

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50214385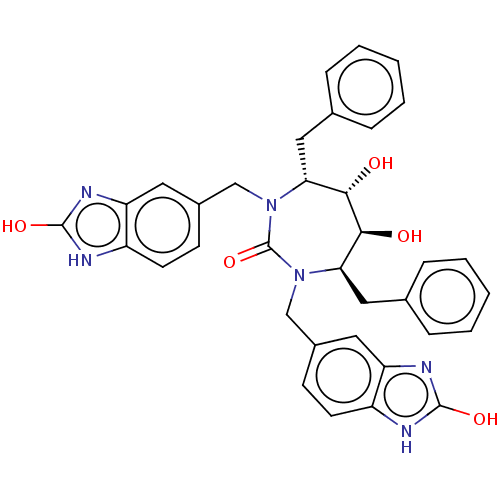 (CHEMBL316681) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against HIV protease | Bioorg Med Chem Lett 6: 2919-2924 (1996) Article DOI: 10.1016/S0960-894X(96)00531-8 BindingDB Entry DOI: 10.7270/Q2QF8SVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073223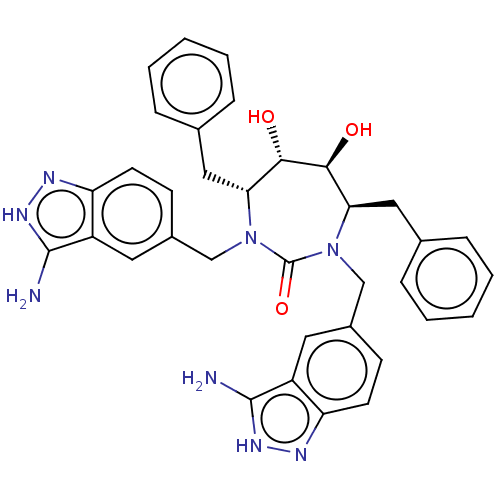 (CHEMBL73240) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of HIV protease | Bioorg Med Chem Lett 8: 715-20 (1999) BindingDB Entry DOI: 10.7270/Q2FQ9VRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50214385 (CHEMBL316681) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of S. cerevisiae glyoxalase-I by using enzymatic assay at each of 6 substrate concentrations between 0.1 mM and... | Bioorg Med Chem Lett 8: 715-20 (1999) BindingDB Entry DOI: 10.7270/Q2FQ9VRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073223 (CHEMBL73240) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV protease | Bioorg Med Chem Lett 9: 2259-62 (1999) BindingDB Entry DOI: 10.7270/Q20864H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50080117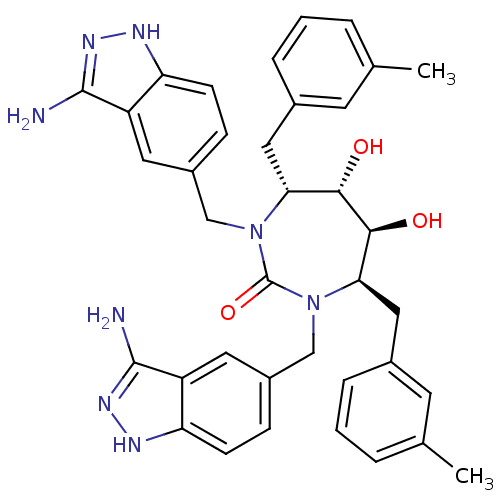 ((4R,5S,6S,7R)-1,3-Bis-(3-amino-1H-indazol-5-ylmeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV protease | Bioorg Med Chem Lett 9: 2259-62 (1999) BindingDB Entry DOI: 10.7270/Q20864H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7084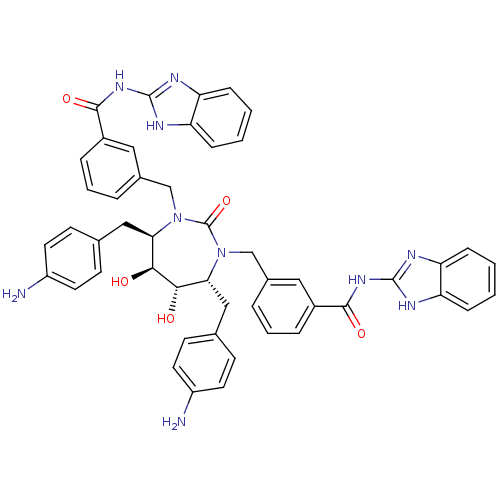 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-4,7-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | -64.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069201 ((4R,5S,6S,7R)-5,6-Dihydroxy-4,7-bis-(4-hydroxy-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description HIV protease inhibition. | Bioorg Med Chem Lett 8: 823-8 (1999) BindingDB Entry DOI: 10.7270/Q2DR2TMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM161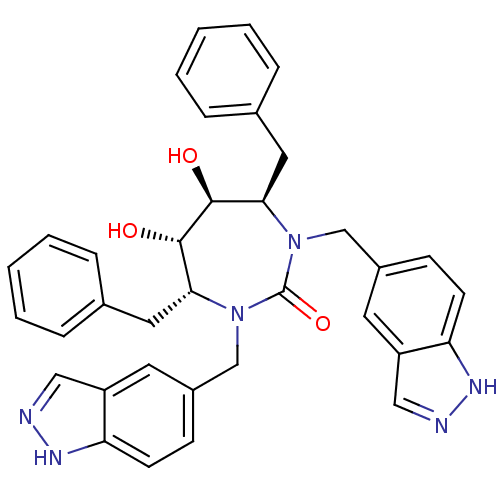 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against HIV protease | Bioorg Med Chem Lett 6: 2919-2924 (1996) Article DOI: 10.1016/S0960-894X(96)00531-8 BindingDB Entry DOI: 10.7270/Q2QF8SVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069033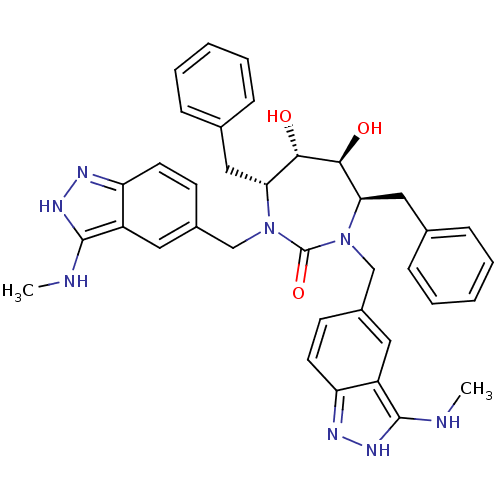 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1,3-bis-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of HIV protease | Bioorg Med Chem Lett 8: 715-20 (1999) BindingDB Entry DOI: 10.7270/Q2FQ9VRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM161 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of HIV protease | Bioorg Med Chem Lett 8: 715-20 (1999) BindingDB Entry DOI: 10.7270/Q2FQ9VRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288430 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1,3-bis-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against HIV protease | Bioorg Med Chem Lett 6: 2919-2924 (1996) Article DOI: 10.1016/S0960-894X(96)00531-8 BindingDB Entry DOI: 10.7270/Q2QF8SVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124714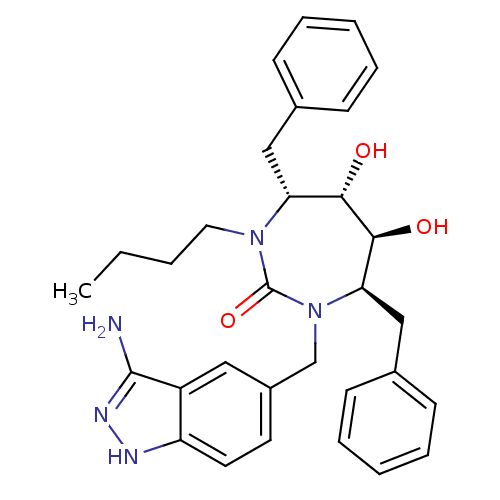 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-4,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM155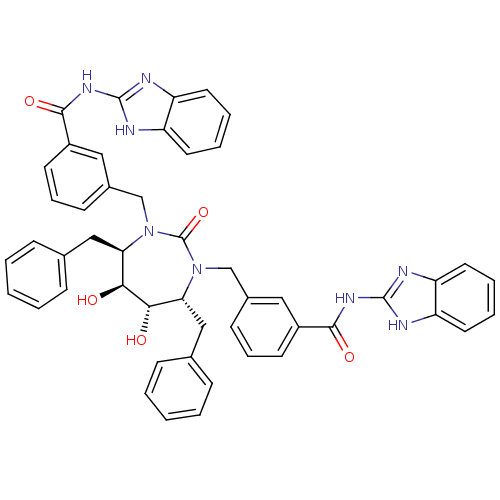 (CHEMBL11266 | N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0240 | -63.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7088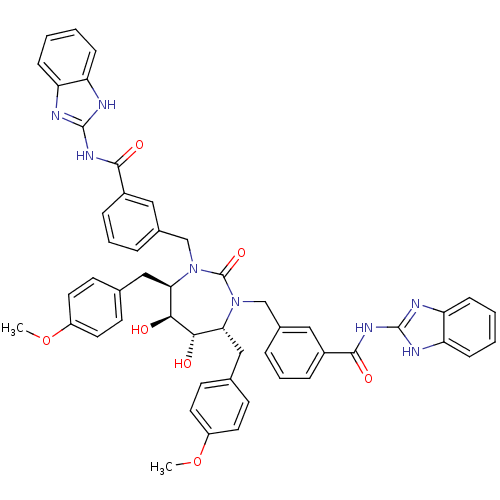 (SD146 Analog 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0260 | -62.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM182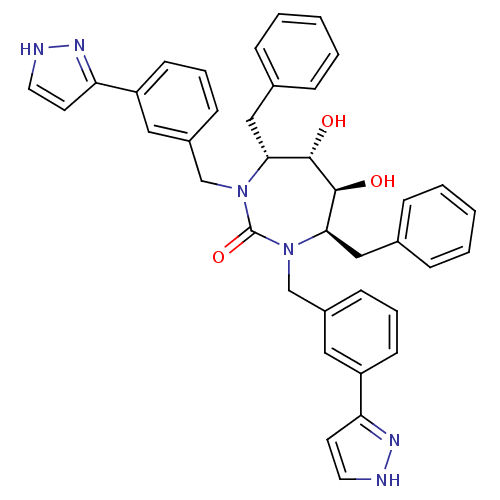 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description HIV protease inhibition. | Bioorg Med Chem Lett 8: 823-8 (1999) BindingDB Entry DOI: 10.7270/Q2DR2TMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065064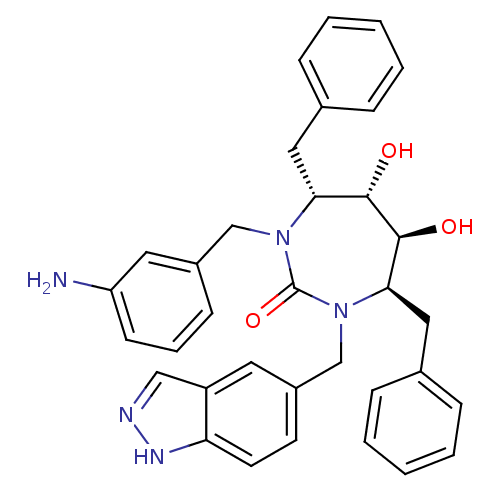 ((4R,5S,6S,7R)-1-(3-Amino-benzyl)-4,7-dibenzyl-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV -1 Protease | Bioorg Med Chem Lett 9: 3217-20 (1999) BindingDB Entry DOI: 10.7270/Q2B27TG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124721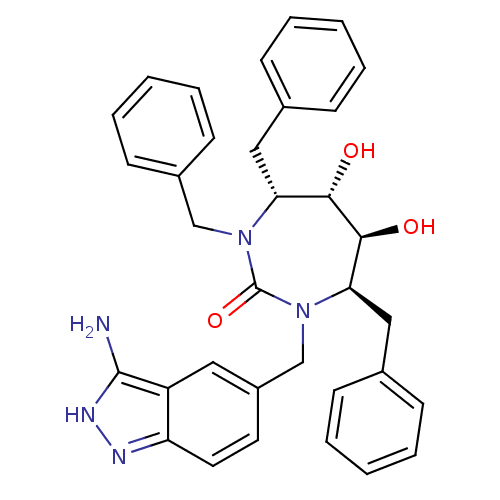 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50080108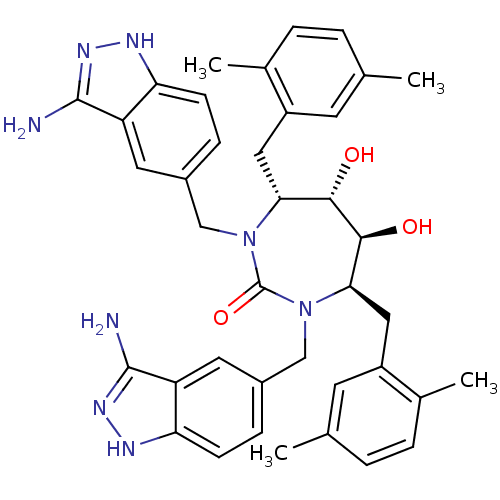 ((4R,5S,6S,7R)-1,3-Bis-(3-amino-1H-indazol-5-ylmeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV protease | Bioorg Med Chem Lett 9: 2259-62 (1999) BindingDB Entry DOI: 10.7270/Q20864H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7086 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-3-{[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0350 | -62.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124716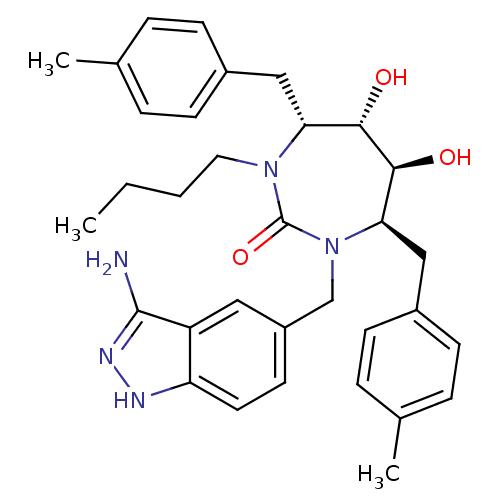 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7085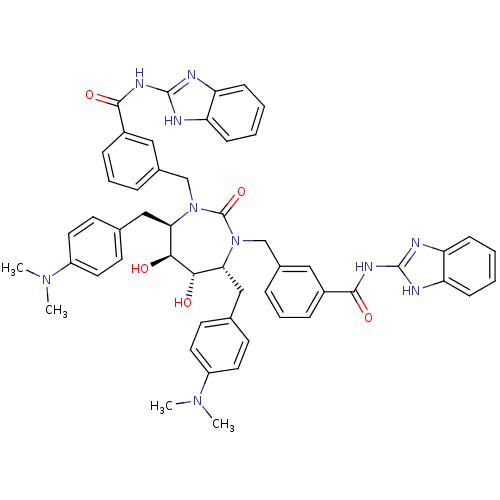 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-3-{[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0380 | -61.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7090 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-3-{[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0380 | -61.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069029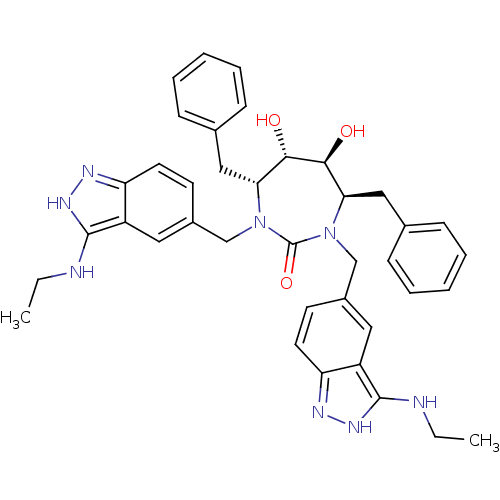 ((4R,5S,6S,7R)-4,7-Dibenzyl-1,3-bis-(3-ethylamino-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of HIV protease | Bioorg Med Chem Lett 8: 715-20 (1999) BindingDB Entry DOI: 10.7270/Q2FQ9VRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124715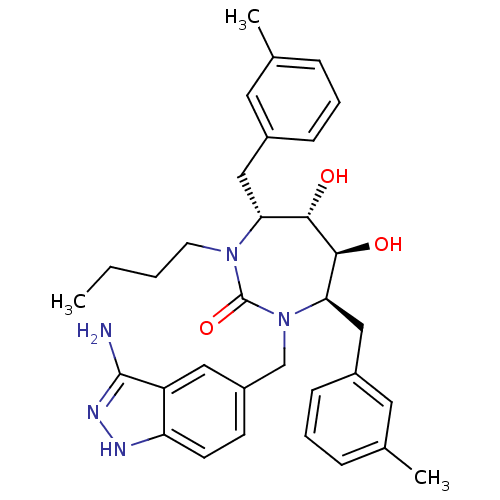 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7091 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-3-{[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0460 | -61.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124722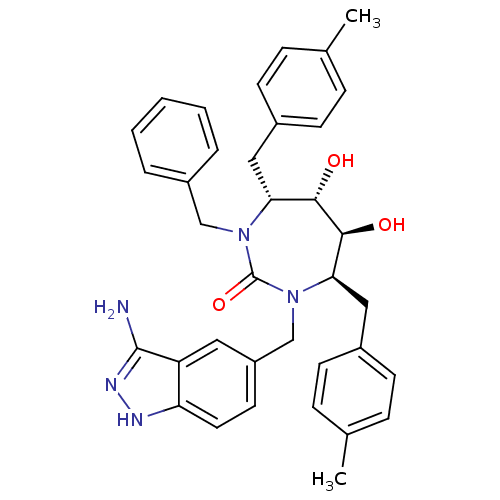 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50082401 ((4R,5S,6S,7R)-1-(3-Amino-benzyl)-4,7-dibenzyl-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV -1 Protease | Bioorg Med Chem Lett 9: 3217-20 (1999) BindingDB Entry DOI: 10.7270/Q2B27TG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069204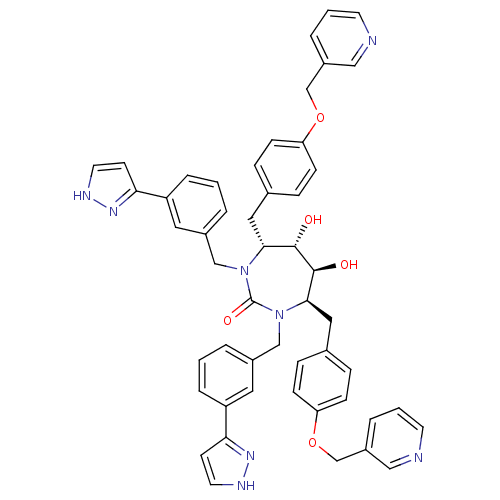 ((4R,5S,6S,7R)-5,6-Dihydroxy-1,3-bis-[3-(1H-pyrazol...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description HIV protease inhibition. | Bioorg Med Chem Lett 8: 823-8 (1999) BindingDB Entry DOI: 10.7270/Q2DR2TMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069199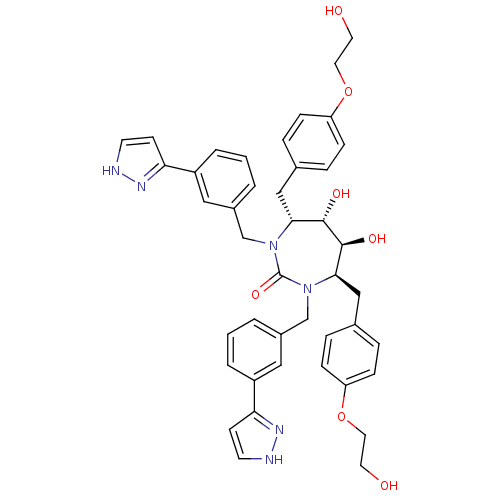 ((4R,5S,6S,7R)-5,6-Dihydroxy-4,7-bis-[4-(2-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description HIV protease inhibition. | Bioorg Med Chem Lett 8: 823-8 (1999) BindingDB Entry DOI: 10.7270/Q2DR2TMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069197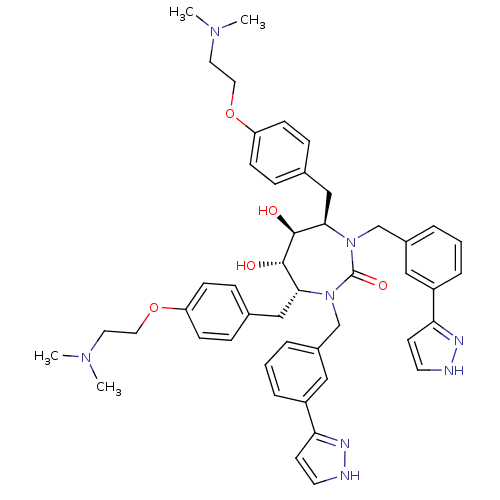 ((4R,5S,6S,7R)-4,7-Bis-[4-(2-dimethylamino-ethoxy)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description HIV protease inhibition. | Bioorg Med Chem Lett 8: 823-8 (1999) BindingDB Entry DOI: 10.7270/Q2DR2TMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50080110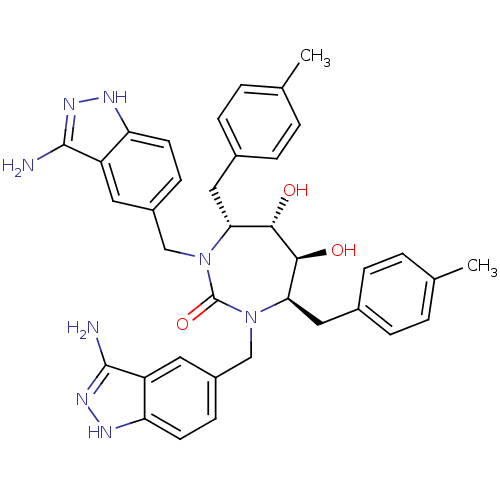 ((4R,5S,6S,7R)-1,3-Bis-(3-amino-1H-indazol-5-ylmeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV protease | Bioorg Med Chem Lett 9: 2259-62 (1999) BindingDB Entry DOI: 10.7270/Q20864H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124718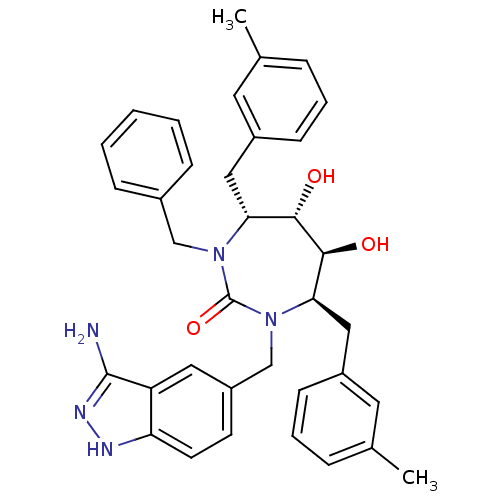 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50080112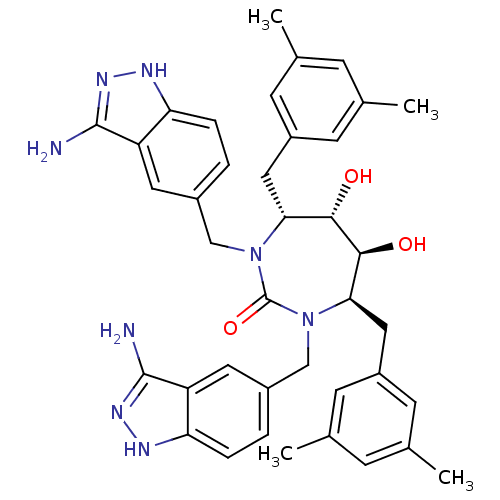 ((4R,5S,6S,7R)-1,3-Bis-(3-amino-1H-indazol-5-ylmeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV protease | Bioorg Med Chem Lett 9: 2259-62 (1999) BindingDB Entry DOI: 10.7270/Q20864H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50286123 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1,3-bis-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibitory activity against HIV protease | Bioorg Med Chem Lett 5: 3027-3032 (1995) Article DOI: 10.1016/0960-894X(95)00520-8 BindingDB Entry DOI: 10.7270/Q2R49QQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50080113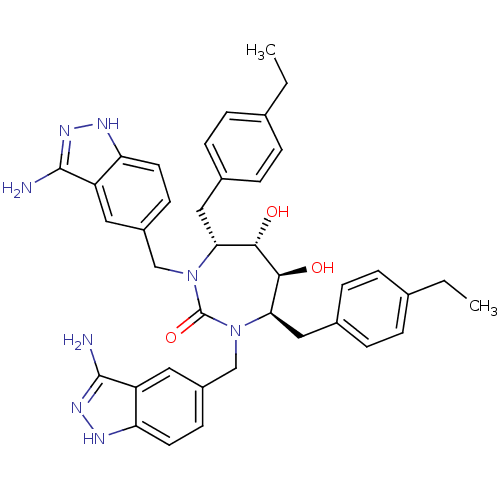 ((4R,5S,6S,7R)-1,3-Bis-(3-amino-1H-indazol-5-ylmeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV protease | Bioorg Med Chem Lett 9: 2259-62 (1999) BindingDB Entry DOI: 10.7270/Q20864H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50080111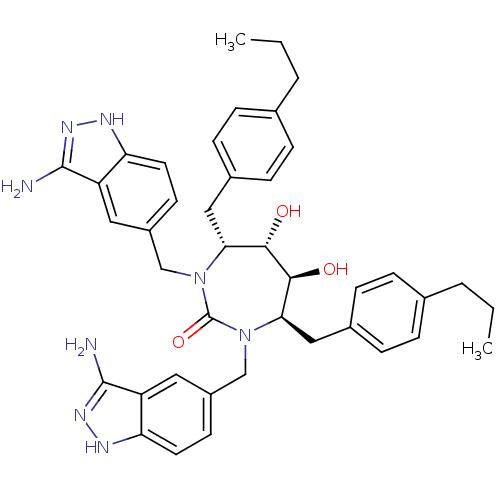 ((4R,5S,6S,7R)-1,3-Bis-(3-amino-1H-indazol-5-ylmeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV protease | Bioorg Med Chem Lett 9: 2259-62 (1999) BindingDB Entry DOI: 10.7270/Q20864H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7089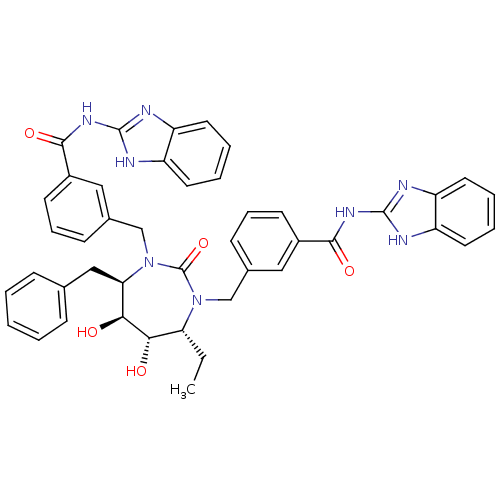 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-3-{[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0690 | -60.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069196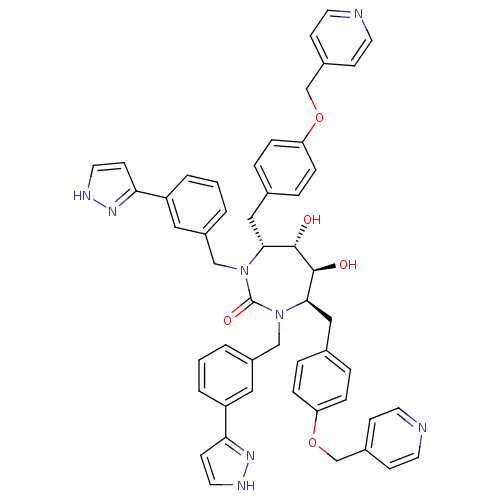 ((4R,5S,6S,7R)-5,6-Dihydroxy-1,3-bis-[3-(1H-pyrazol...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description HIV protease inhibition. | Bioorg Med Chem Lett 8: 823-8 (1999) BindingDB Entry DOI: 10.7270/Q2DR2TMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124723 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069203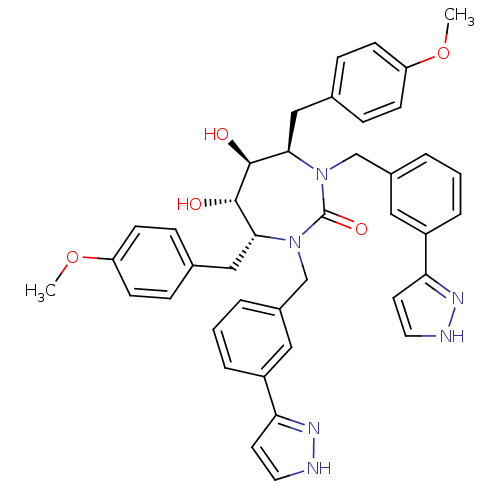 ((4R,5S,6S,7R)-5,6-Dihydroxy-4,7-bis-(4-methoxy-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description HIV protease inhibition. | Bioorg Med Chem Lett 8: 823-8 (1999) BindingDB Entry DOI: 10.7270/Q2DR2TMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069030 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1,3-bis-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of HIV protease | Bioorg Med Chem Lett 8: 715-20 (1999) BindingDB Entry DOI: 10.7270/Q2FQ9VRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288424 ((4R,5S,6S,7R)-1,3-Bis-(1H-benzotriazol-5-ylmethyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against HIV protease | Bioorg Med Chem Lett 6: 2919-2924 (1996) Article DOI: 10.1016/S0960-894X(96)00531-8 BindingDB Entry DOI: 10.7270/Q2QF8SVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50082400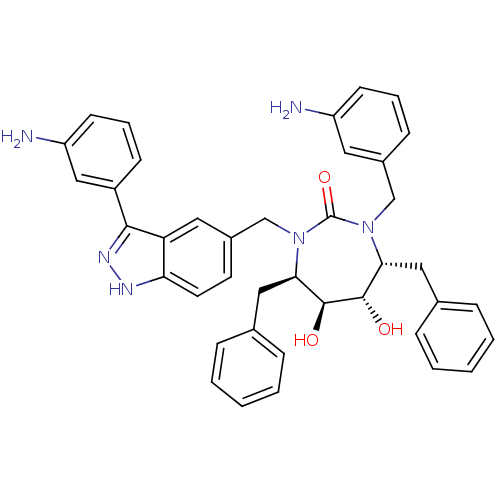 ((4R,5S,6S,7R)-1-(3-Amino-benzyl)-3-[3-(3-amino-phe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV -1 Protease | Bioorg Med Chem Lett 9: 3217-20 (1999) BindingDB Entry DOI: 10.7270/Q2B27TG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50124717 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity of the compound against HIV-Protease was determined | Bioorg Med Chem Lett 13: 605-8 (2003) BindingDB Entry DOI: 10.7270/Q27D2THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7075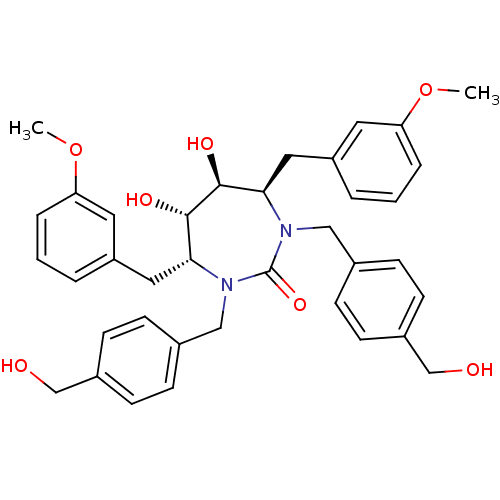 ((4R,5S,6S,7R)-5,6-dihydroxy-1,3-bis({[4-(hydroxyme...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.110 | -59.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069206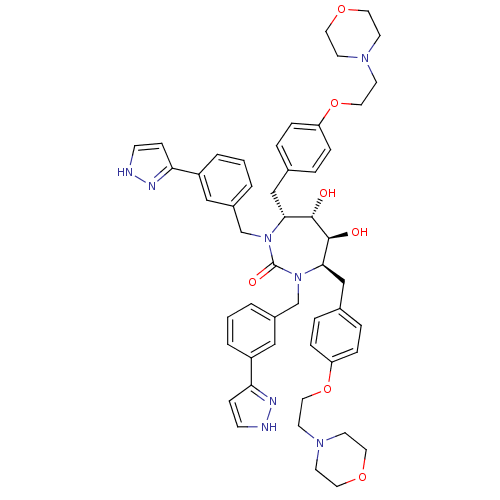 ((4R,5S,6S,7R)-5,6-Dihydroxy-4,7-bis-[4-(2-morpholi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description HIV protease inhibition. | Bioorg Med Chem Lett 8: 823-8 (1999) BindingDB Entry DOI: 10.7270/Q2DR2TMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50082397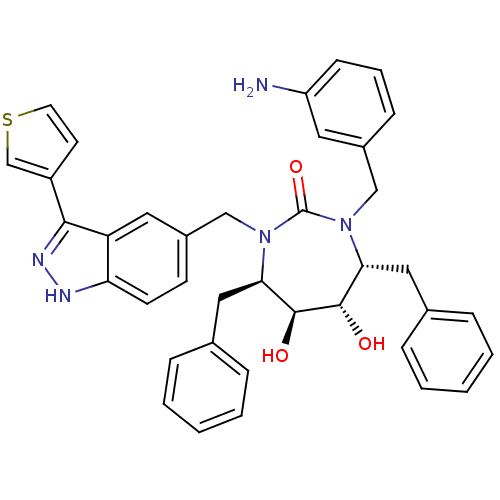 ((4R,5S,6S,7R)-1-(3-Amino-benzyl)-4,7-dibenzyl-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV -1 Protease | Bioorg Med Chem Lett 9: 3217-20 (1999) BindingDB Entry DOI: 10.7270/Q2B27TG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288420 (5-[4,7-dibenzyl-5,6-dihydroxy-2-oxo-3-(2-oxo-2,3-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against HIV protease | Bioorg Med Chem Lett 6: 2919-2924 (1996) Article DOI: 10.1016/S0960-894X(96)00531-8 BindingDB Entry DOI: 10.7270/Q2QF8SVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50080115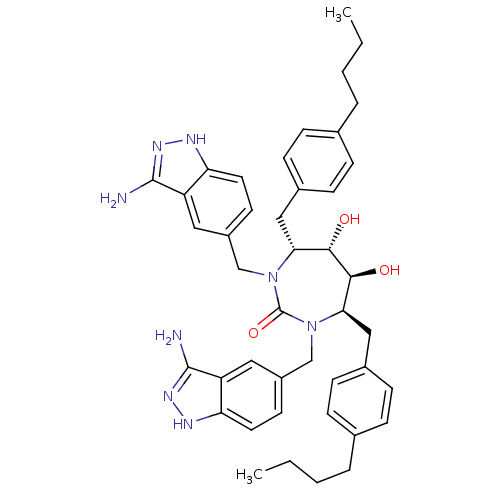 ((4R,5S,6S,7R)-1,3-Bis-(3-amino-1H-indazol-5-ylmeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV protease | Bioorg Med Chem Lett 9: 2259-62 (1999) BindingDB Entry DOI: 10.7270/Q20864H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50082403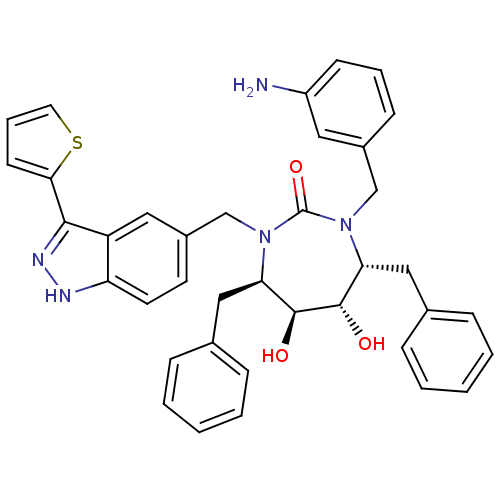 ((4R,5S,6S,7R)-1-(3-Amino-benzyl)-4,7-dibenzyl-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for HIV -1 Protease | Bioorg Med Chem Lett 9: 3217-20 (1999) BindingDB Entry DOI: 10.7270/Q2B27TG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 297 total ) | Next | Last >> |