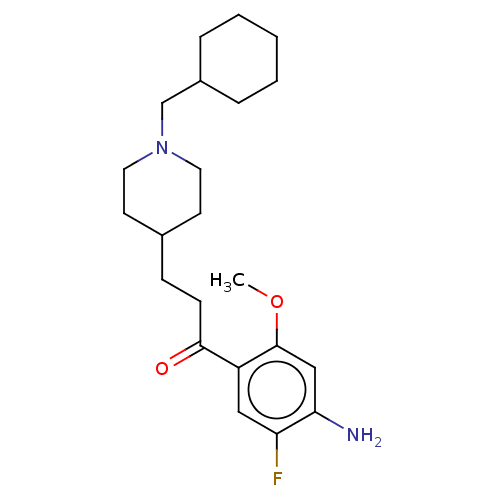 (1-(4-amino-5-fluoro-2-methoxyphenyl)-3-[1-(cyclohe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079366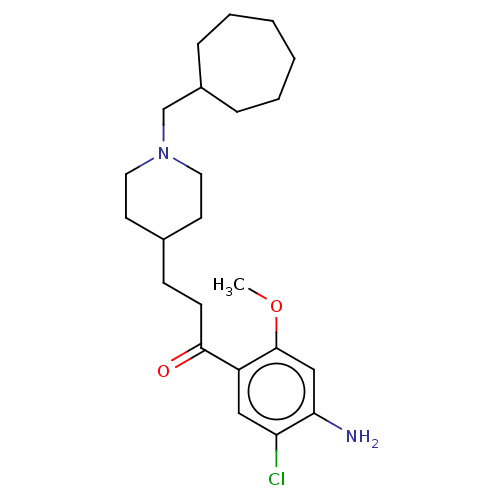 (CHEMBL3416997 | US9663465, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327461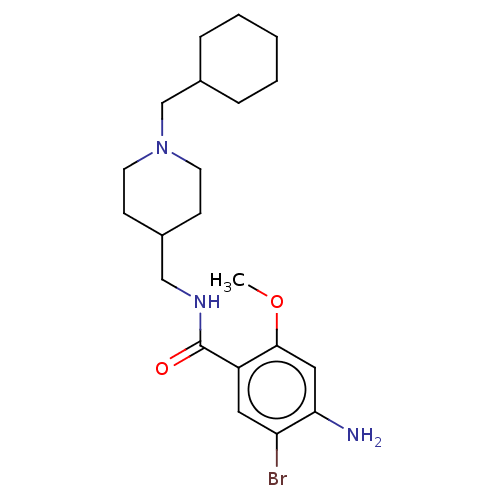 (4-amino-5-bromo-N-[[1-(cyclohexylmethyl)-4-piperid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079362 (CHEMBL3417009 | US9663465, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079369 (CHEMBL3417000 | US9663465, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079368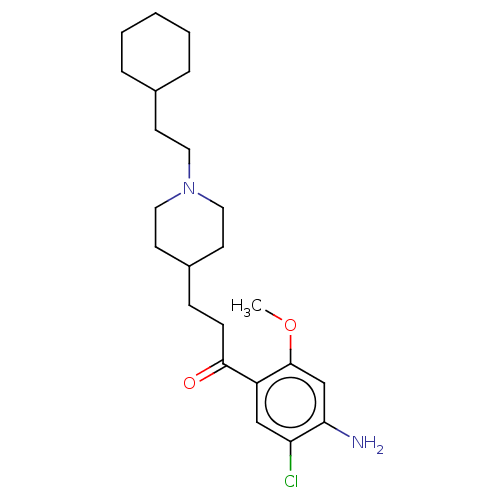 (CHEMBL3416999 | US9663465, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079364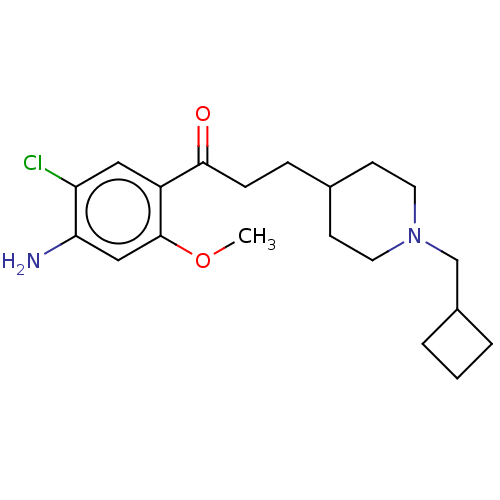 (CHEMBL3416995 | US9663465, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327445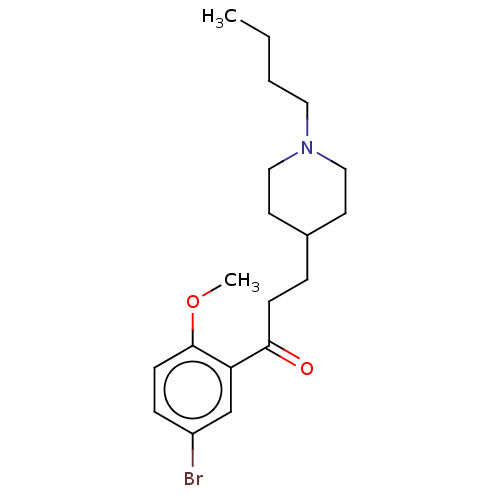 (1-(4-amino-5-bromo-2-methoxyphenyl)-3-[1-butyl-4-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327465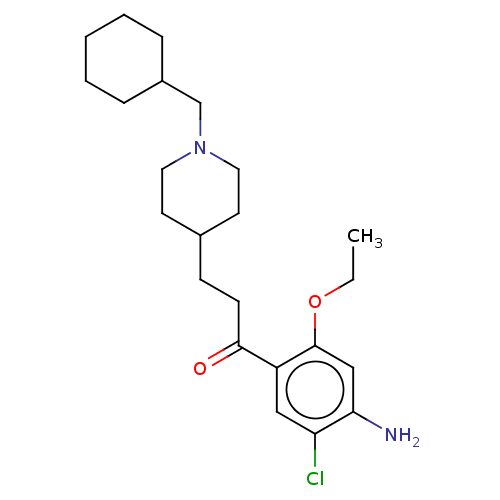 (1-(4-amino-5-chloro-2-éthoxyphenyl)-3-[1-(cyclohex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327467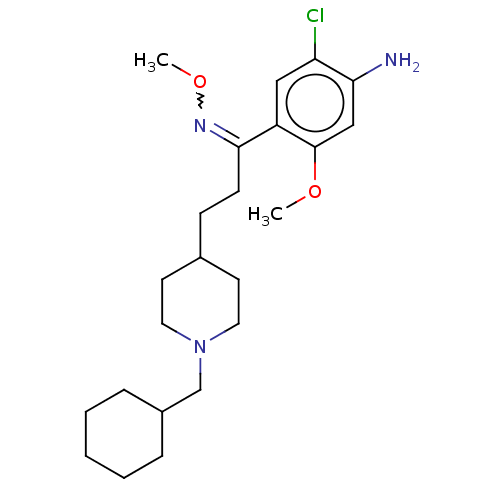 ( 2-chloro-4-[[2-[1-(cyclohexylmethyl)-4-piperidyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327462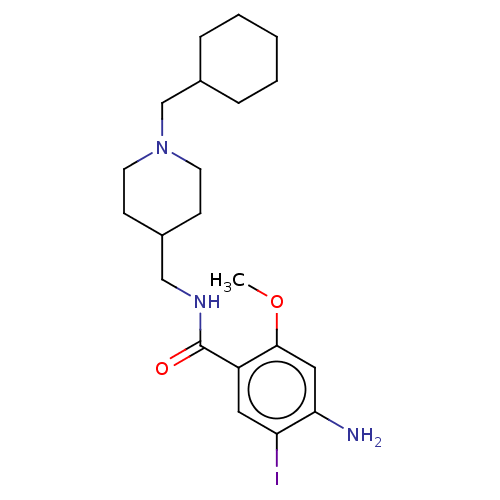 ( 4-amino-5-iodo-N-[[1-(cyclohexylmethyl)-4-piperid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079363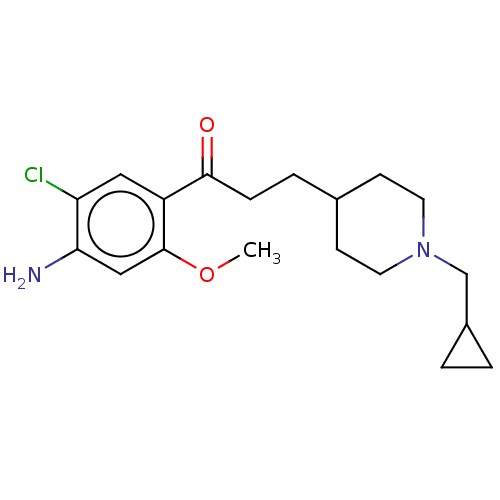 (CHEMBL3414597 | US9663465, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 14.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327457 (1-(4-amino-5-bromo-2-methoxyphenyl)-3-[1-(cyclohex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327458 ( 1-(4-amino-5-iodo-2-methoxyphenyl)-3-[1-(cyclohex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50122872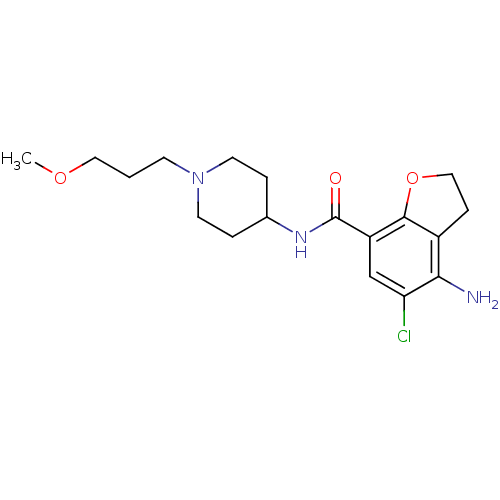 (4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | US Patent | 44.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327446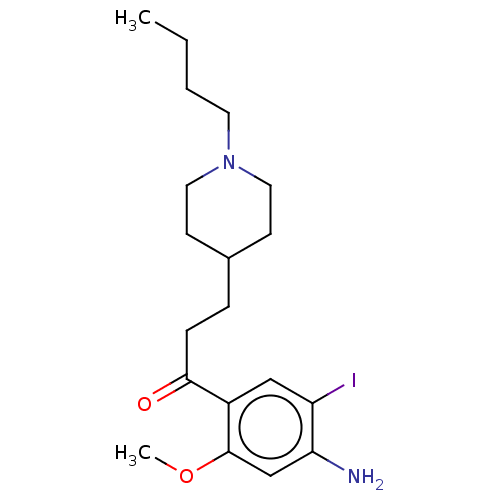 (1-(4-amino-5-iodo-2-methoxyphenyl)-3-[1-butyl-4-pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 44.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327444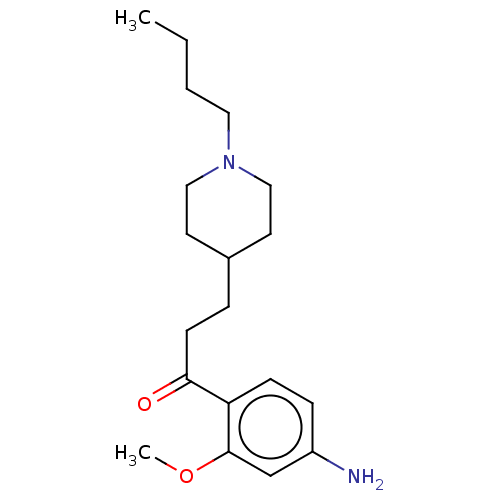 (1-(4-amino-2-methoxyphenyl)-3-[1-butyl-4-piperidyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 88.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327447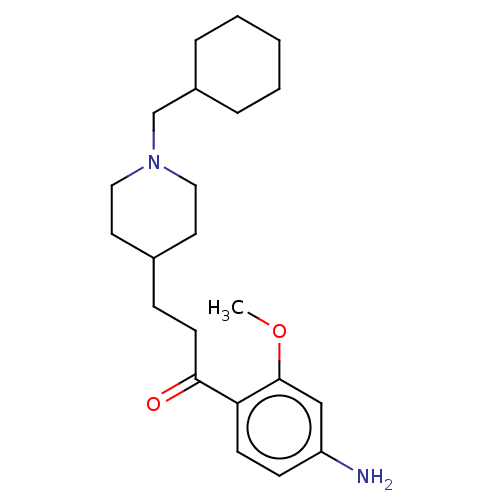 (1-(4-amino-2-methoxyphenyl)-3-[1-(cyclohexylmethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327453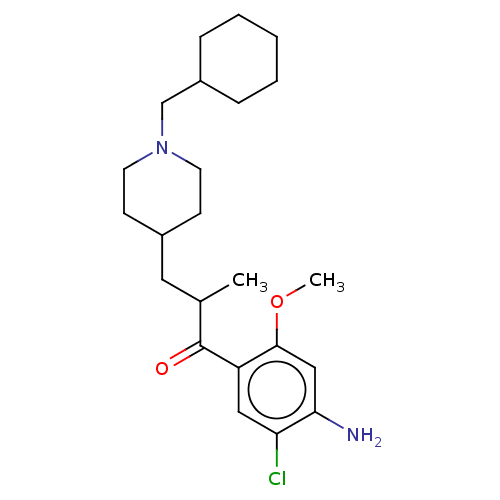 (1-(4-amino-5-chloro-2-methoxyphenyl)-3-[1-(cyclohe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327464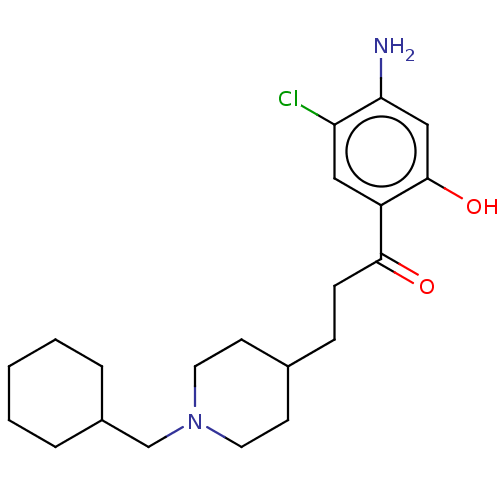 (1-(4-amino-5-chloro-2-hydroxyphenyl)-3-[1-(cyclohe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM327448 (1-(4-amino-5-fluoro-2-methoxyphenyl)-3-[1-(cyclohe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM327447 (1-(4-amino-2-methoxyphenyl)-3-[1-(cyclohexylmethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079366 (CHEMBL3416997 | US9663465, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079362 (CHEMBL3417009 | US9663465, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079365 (CHEMBL3416996 | US9663465, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM327457 (1-(4-amino-5-bromo-2-methoxyphenyl)-3-[1-(cyclohex...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM327456 (1-(4-amino-5-chloro-2-methoxyphenyl)-3-[1-[(piperi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM327453 (1-(4-amino-5-chloro-2-methoxyphenyl)-3-[1-(cyclohe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 201 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079368 (CHEMBL3416999 | US9663465, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 222 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM327458 ( 1-(4-amino-5-iodo-2-methoxyphenyl)-3-[1-(cyclohex...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM327467 ( 2-chloro-4-[[2-[1-(cyclohexylmethyl)-4-piperidyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079369 (CHEMBL3417000 | US9663465, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 321 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM327465 (1-(4-amino-5-chloro-2-éthoxyphenyl)-3-[1-(cyclohex...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 395 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM327464 (1-(4-amino-5-chloro-2-hydroxyphenyl)-3-[1-(cyclohe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 411 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM327445 (1-(4-amino-5-bromo-2-methoxyphenyl)-3-[1-butyl-4-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 445 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079364 (CHEMBL3416995 | US9663465, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 577 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM327466 (1-[4-amino-5-chloro-2-(2-fluoroéthoxy)phenyl]-3-[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 625 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM327446 (1-(4-amino-5-iodo-2-methoxyphenyl)-3-[1-butyl-4-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 658 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM327444 (1-(4-amino-2-methoxyphenyl)-3-[1-butyl-4-piperidyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 748 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079363 (CHEMBL3414597 | US9663465, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 937 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079375 (CHEMBL3417008 | US9663465, 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM327460 (4-amino-5-chloro-[[1-(cyclohexylmethyl)-4-piperidy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM327462 ( 4-amino-5-iodo-N-[[1-(cyclohexylmethyl)-4-piperid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM327461 (4-amino-5-bromo-N-[[1-(cyclohexylmethyl)-4-piperid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||