

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insulin receptor (Homo sapiens (Human)) | BDBM50256480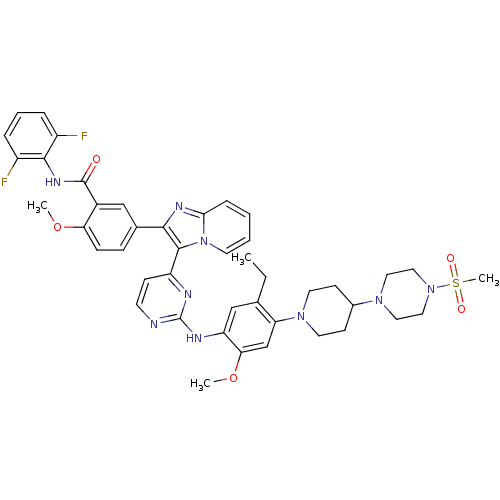 (CHEMBL466397 | N-(2,6-difluorophenyl)-5-(3-(2-(5-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to insulin receptor by liquid scintillation counting | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256480 (CHEMBL466397 | N-(2,6-difluorophenyl)-5-(3-(2-(5-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to IGF1R by liquid scintillation counting | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50256478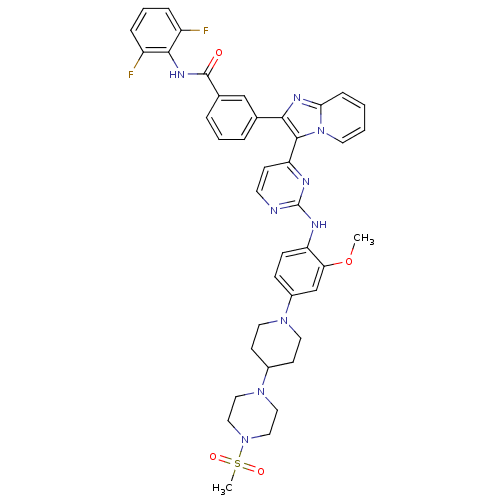 (CHEMBL507714 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to insulin receptor by liquid scintillation counting | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256478 (CHEMBL507714 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to IGF1R by liquid scintillation counting | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21726 (2-Trifluoromethyltetradecanoyl-CoA, 3) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 900 | -35.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21729 (((2R,3S)-3-Fluoro-2-methylhexadecanoyl-CoA, 5) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | -34.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21730 ((2S,3R)-3-fluoro-2-methylhexadecanoyl-CoA, 6) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase VIM-2 (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM153698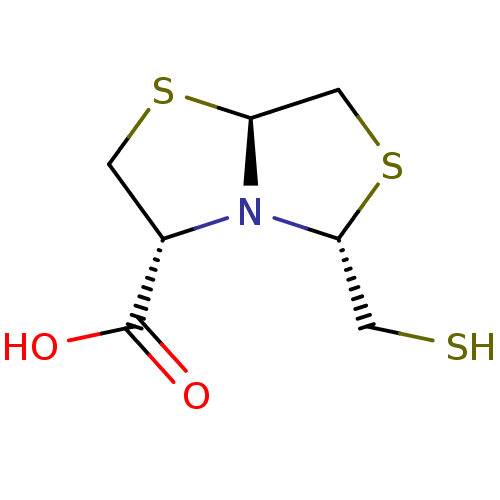 (L-CS319) | PDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metallo-beta-lactamase VIM-2 (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM153700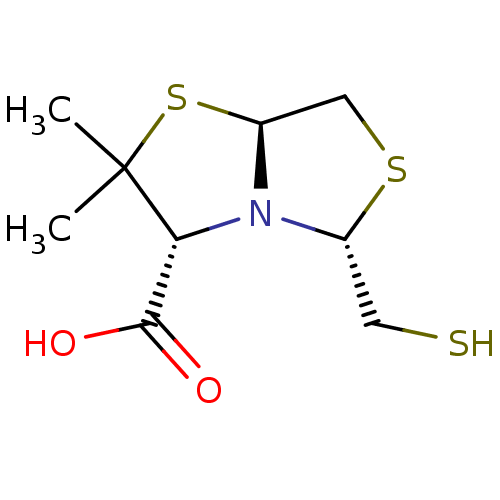 (L-VC26) | PDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21731 (THC-CoA Analogue, 7) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.80E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-24 (Klebsiella pneumoniae (Enterobacteria)) | BDBM153700 (L-VC26) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21732 ((R)-ibuprofenoyl-CoA, 20) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 5.40E+3 | -31.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase VIM-2 (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM153699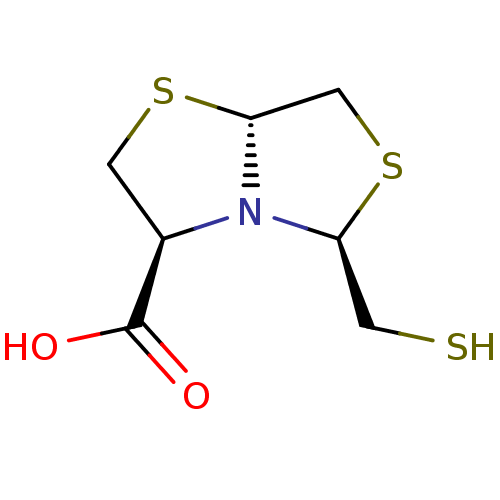 (D-CS319) | PDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-24 (Klebsiella pneumoniae (Enterobacteria)) | BDBM153698 (L-CS319) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| VIM-24 (Klebsiella pneumoniae (Enterobacteria)) | BDBM153699 (D-CS319) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-24 (Klebsiella pneumoniae (Enterobacteria)) | BDBM153701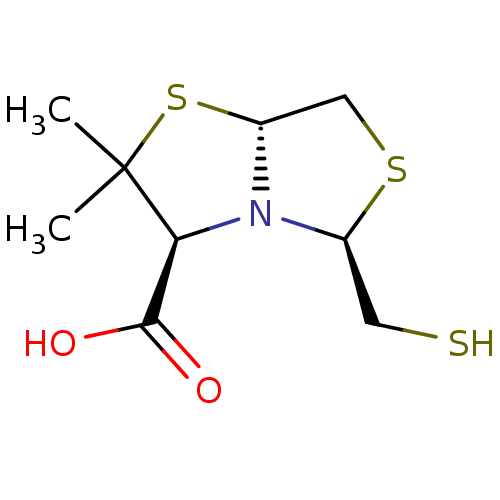 (D-VC26) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase VIM-2 (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM153701 (D-VC26) | PDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21733 ((S)-ibuprofenoyl-CoA, 20) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.92E+4 | -28.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21728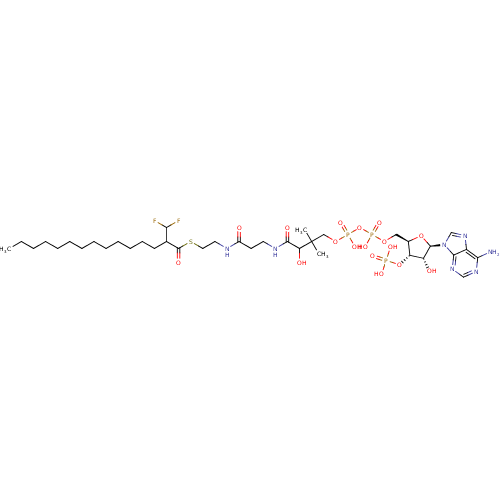 (2-Difluoromethylpentadecanoyl-CoA, 4) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | -27.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21735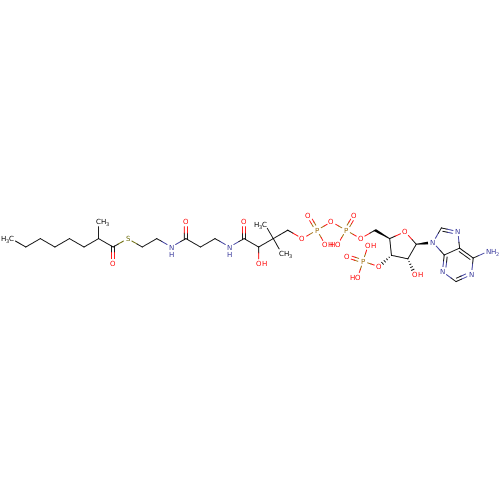 (2-methyloctanoyl CoA, 21 | {[(2R,3S,4R,5R)-5-(6-am...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.50E+4 | -25.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21734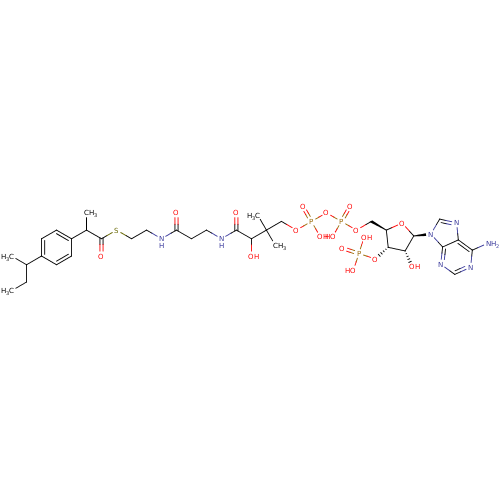 ((Rac)-ibuprofenoyl-CoA, 20 | racemic mixture) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 5.60E+4 | -25.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetyllactosaminide alpha-1,3-galactosyltransferase (Mus musculus) | BDBM50118213 (5'-UTP | CHEMBL336296 | H4utp | UTP | uridine 5'-(...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Kinetic constant for galactosyltransferase inhibition was determined | J Med Chem 30: 1383-91 (1987) BindingDB Entry DOI: 10.7270/Q2HX1D8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21736 (2-methylmyristoyl CoA, 22) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 1.37E+5 | -22.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetyllactosaminide alpha-1,3-galactosyltransferase (Mus musculus) | BDBM50367549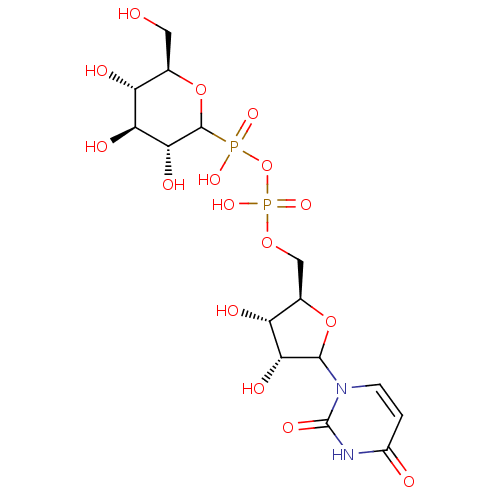 (CHEMBL604428) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Kinetic constant for galactosyltransferase inhibition was determined | J Med Chem 30: 1383-91 (1987) BindingDB Entry DOI: 10.7270/Q2HX1D8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256467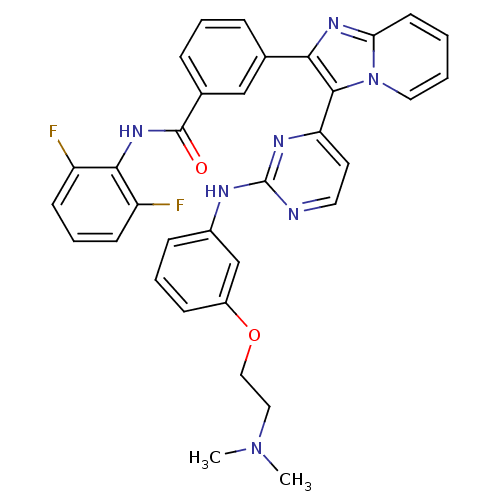 (CHEMBL448668 | N-(2,6-difluorophenyl)-3-(3-(2-(3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256469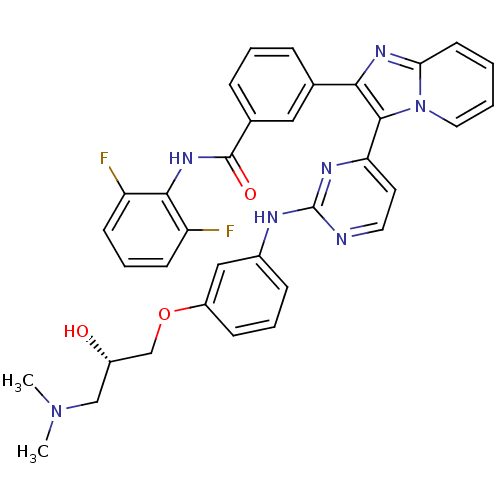 ((S)-N-(2,6-difluorophenyl)-3-(3-(2-(3-(3-(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256474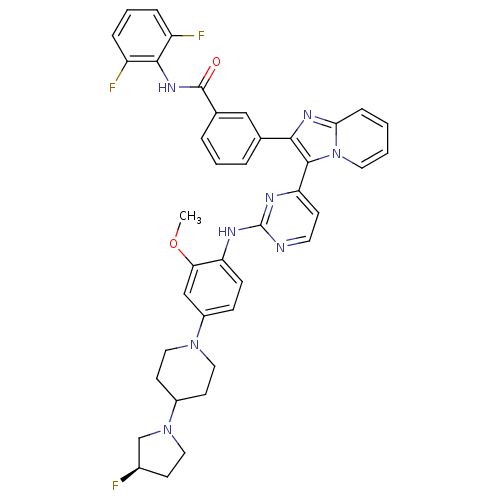 ((R)-N-(2,6-difluorophenyl)-3-(3-(2-(4-(4-(3-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256475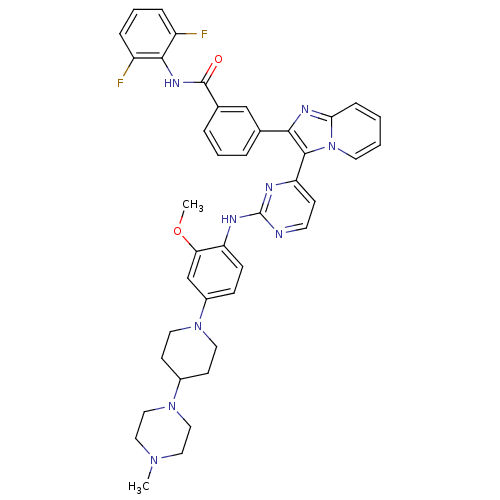 (CHEMBL449110 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256473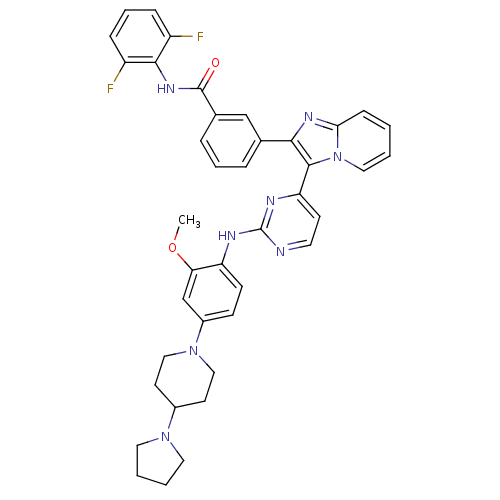 (CHEMBL502198 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256472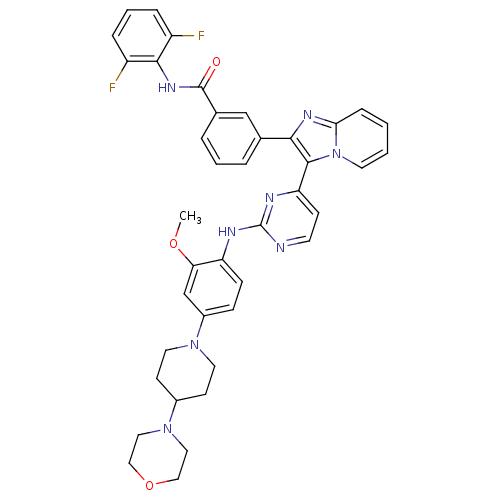 (CHEMBL502652 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256470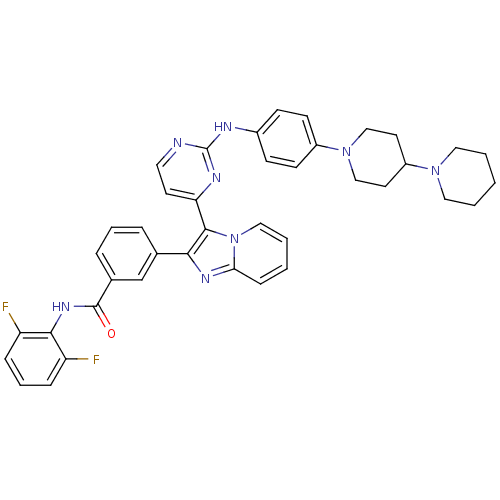 (3-(3-(2-(4-(1,4'-bipiperidin-1'-yl)phenylamino)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256471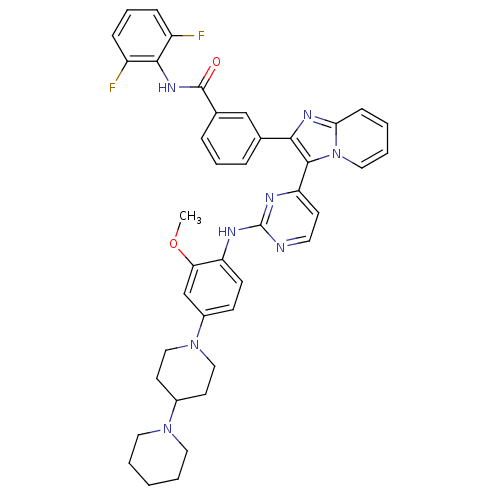 (3-(3-(2-(4-(1,4'-bipiperidin-1'-yl)-2-methoxypheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256410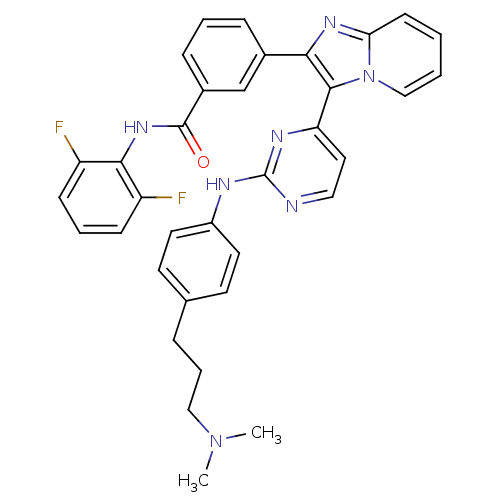 (CHEMBL447668 | N-(2,6-difluorophenyl)-3-(3-(2-(3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544399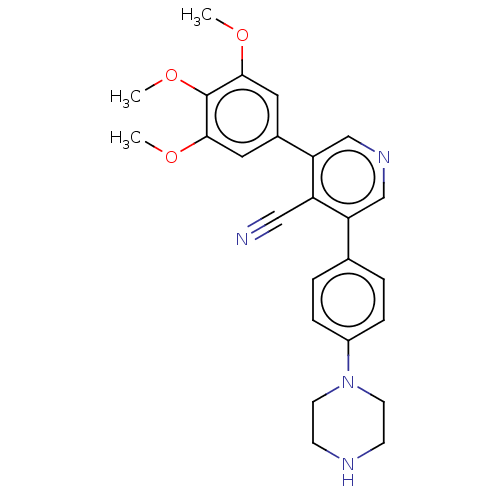 (CHEMBL4638273) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256477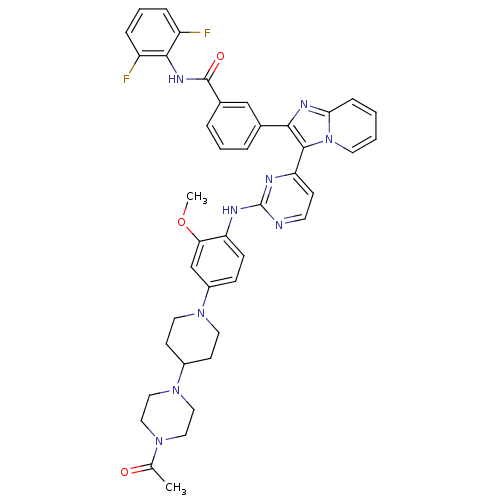 (3-(3-(2-(4-(4-(4-acetylpiperazin-1-yl)piperidin-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256468 (CHEMBL500003 | N-(2,6-difluorophenyl)-3-(3-(2-(4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544408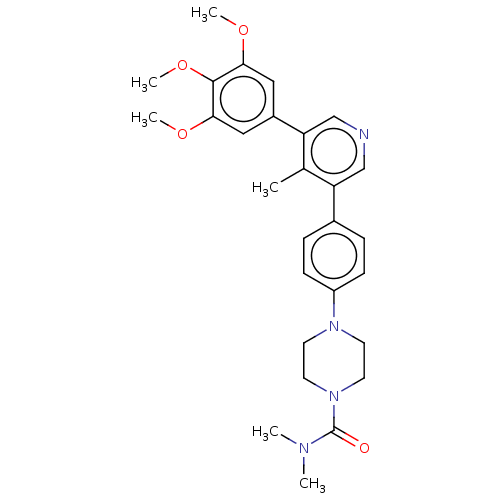 (CHEMBL4641207) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256476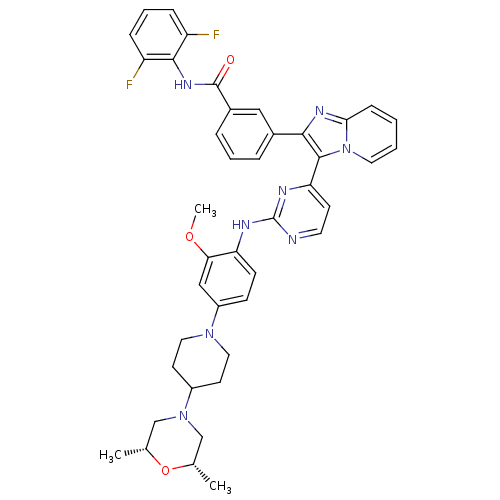 (CHEMBL454796 | N-(2,6-difluorophenyl)-3-(3-(2-(4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50256478 (CHEMBL507714 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged insulin receptor expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544414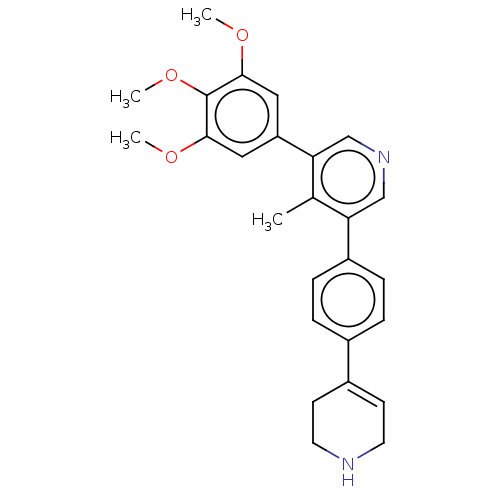 (CHEMBL4641530) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256478 (CHEMBL507714 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544419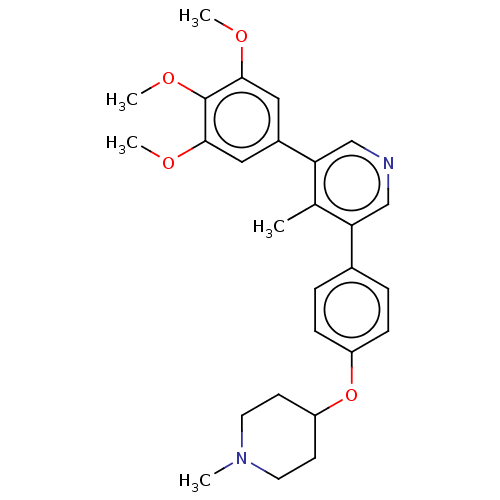 (CHEMBL4647724) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528194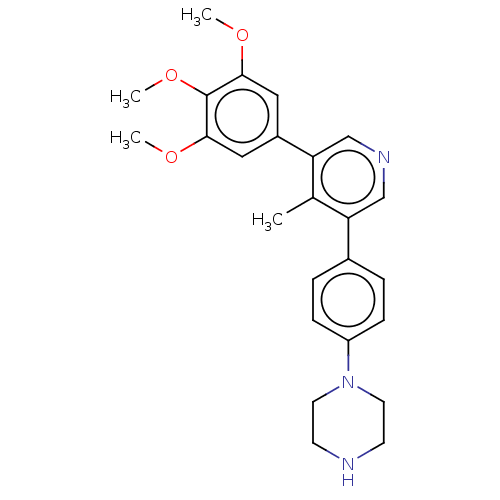 (CHEMBL4517408) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Competitive displacement of PBI-6908 from nanoluciferase-fused ALK2 G328V mutant (unknown origin) expressed in HEK293 cells incubated for 2 hrs by Na... | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544418 (CHEMBL4648102) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256466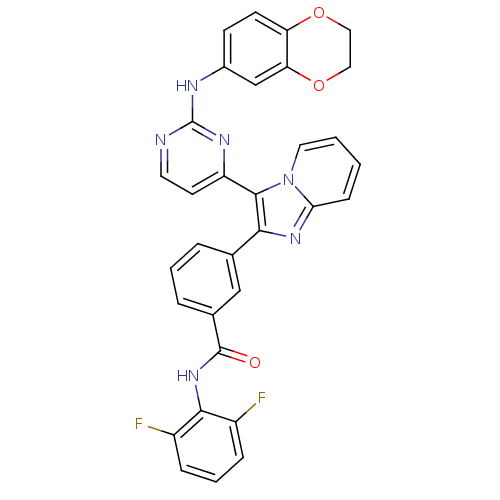 (CHEMBL444031 | N-(2,6-difluorophenyl)-3-(3-(2-(2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50256479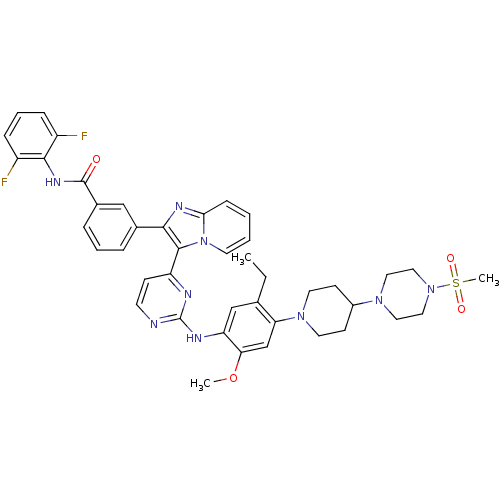 (CHEMBL448929 | N-(2,6-difluorophenyl)-3-(3-(2-(5-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged insulin receptor expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256479 (CHEMBL448929 | N-(2,6-difluorophenyl)-3-(3-(2-(5-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544404 (CHEMBL4644571) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544413 (CHEMBL4633241) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528194 (CHEMBL4517408) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Competitive displacement of PBI-6908 from nanoluciferase-fused ALK2 Q207D mutant (unknown origin) expressed in HEK293 cells incubated for 2 hrs by Na... | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 2146 total ) | Next | Last >> |