 Found 244 hits with Last Name = 'macinnes' and Initial = 'a'
Found 244 hits with Last Name = 'macinnes' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357243
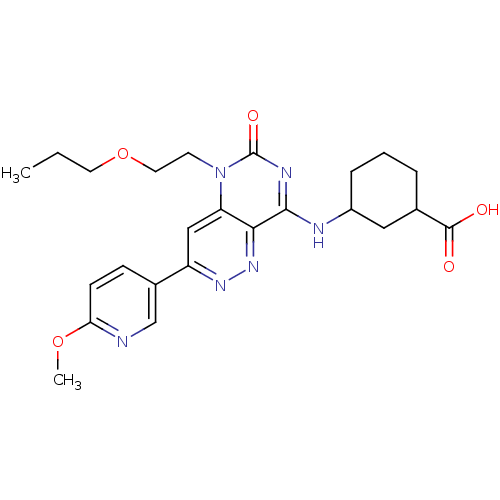
(CHEMBL1916483)Show SMILES CCCOCCn1c2cc(nnc2c(NC2CCCC(C2)C(O)=O)nc1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H30N6O5/c1-3-10-35-11-9-30-19-13-18(16-7-8-20(34-2)25-14-16)28-29-21(19)22(27-24(30)33)26-17-6-4-5-15(12-17)23(31)32/h7-8,13-15,17H,3-6,9-12H2,1-2H3,(H,31,32)(H,26,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357238
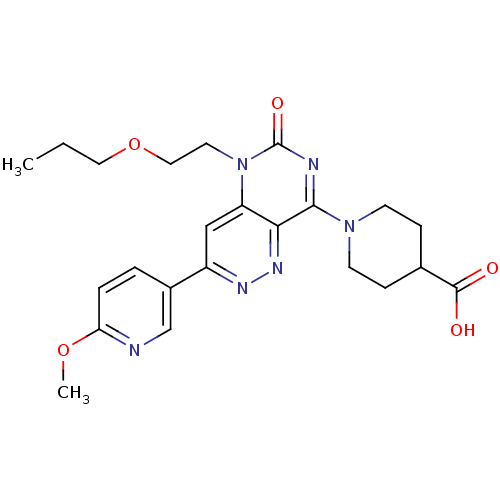
(CHEMBL1916488)Show SMILES CCCOCCn1c2cc(nnc2c(nc1=O)N1CCC(CC1)C(O)=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C23H28N6O5/c1-3-11-34-12-10-29-18-13-17(16-4-5-19(33-2)24-14-16)26-27-20(18)21(25-23(29)32)28-8-6-15(7-9-28)22(30)31/h4-5,13-15H,3,6-12H2,1-2H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357234
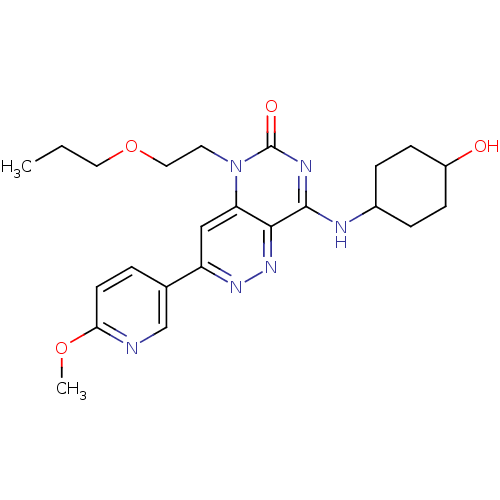
(CHEMBL1916475)Show SMILES CCCOCCn1c2cc(nnc2c(NC2CCC(O)CC2)nc1=O)-c1ccc(OC)nc1 |(22.74,-11.54,;21.41,-12.31,;20.07,-11.54,;18.74,-12.31,;18.74,-13.85,;17.41,-14.62,;17.41,-16.16,;18.74,-16.94,;20.06,-16.18,;21.39,-16.94,;21.39,-18.48,;20.06,-19.25,;18.73,-18.48,;17.41,-19.24,;17.41,-20.78,;16.08,-21.56,;14.75,-20.78,;13.42,-21.54,;13.41,-23.08,;12.08,-23.85,;14.75,-23.86,;16.09,-23.09,;16.08,-18.48,;16.08,-16.94,;14.74,-16.18,;22.71,-16.16,;22.71,-14.63,;24.03,-13.85,;25.38,-14.61,;26.71,-13.84,;28.04,-14.6,;25.38,-16.16,;24.05,-16.93,)| Show InChI InChI=1S/C23H30N6O4/c1-3-11-33-12-10-29-19-13-18(15-4-9-20(32-2)24-14-15)27-28-21(19)22(26-23(29)31)25-16-5-7-17(30)8-6-16/h4,9,13-14,16-17,30H,3,5-8,10-12H2,1-2H3,(H,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357240
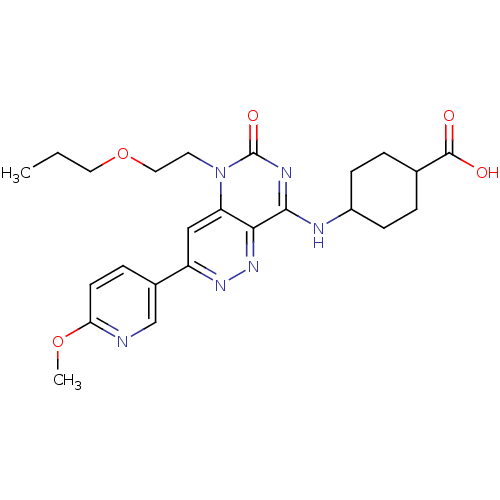
(CHEMBL1916486)Show SMILES CCCOCCn1c2cc(nnc2c(NC2CCC(CC2)C(O)=O)nc1=O)-c1ccc(OC)nc1 |(2.15,6.01,;.82,5.24,;-.51,6.01,;-1.85,5.24,;-1.85,3.69,;-3.18,2.92,;-3.18,1.38,;-1.85,.61,;-.53,1.37,;.8,.61,;.8,-.93,;-.53,-1.7,;-1.86,-.93,;-3.18,-1.7,;-3.18,-3.24,;-4.51,-4.01,;-5.85,-3.24,;-7.18,-4,;-7.18,-5.54,;-5.85,-6.32,;-4.51,-5.55,;-8.52,-6.31,;-9.85,-5.54,;-8.52,-7.85,;-4.51,-.93,;-4.51,.61,;-5.85,1.37,;2.12,1.39,;2.12,2.92,;3.44,3.7,;4.79,2.93,;6.12,3.71,;7.45,2.95,;4.79,1.39,;3.46,.62,)| Show InChI InChI=1S/C24H30N6O5/c1-3-11-35-12-10-30-19-13-18(16-6-9-20(34-2)25-14-16)28-29-21(19)22(27-24(30)33)26-17-7-4-15(5-8-17)23(31)32/h6,9,13-15,17H,3-5,7-8,10-12H2,1-2H3,(H,31,32)(H,26,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357241
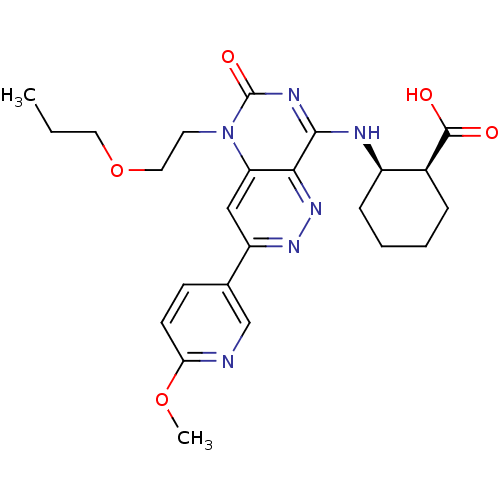
(CHEMBL1916485)Show SMILES CCCOCCn1c2cc(nnc2c(N[C@@H]2CCCC[C@@H]2C(O)=O)nc1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C24H30N6O5/c1-3-11-35-12-10-30-19-13-18(15-8-9-20(34-2)25-14-15)28-29-21(19)22(27-24(30)33)26-17-7-5-4-6-16(17)23(31)32/h8-9,13-14,16-17H,3-7,10-12H2,1-2H3,(H,31,32)(H,26,27,33)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357250
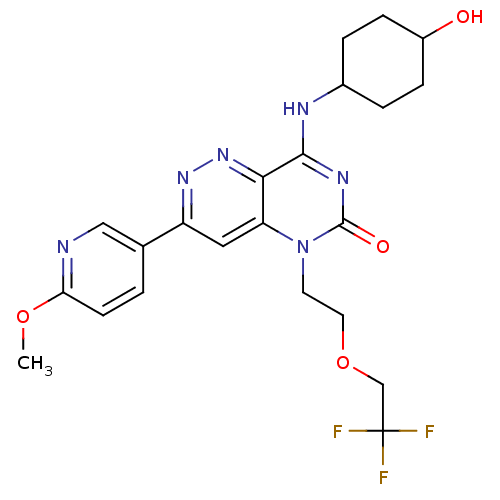
(CHEMBL1916476)Show SMILES COc1ccc(cn1)-c1cc2n(CCOCC(F)(F)F)c(=O)nc(NC3CCC(O)CC3)c2nn1 |(6.09,-33.21,;4.75,-32.45,;3.42,-33.22,;2.07,-32.46,;.75,-33.24,;.75,-34.77,;2.09,-35.54,;3.42,-34.77,;-.57,-35.55,;-1.9,-34.79,;-3.22,-35.55,;-4.55,-34.77,;-4.55,-33.23,;-3.22,-32.46,;-3.22,-30.92,;-1.88,-30.15,;-.55,-30.92,;-.55,-32.46,;.78,-30.15,;.78,-31.69,;-5.88,-35.55,;-7.22,-34.79,;-5.88,-37.09,;-4.55,-37.85,;-4.55,-39.4,;-5.88,-40.17,;-7.21,-39.39,;-8.54,-40.16,;-8.55,-41.7,;-9.88,-42.46,;-7.21,-42.47,;-5.87,-41.7,;-3.23,-37.09,;-1.9,-37.86,;-.57,-37.09,)| Show InChI InChI=1S/C22H25F3N6O4/c1-34-18-7-2-13(11-26-18)16-10-17-19(30-29-16)20(27-14-3-5-15(32)6-4-14)28-21(33)31(17)8-9-35-12-22(23,24)25/h2,7,10-11,14-15,32H,3-6,8-9,12H2,1H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300970
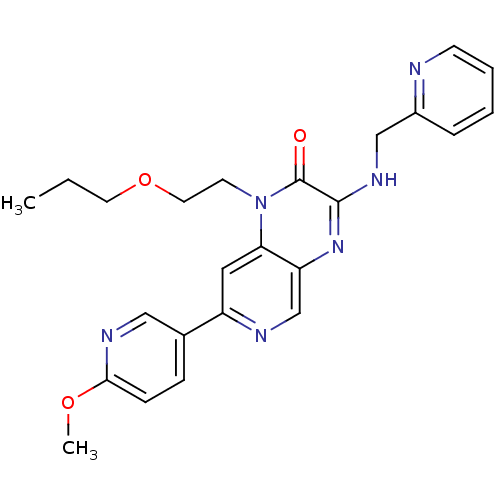
(7-(6-methoxypyridin-3-yl)-1-(2-propoxyethyl)-3-(py...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCc2ccccn2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H26N6O3/c1-3-11-33-12-10-30-21-13-19(17-7-8-22(32-2)27-14-17)26-16-20(21)29-23(24(30)31)28-15-18-6-4-5-9-25-18/h4-9,13-14,16H,3,10-12,15H2,1-2H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357239
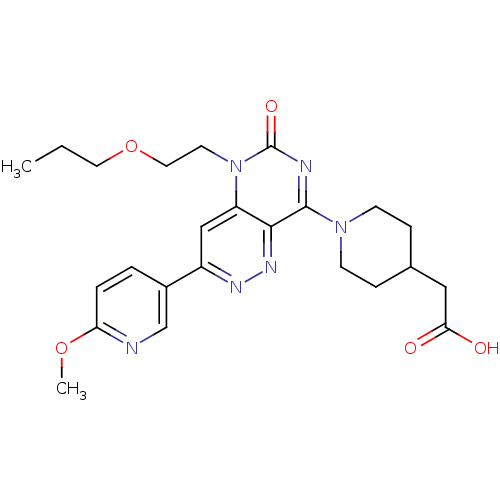
(CHEMBL1916487)Show SMILES CCCOCCn1c2cc(nnc2c(nc1=O)N1CCC(CC(O)=O)CC1)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H30N6O5/c1-3-11-35-12-10-30-19-14-18(17-4-5-20(34-2)25-15-17)27-28-22(19)23(26-24(30)33)29-8-6-16(7-9-29)13-21(31)32/h4-5,14-16H,3,6-13H2,1-2H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357254
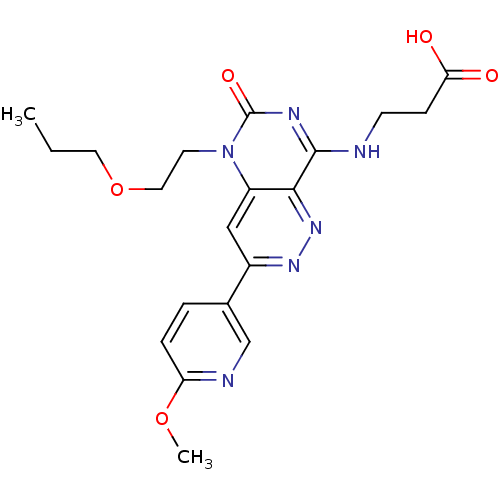
(CHEMBL1916303)Show SMILES CCCOCCn1c2cc(nnc2c(NCCC(O)=O)nc1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C20H24N6O5/c1-3-9-31-10-8-26-15-11-14(13-4-5-16(30-2)22-12-13)24-25-18(15)19(23-20(26)29)21-7-6-17(27)28/h4-5,11-12H,3,6-10H2,1-2H3,(H,27,28)(H,21,23,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300953
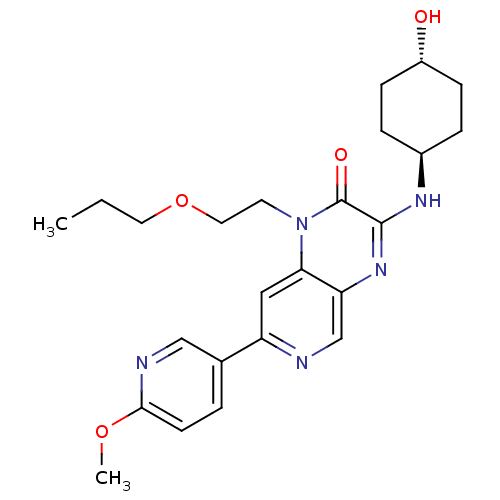
(3-[(trans-4-hydroxycyclohexyl)amino]-7-(6-methoxyp...)Show SMILES CCCOCCn1c2cc(ncc2nc(N[C@H]2CC[C@H](O)CC2)c1=O)-c1ccc(OC)nc1 |r,wU:16.16,wD:19.20,(24.97,-.51,;23.64,-1.28,;23.64,-2.82,;22.3,-3.59,;22.3,-5.13,;20.97,-5.9,;20.97,-7.44,;22.3,-8.21,;23.62,-7.45,;24.95,-8.21,;24.95,-9.75,;23.62,-10.52,;22.3,-9.75,;20.97,-10.52,;19.62,-9.75,;18.29,-10.51,;16.96,-9.74,;15.63,-10.51,;14.29,-9.73,;14.3,-8.19,;12.97,-7.41,;15.64,-7.43,;16.97,-8.2,;19.64,-8.21,;18.31,-7.44,;26.28,-7.43,;27.61,-8.2,;28.94,-7.43,;28.94,-5.89,;30.27,-5.11,;31.61,-5.87,;27.59,-5.12,;26.27,-5.9,)| Show InChI InChI=1S/C24H31N5O4/c1-3-11-33-12-10-29-21-13-19(16-4-9-22(32-2)26-14-16)25-15-20(21)28-23(24(29)31)27-17-5-7-18(30)8-6-17/h4,9,13-15,17-18,30H,3,5-8,10-12H2,1-2H3,(H,27,28)/t17-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357263

(CHEMBL1916293)Show SMILES CCCOCCn1c2cc(ncc2c(NC2CCC(O)CC2)nc1=O)-c1ccc(OC)nc1 |(28.17,-7.71,;26.83,-8.48,;25.5,-7.71,;24.17,-8.48,;24.17,-10.02,;22.83,-10.79,;22.83,-12.33,;24.16,-13.11,;25.48,-12.34,;26.81,-13.1,;26.82,-14.65,;25.49,-15.41,;24.16,-14.65,;22.83,-15.41,;22.83,-16.95,;21.5,-17.72,;20.17,-16.95,;18.84,-17.71,;18.84,-19.25,;17.5,-20.02,;20.17,-20.02,;21.51,-19.26,;21.5,-14.65,;21.5,-13.11,;20.17,-12.34,;28.14,-12.33,;28.13,-10.79,;29.46,-10.01,;30.8,-10.78,;32.13,-10,;33.47,-10.77,;30.81,-12.32,;29.48,-13.1,)| Show InChI InChI=1S/C24H31N5O4/c1-3-11-33-12-10-29-21-13-20(16-4-9-22(32-2)26-14-16)25-15-19(21)23(28-24(29)31)27-17-5-7-18(30)8-6-17/h4,9,13-15,17-18,30H,3,5-8,10-12H2,1-2H3,(H,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300953

(3-[(trans-4-hydroxycyclohexyl)amino]-7-(6-methoxyp...)Show SMILES CCCOCCn1c2cc(ncc2nc(N[C@H]2CC[C@H](O)CC2)c1=O)-c1ccc(OC)nc1 |r,wU:16.16,wD:19.20,(24.97,-.51,;23.64,-1.28,;23.64,-2.82,;22.3,-3.59,;22.3,-5.13,;20.97,-5.9,;20.97,-7.44,;22.3,-8.21,;23.62,-7.45,;24.95,-8.21,;24.95,-9.75,;23.62,-10.52,;22.3,-9.75,;20.97,-10.52,;19.62,-9.75,;18.29,-10.51,;16.96,-9.74,;15.63,-10.51,;14.29,-9.73,;14.3,-8.19,;12.97,-7.41,;15.64,-7.43,;16.97,-8.2,;19.64,-8.21,;18.31,-7.44,;26.28,-7.43,;27.61,-8.2,;28.94,-7.43,;28.94,-5.89,;30.27,-5.11,;31.61,-5.87,;27.59,-5.12,;26.27,-5.9,)| Show InChI InChI=1S/C24H31N5O4/c1-3-11-33-12-10-29-21-13-19(16-4-9-22(32-2)26-14-16)25-15-20(21)28-23(24(29)31)27-17-5-7-18(30)8-6-17/h4,9,13-15,17-18,30H,3,5-8,10-12H2,1-2H3,(H,27,28)/t17-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357244
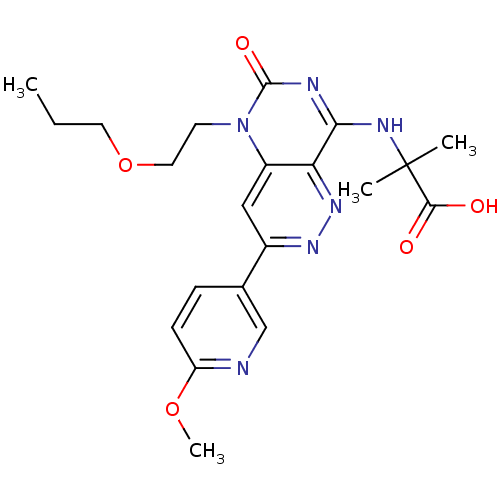
(CHEMBL1916482)Show SMILES CCCOCCn1c2cc(nnc2c(NC(C)(C)C(O)=O)nc1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C21H26N6O5/c1-5-9-32-10-8-27-15-11-14(13-6-7-16(31-4)22-12-13)25-26-17(15)18(23-20(27)30)24-21(2,3)19(28)29/h6-7,11-12H,5,8-10H2,1-4H3,(H,28,29)(H,23,24,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300964
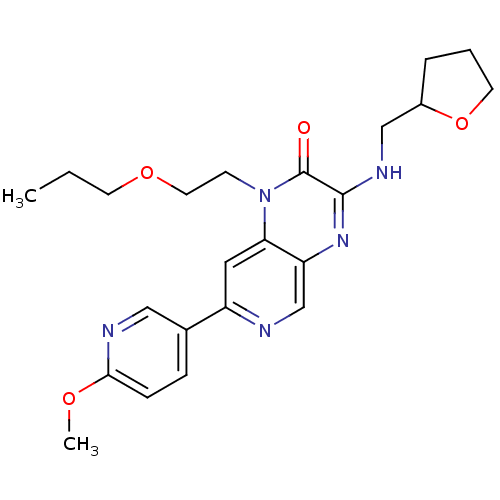
(CHEMBL584270 | rac-7-(6-methoxypyridin-3-yl)-1-(2-...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC2CCCO2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C23H29N5O4/c1-3-9-31-11-8-28-20-12-18(16-6-7-21(30-2)25-13-16)24-15-19(20)27-22(23(28)29)26-14-17-5-4-10-32-17/h6-7,12-13,15,17H,3-5,8-11,14H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357237
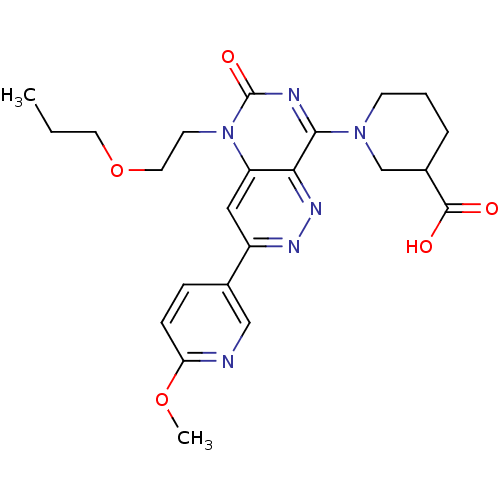
(CHEMBL1916489)Show SMILES CCCOCCn1c2cc(nnc2c(nc1=O)N1CCCC(C1)C(O)=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C23H28N6O5/c1-3-10-34-11-9-29-18-12-17(15-6-7-19(33-2)24-13-15)26-27-20(18)21(25-23(29)32)28-8-4-5-16(14-28)22(30)31/h6-7,12-13,16H,3-5,8-11,14H2,1-2H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300972
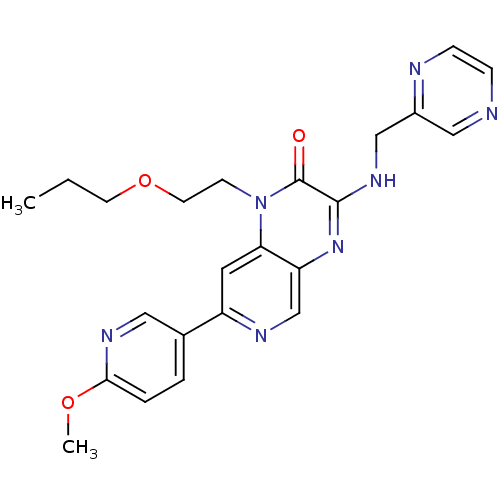
(7-(6-methoxypyridin-3-yl)-1-(2-propoxyethyl)-3-(py...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCc2cnccn2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C23H25N7O3/c1-3-9-33-10-8-30-20-11-18(16-4-5-21(32-2)27-12-16)26-15-19(20)29-22(23(30)31)28-14-17-13-24-6-7-25-17/h4-7,11-13,15H,3,8-10,14H2,1-2H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50296256
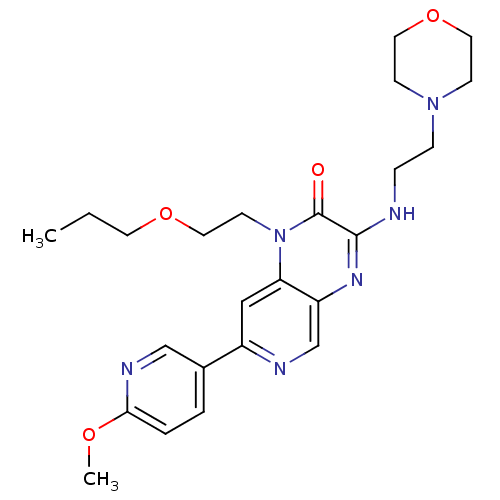
(7-(6-methoxypyridin-3-yl)-3-(2-morpholinoethylamin...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCCN2CCOCC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H32N6O4/c1-3-11-33-14-10-30-21-15-19(18-4-5-22(32-2)27-16-18)26-17-20(21)28-23(24(30)31)25-6-7-29-8-12-34-13-9-29/h4-5,15-17H,3,6-14H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50296256

(7-(6-methoxypyridin-3-yl)-3-(2-morpholinoethylamin...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCCN2CCOCC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H32N6O4/c1-3-11-33-14-10-30-21-15-19(18-4-5-22(32-2)27-16-18)26-17-20(21)28-23(24(30)31)25-6-7-29-8-12-34-13-9-29/h4-5,15-17H,3,6-14H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 4092-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.004
BindingDB Entry DOI: 10.7270/Q2ZG6S9Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300976
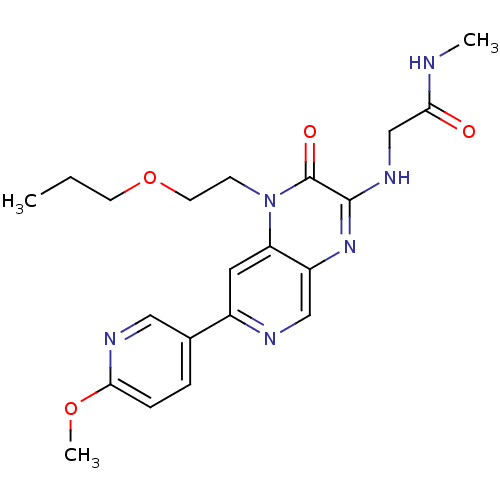
(2-(7-(6-methoxypyridin-3-yl)-2-oxo-1-(2-propoxyeth...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC(=O)NC)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C21H26N6O4/c1-4-8-31-9-7-27-17-10-15(14-5-6-19(30-3)24-11-14)23-12-16(17)26-20(21(27)29)25-13-18(28)22-2/h5-6,10-12H,4,7-9,13H2,1-3H3,(H,22,28)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300983
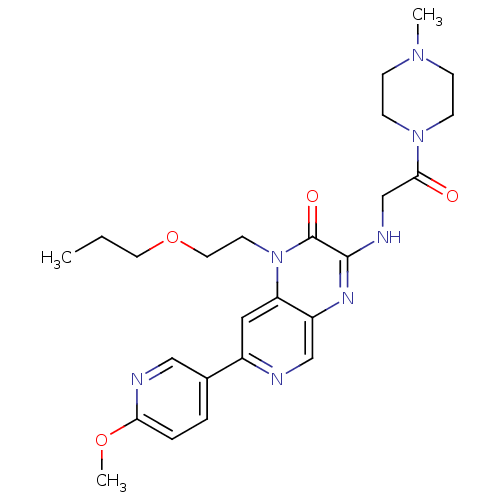
(7-(6-methoxypyridin-3-yl)-3-(2-(4-methylpiperazin-...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC(=O)N2CCN(C)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C25H33N7O4/c1-4-12-36-13-11-32-21-14-19(18-5-6-22(35-3)27-15-18)26-16-20(21)29-24(25(32)34)28-17-23(33)31-9-7-30(2)8-10-31/h5-6,14-16H,4,7-13,17H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300977
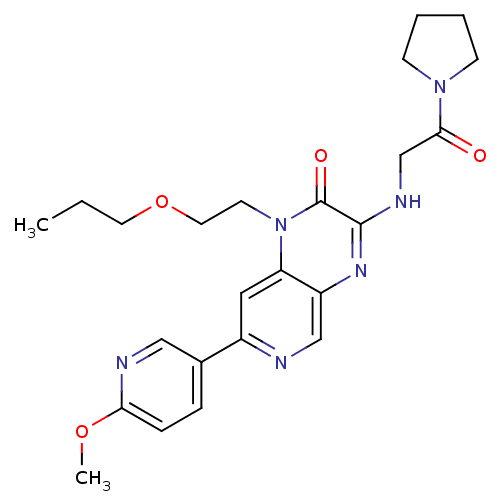
(7-(6-methoxypyridin-3-yl)-3-(2-oxo-2-(pyrrolidin-1...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC(=O)N2CCCC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H30N6O4/c1-3-11-34-12-10-30-20-13-18(17-6-7-21(33-2)26-14-17)25-15-19(20)28-23(24(30)32)27-16-22(31)29-8-4-5-9-29/h6-7,13-15H,3-5,8-12,16H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357264
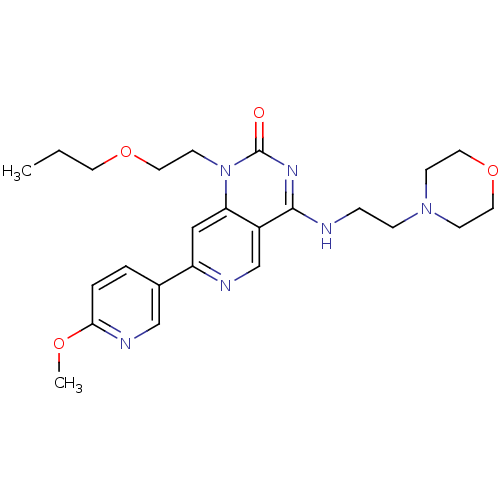
(CHEMBL1916291)Show SMILES CCCOCCn1c2cc(ncc2c(NCCN2CCOCC2)nc1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H32N6O4/c1-3-11-33-14-10-30-21-15-20(18-4-5-22(32-2)27-16-18)26-17-19(21)23(28-24(30)31)25-6-7-29-8-12-34-13-9-29/h4-5,15-17H,3,6-14H2,1-2H3,(H,25,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357258

(CHEMBL1916299)Show SMILES CCCOCCn1c2cc(nnc2c(NCCN2CCOCC2)nc1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C23H31N7O4/c1-3-11-33-14-10-30-19-15-18(17-4-5-20(32-2)25-16-17)27-28-21(19)22(26-23(30)31)24-6-7-29-8-12-34-13-9-29/h4-5,15-16H,3,6-14H2,1-2H3,(H,24,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300989
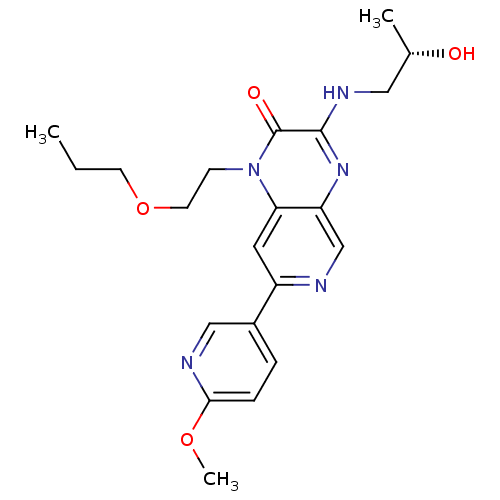
((S)-3-(2-hydroxypropylamino)-7-(6-methoxypyridin-3...)Show SMILES CCCOCCn1c2cc(ncc2nc(NC[C@H](C)O)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H27N5O4/c1-4-8-30-9-7-26-18-10-16(15-5-6-19(29-3)23-12-15)22-13-17(18)25-20(21(26)28)24-11-14(2)27/h5-6,10,12-14,27H,4,7-9,11H2,1-3H3,(H,24,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357235
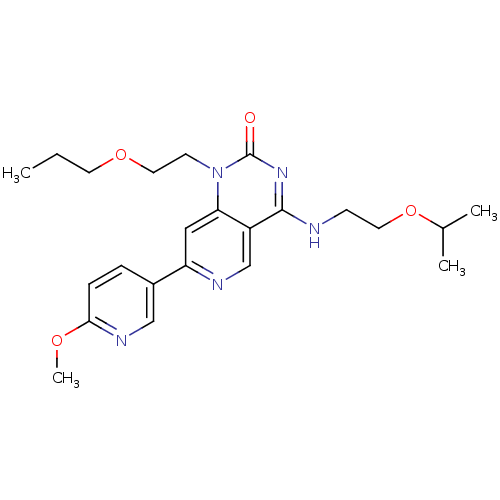
(CHEMBL1916290)Show SMILES CCCOCCn1c2cc(ncc2c(NCCOC(C)C)nc1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C23H31N5O4/c1-5-10-31-12-9-28-20-13-19(17-6-7-21(30-4)26-14-17)25-15-18(20)22(27-23(28)29)24-8-11-32-16(2)3/h6-7,13-16H,5,8-12H2,1-4H3,(H,24,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357236
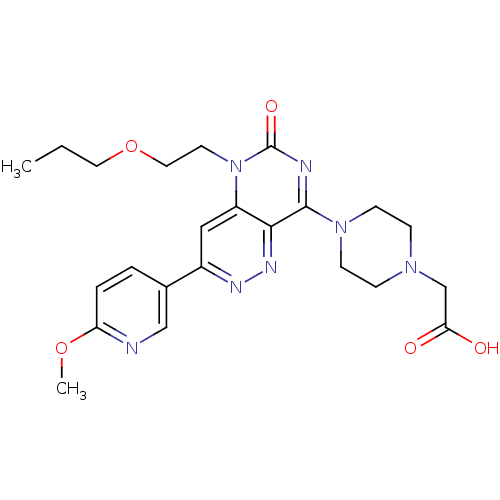
(CHEMBL1916490)Show SMILES CCCOCCn1c2cc(nnc2c(nc1=O)N1CCN(CC(O)=O)CC1)-c1ccc(OC)nc1 Show InChI InChI=1S/C23H29N7O5/c1-3-11-35-12-10-30-18-13-17(16-4-5-19(34-2)24-14-16)26-27-21(18)22(25-23(30)33)29-8-6-28(7-9-29)15-20(31)32/h4-5,13-14H,3,6-12,15H2,1-2H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357262
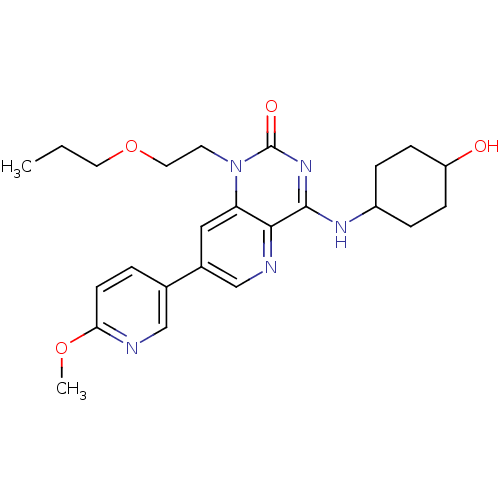
(CHEMBL1916295)Show SMILES CCCOCCn1c2cc(cnc2c(NC2CCC(O)CC2)nc1=O)-c1ccc(OC)nc1 |(25.6,-22.57,;24.26,-23.34,;22.93,-22.57,;21.6,-23.34,;21.6,-24.88,;20.26,-25.65,;20.26,-27.19,;21.59,-27.97,;22.91,-27.21,;24.24,-27.97,;24.25,-29.51,;22.92,-30.28,;21.59,-29.51,;20.26,-30.27,;20.26,-31.81,;18.93,-32.59,;17.6,-31.81,;16.27,-32.57,;16.27,-34.11,;14.93,-34.88,;17.6,-34.89,;18.94,-34.12,;18.93,-29.51,;18.93,-27.97,;17.6,-27.2,;25.57,-27.19,;25.56,-25.65,;26.89,-24.88,;28.23,-25.64,;29.56,-24.87,;30.9,-25.63,;28.23,-27.18,;26.9,-27.96,)| Show InChI InChI=1S/C24H31N5O4/c1-3-11-33-12-10-29-20-13-17(16-4-9-21(32-2)25-14-16)15-26-22(20)23(28-24(29)31)27-18-5-7-19(30)8-6-18/h4,9,13-15,18-19,30H,3,5-8,10-12H2,1-2H3,(H,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300991
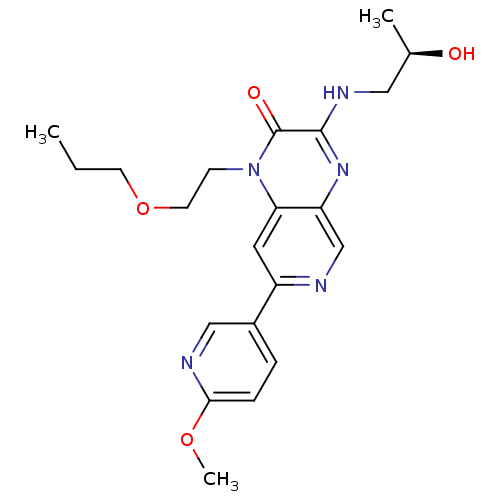
((R)-3-(2-hydroxypropylamino)-7-(6-methoxypyridin-3...)Show SMILES CCCOCCn1c2cc(ncc2nc(NC[C@@H](C)O)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H27N5O4/c1-4-8-30-9-7-26-18-10-16(15-5-6-19(29-3)23-12-15)22-13-17(18)25-20(21(26)28)24-11-14(2)27/h5-6,10,12-14,27H,4,7-9,11H2,1-3H3,(H,24,25)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300962
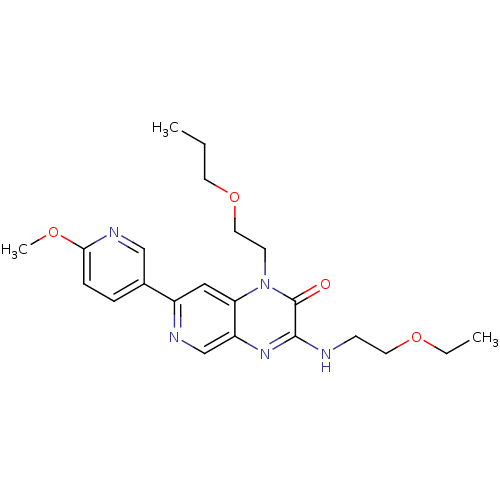
(3-(2-ethoxyethylamino)-7-(6-methoxypyridin-3-yl)-1...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCCOCC)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C22H29N5O4/c1-4-10-31-12-9-27-19-13-17(16-6-7-20(29-3)25-14-16)24-15-18(19)26-21(22(27)28)23-8-11-30-5-2/h6-7,13-15H,4-5,8-12H2,1-3H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
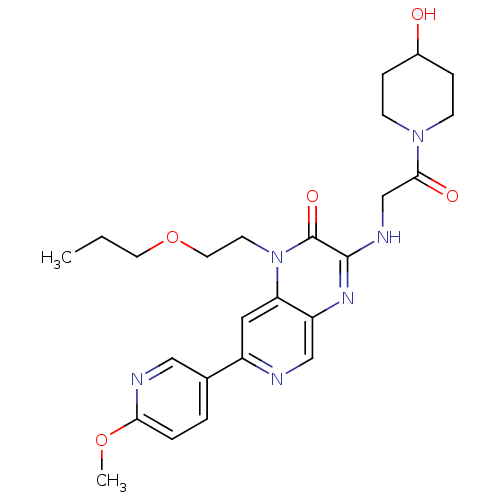
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300978
(3-(2-(4-hydroxypiperidin-1-yl)-2-oxoethylamino)-7-...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC(=O)N2CCC(O)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C25H32N6O5/c1-3-11-36-12-10-31-21-13-19(17-4-5-22(35-2)27-14-17)26-15-20(21)29-24(25(31)34)28-16-23(33)30-8-6-18(32)7-9-30/h4-5,13-15,18,32H,3,6-12,16H2,1-2H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357245
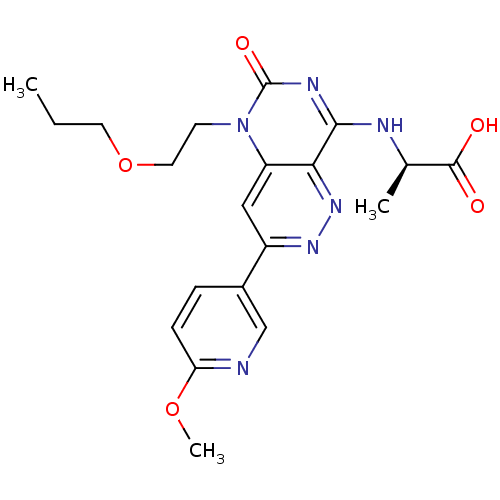
(CHEMBL1916481)Show SMILES CCCOCCn1c2cc(nnc2c(N[C@H](C)C(O)=O)nc1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C20H24N6O5/c1-4-8-31-9-7-26-15-10-14(13-5-6-16(30-3)21-11-13)24-25-17(15)18(23-20(26)29)22-12(2)19(27)28/h5-6,10-12H,4,7-9H2,1-3H3,(H,27,28)(H,22,23,29)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300985
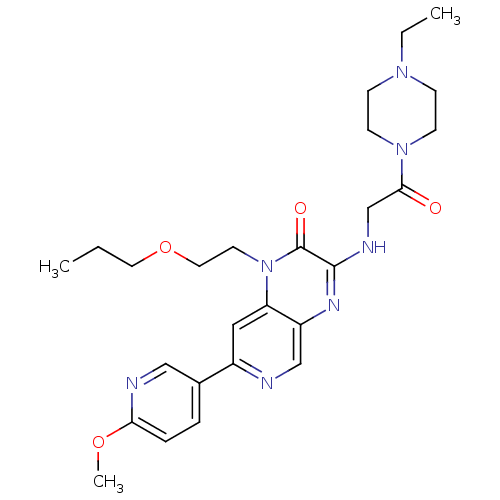
(3-(2-(4-ethylpiperazin-1-yl)-2-oxoethylamino)-7-(6...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC(=O)N2CCN(CC)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C26H35N7O4/c1-4-13-37-14-12-33-22-15-20(19-6-7-23(36-3)28-16-19)27-17-21(22)30-25(26(33)35)29-18-24(34)32-10-8-31(5-2)9-11-32/h6-7,15-17H,4-5,8-14,18H2,1-3H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300948
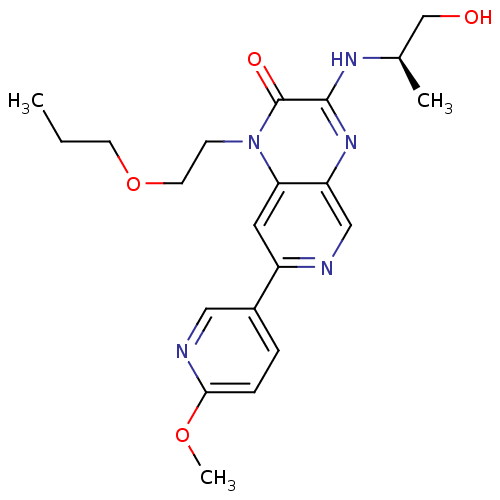
((R)-3-(1-hydroxypropan-2-ylamino)-7-(6-methoxypyri...)Show SMILES CCCOCCn1c2cc(ncc2nc(N[C@H](C)CO)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H27N5O4/c1-4-8-30-9-7-26-18-10-16(15-5-6-19(29-3)23-11-15)22-12-17(18)25-20(21(26)28)24-14(2)13-27/h5-6,10-12,14,27H,4,7-9,13H2,1-3H3,(H,24,25)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300963
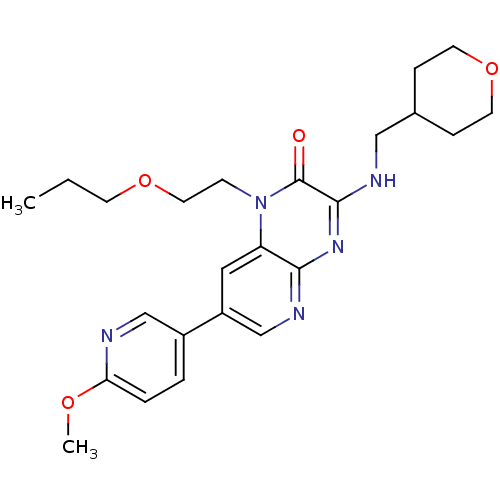
(7-(6-methoxypyridin-3-yl)-1-(2-propoxyethyl)-3-((t...)Show SMILES CCCOCCn1c2cc(cnc2nc(NCC2CCOCC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H31N5O4/c1-3-9-32-12-8-29-20-13-19(18-4-5-21(31-2)25-15-18)16-27-22(20)28-23(24(29)30)26-14-17-6-10-33-11-7-17/h4-5,13,15-17H,3,6-12,14H2,1-2H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300980
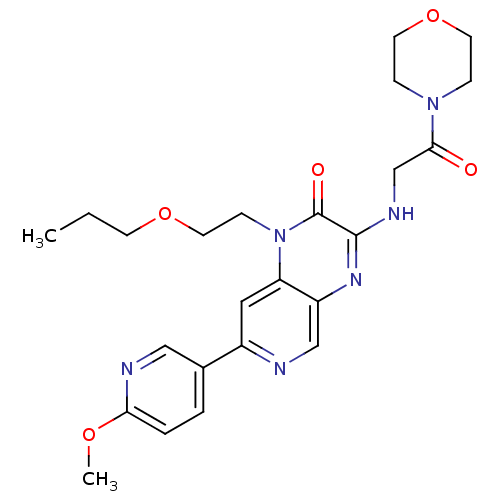
(7-(6-methoxypyridin-3-yl)-3-(2-morpholino-2-oxoeth...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCC(=O)N2CCOCC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H30N6O5/c1-3-9-34-12-8-30-20-13-18(17-4-5-21(33-2)26-14-17)25-15-19(20)28-23(24(30)32)27-16-22(31)29-6-10-35-11-7-29/h4-5,13-15H,3,6-12,16H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300961
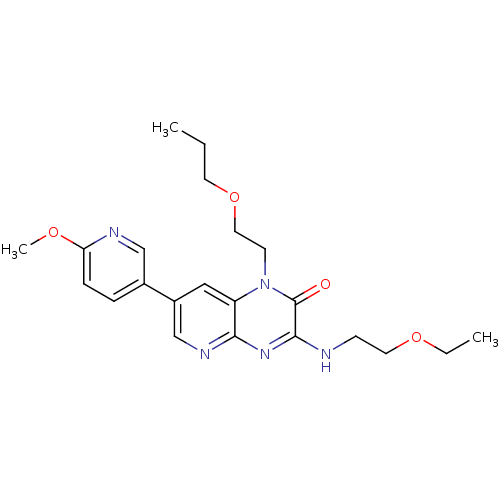
(3-(2-ethoxyethylamino)-7-(6-methoxypyridin-3-yl)-1...)Show SMILES CCCOCCn1c2cc(cnc2nc(NCCOCC)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C22H29N5O4/c1-4-10-31-12-9-27-18-13-17(16-6-7-19(29-3)24-14-16)15-25-20(18)26-21(22(27)28)23-8-11-30-5-2/h6-7,13-15H,4-5,8-12H2,1-3H3,(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50308556
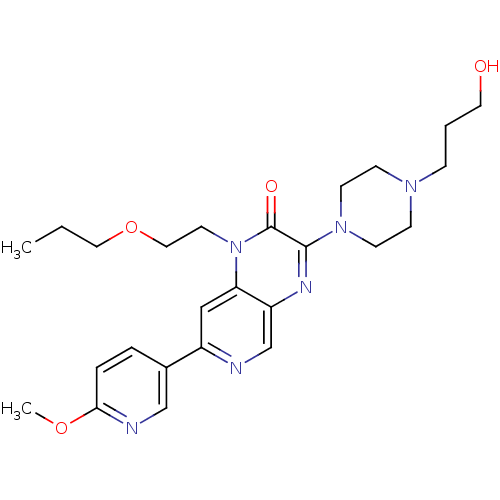
(3-(4-(3-hydroxypropyl)piperazin-1-yl)-1-(2-propoxy...)Show SMILES CCCOCCn1c2cc(ncc2nc(N2CCN(CCCO)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C25H34N6O4/c1-3-14-35-15-12-31-22-16-20(19-5-6-23(34-2)27-17-19)26-18-21(22)28-24(25(31)33)30-10-8-29(9-11-30)7-4-13-32/h5-6,16-18,32H,3-4,7-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assay |
J Med Chem 53: 2656-60 (2010)
Article DOI: 10.1021/jm901781q
BindingDB Entry DOI: 10.7270/Q2VM4CCK |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50308557
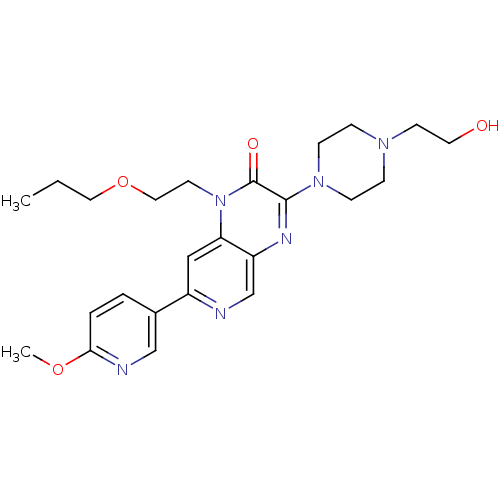
(3-(4-(2-hydroxyethyl)piperazin-1-yl)-1-(2-propoxye...)Show SMILES CCCOCCn1c2cc(ncc2nc(N2CCN(CCO)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H32N6O4/c1-3-13-34-14-11-30-21-15-19(18-4-5-22(33-2)26-16-18)25-17-20(21)27-23(24(30)32)29-8-6-28(7-9-29)10-12-31/h4-5,15-17,31H,3,6-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50308557

(3-(4-(2-hydroxyethyl)piperazin-1-yl)-1-(2-propoxye...)Show SMILES CCCOCCn1c2cc(ncc2nc(N2CCN(CCO)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H32N6O4/c1-3-13-34-14-11-30-21-15-19(18-4-5-22(33-2)26-16-18)25-17-20(21)27-23(24(30)32)29-8-6-28(7-9-29)10-12-31/h4-5,15-17,31H,3,6-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assay |
J Med Chem 53: 2656-60 (2010)
Article DOI: 10.1021/jm901781q
BindingDB Entry DOI: 10.7270/Q2VM4CCK |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50308563
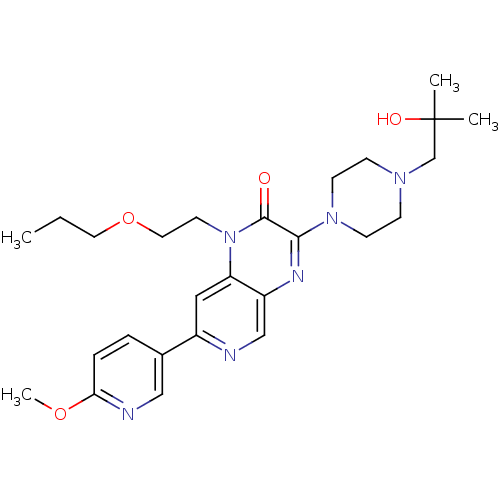
(3-(4-(2-hydroxy-2-methylpropyl)piperazin-1-yl)-1-(...)Show SMILES CCCOCCn1c2cc(ncc2nc(N2CCN(CC(C)(C)O)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C26H36N6O4/c1-5-13-36-14-12-32-22-15-20(19-6-7-23(35-4)28-16-19)27-17-21(22)29-24(25(32)33)31-10-8-30(9-11-31)18-26(2,3)34/h6-7,15-17,34H,5,8-14,18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assay |
J Med Chem 53: 2656-60 (2010)
Article DOI: 10.1021/jm901781q
BindingDB Entry DOI: 10.7270/Q2VM4CCK |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50308558

(3-(4-ethylpiperazin-1-yl)-1-(2-propoxyethyl)-7-(6-...)Show SMILES CCCOCCn1c2cc(ncc2nc(N2CCN(CC)CC2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C24H32N6O3/c1-4-13-33-14-12-30-21-15-19(18-6-7-22(32-3)26-16-18)25-17-20(21)27-23(24(30)31)29-10-8-28(5-2)9-11-29/h6-7,15-17H,4-5,8-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assay |
J Med Chem 53: 2656-60 (2010)
Article DOI: 10.1021/jm901781q
BindingDB Entry DOI: 10.7270/Q2VM4CCK |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357256
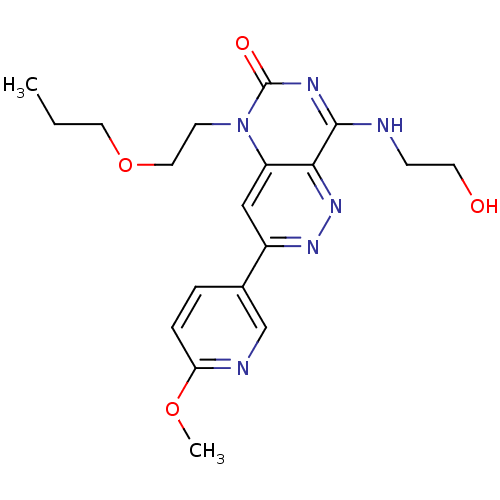
(CHEMBL1916301)Show SMILES CCCOCCn1c2cc(nnc2c(NCCO)nc1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C19H24N6O4/c1-3-9-29-10-7-25-15-11-14(13-4-5-16(28-2)21-12-13)23-24-17(15)18(20-6-8-26)22-19(25)27/h4-5,11-12,26H,3,6-10H2,1-2H3,(H,20,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50308564

((R)-3-(4-(2-hydroxypropyl)piperazin-1-yl)-1-(2-iso...)Show SMILES CCCOCCn1c2cc(ncc2nc(N2CCN(C[C@@H](C)O)CC2)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C25H34N6O4/c1-4-12-35-13-11-31-22-14-20(19-5-6-23(34-3)27-15-19)26-16-21(22)28-24(25(31)33)30-9-7-29(8-10-30)17-18(2)32/h5-6,14-16,18,32H,4,7-13,17H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assay |
J Med Chem 53: 2656-60 (2010)
Article DOI: 10.1021/jm901781q
BindingDB Entry DOI: 10.7270/Q2VM4CCK |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300950
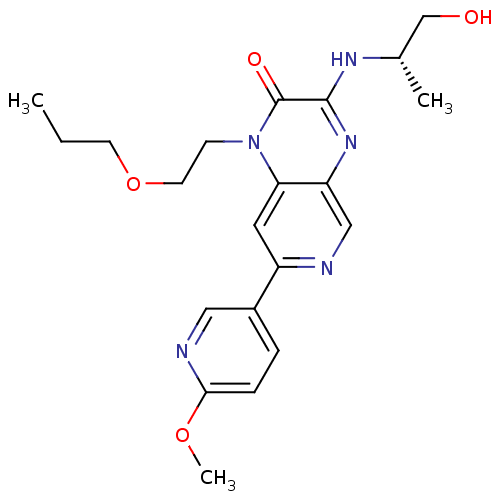
((S)-3-(1-hydroxypropan-2-ylamino)-7-(6-methoxypyri...)Show SMILES CCCOCCn1c2cc(ncc2nc(N[C@@H](C)CO)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H27N5O4/c1-4-8-30-9-7-26-18-10-16(15-5-6-19(29-3)23-11-15)22-12-17(18)25-20(21(26)28)24-14(2)13-27/h5-6,10-12,14,27H,4,7-9,13H2,1-3H3,(H,24,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300951
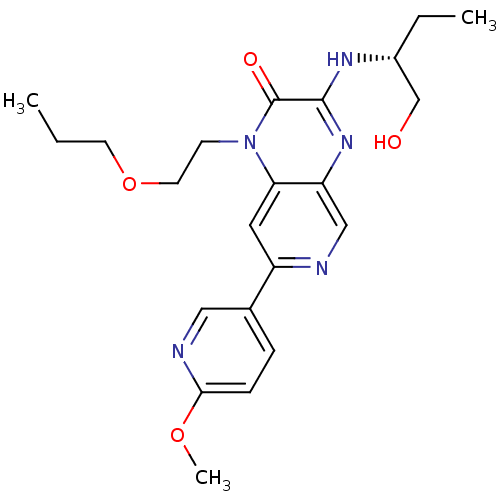
((R)-3-(1-hydroxybutan-2-ylamino)-7-(6-methoxypyrid...)Show SMILES CCCOCCn1c2cc(ncc2nc(N[C@H](CC)CO)c1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C22H29N5O4/c1-4-9-31-10-8-27-19-11-17(15-6-7-20(30-3)24-12-15)23-13-18(19)26-21(22(27)29)25-16(5-2)14-28/h6-7,11-13,16,28H,4-5,8-10,14H2,1-3H3,(H,25,26)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300957
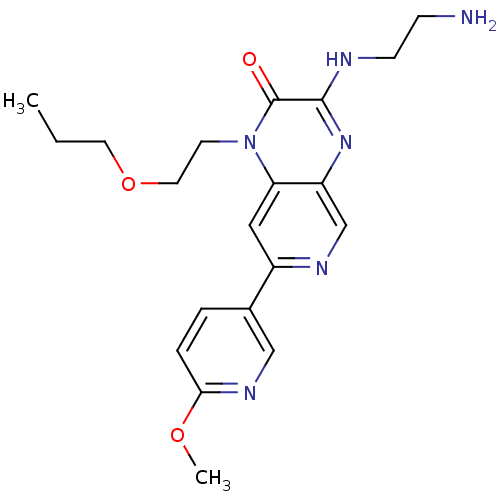
(3-(2-aminoethylamino)-7-(6-methoxypyridin-3-yl)-1-...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCCN)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C20H26N6O3/c1-3-9-29-10-8-26-17-11-15(14-4-5-18(28-2)24-12-14)23-13-16(17)25-19(20(26)27)22-7-6-21/h4-5,11-13H,3,6-10,21H2,1-2H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300957

(3-(2-aminoethylamino)-7-(6-methoxypyridin-3-yl)-1-...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCCN)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C20H26N6O3/c1-3-9-29-10-8-26-17-11-15(14-4-5-18(28-2)24-12-14)23-13-16(17)25-19(20(26)27)22-7-6-21/h4-5,11-13H,3,6-10,21H2,1-2H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assay |
J Med Chem 53: 2656-60 (2010)
Article DOI: 10.1021/jm901781q
BindingDB Entry DOI: 10.7270/Q2VM4CCK |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300987
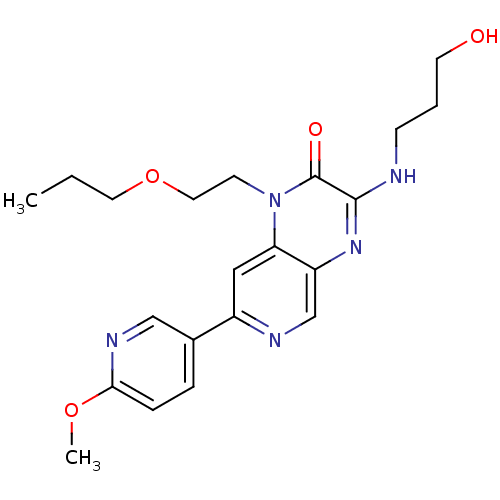
(3-(3-hydroxypropylamino)-7-(6-methoxypyridin-3-yl)...)Show SMILES CCCOCCn1c2cc(ncc2nc(NCCCO)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C21H27N5O4/c1-3-10-30-11-8-26-18-12-16(15-5-6-19(29-2)24-13-15)23-14-17(18)25-20(21(26)28)22-7-4-9-27/h5-6,12-14,27H,3-4,7-11H2,1-2H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50357242
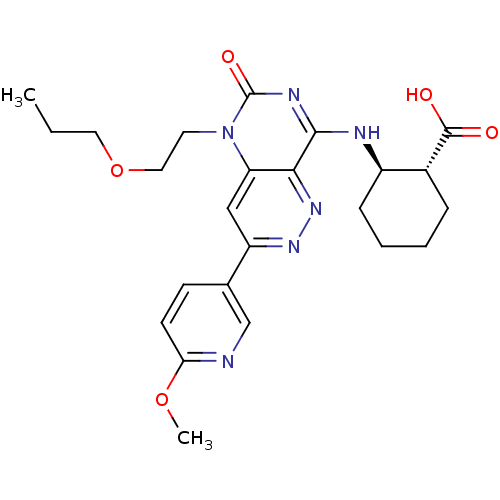
(CHEMBL1916484)Show SMILES CCCOCCn1c2cc(nnc2c(N[C@@H]2CCCC[C@H]2C(O)=O)nc1=O)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C24H30N6O5/c1-3-11-35-12-10-30-19-13-18(15-8-9-20(34-2)25-14-15)28-29-21(19)22(27-24(30)33)26-17-7-5-4-6-16(17)23(31)32/h8-9,13-14,16-17H,3-7,10-12H2,1-2H3,(H,31,32)(H,26,27,33)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 21: 6348-52 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.106
BindingDB Entry DOI: 10.7270/Q2J67HB0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50300971
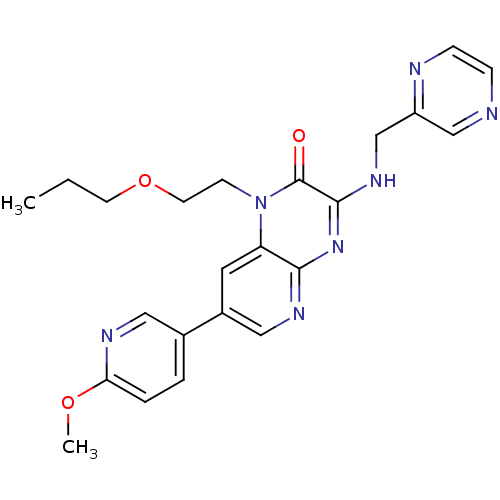
(7-(6-methoxypyridin-3-yl)-1-(2-propoxyethyl)-3-(py...)Show SMILES CCCOCCn1c2cc(cnc2nc(NCc2cnccn2)c1=O)-c1ccc(OC)nc1 Show InChI InChI=1S/C23H25N7O3/c1-3-9-33-10-8-30-19-11-17(16-4-5-20(32-2)26-12-16)13-27-21(19)29-22(23(30)31)28-15-18-14-24-6-7-25-18/h4-7,11-14H,3,8-10,15H2,1-2H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
Bioorg Med Chem Lett 19: 5209-13 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.019
BindingDB Entry DOI: 10.7270/Q2H41RHV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data