 Found 471 hits with Last Name = 'brown' and Initial = 'bs'
Found 471 hits with Last Name = 'brown' and Initial = 'bs' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85530
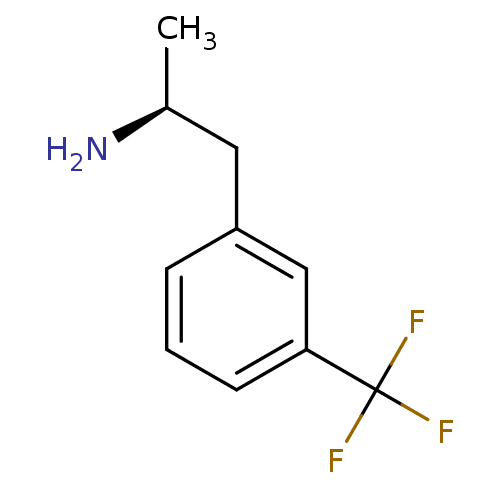
(Nor-d-fenfluramine | Nor-dexfenfluramine | Norfenf...)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85530

(Nor-d-fenfluramine | Nor-dexfenfluramine | Norfenf...)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85598
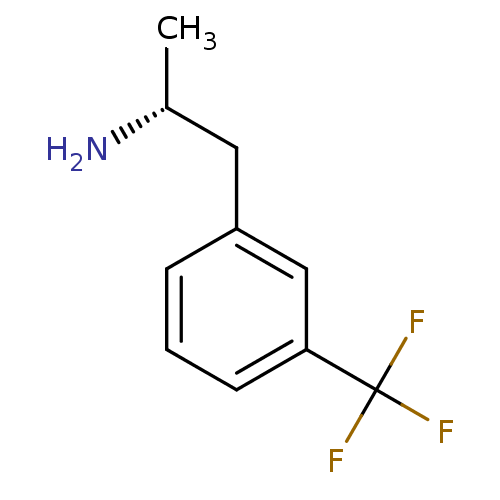
(l-norfenfluramine)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85598

(l-norfenfluramine)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85530

(Nor-d-fenfluramine | Nor-dexfenfluramine | Norfenf...)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85598

(l-norfenfluramine)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85597
(CAS_37577-24-5 | NSC_65801 | l-Fenfluramine)Show InChI InChI=1S/C12H16F3N/c1-3-16-9(2)7-10-5-4-6-11(8-10)12(13,14)15/h4-6,8-9,16H,3,7H2,1-2H3/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85597

(CAS_37577-24-5 | NSC_65801 | l-Fenfluramine)Show InChI InChI=1S/C12H16F3N/c1-3-16-9(2)7-10-5-4-6-11(8-10)12(13,14)15/h4-6,8-9,16H,3,7H2,1-2H3/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85597

(CAS_37577-24-5 | NSC_65801 | l-Fenfluramine)Show InChI InChI=1S/C12H16F3N/c1-3-16-9(2)7-10-5-4-6-11(8-10)12(13,14)15/h4-6,8-9,16H,3,7H2,1-2H3/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85596

(CAS_3239-45-0 | NSC_65801 | d-Fenfluramine)Show InChI InChI=1S/C12H16F3N/c1-3-16-9(2)7-10-5-4-6-11(8-10)12(13,14)15/h4-6,8-9,16H,3,7H2,1-2H3/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85596

(CAS_3239-45-0 | NSC_65801 | d-Fenfluramine)Show InChI InChI=1S/C12H16F3N/c1-3-16-9(2)7-10-5-4-6-11(8-10)12(13,14)15/h4-6,8-9,16H,3,7H2,1-2H3/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50506948
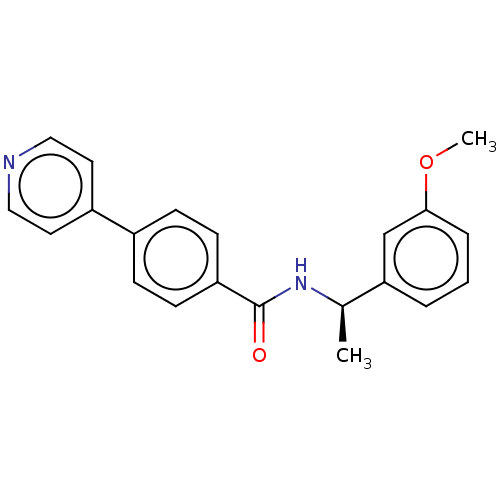
(CHEMBL4448806)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccncc1 |r| Show InChI InChI=1S/C21H20N2O2/c1-15(19-4-3-5-20(14-19)25-2)23-21(24)18-8-6-16(7-9-18)17-10-12-22-13-11-17/h3-15H,1-2H3,(H,23,24)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) incubated for 2 hrs by AbbVie kinase panel assay |
J Med Chem 61: 11074-11100 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01098
BindingDB Entry DOI: 10.7270/Q2RB77XJ |
More data for this
Ligand-Target Pair | |
cGMP-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50506948

(CHEMBL4448806)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccncc1 |r| Show InChI InChI=1S/C21H20N2O2/c1-15(19-4-3-5-20(14-19)25-2)23-21(24)18-8-6-16(7-9-18)17-10-12-22-13-11-17/h3-15H,1-2H3,(H,23,24)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of PKG1A (unknown origin) incubated for 2 hrs by AbbVie kinase panel assay |
J Med Chem 61: 11074-11100 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01098
BindingDB Entry DOI: 10.7270/Q2RB77XJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50506946

(CHEMBL4472858)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccncc1F |r| Show InChI InChI=1S/C21H19FN2O2/c1-14(17-4-3-5-18(12-17)26-2)24-21(25)16-8-6-15(7-9-16)19-10-11-23-13-20(19)22/h3-14H,1-2H3,(H,24,25)/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... |
J Med Chem 61: 11074-11100 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01098
BindingDB Entry DOI: 10.7270/Q2RB77XJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50506948

(CHEMBL4448806)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccncc1 |r| Show InChI InChI=1S/C21H20N2O2/c1-15(19-4-3-5-20(14-19)25-2)23-21(24)18-8-6-16(7-9-18)17-10-12-22-13-11-17/h3-15H,1-2H3,(H,23,24)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (unknown origin) incubated for 2 hrs by AbbVie kinase panel assay |
J Med Chem 61: 11074-11100 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01098
BindingDB Entry DOI: 10.7270/Q2RB77XJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50506950
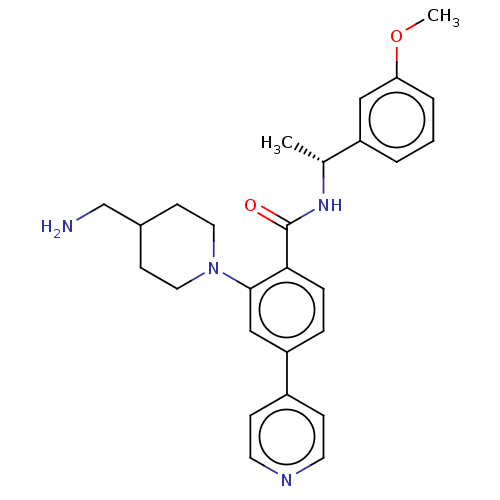
(CHEMBL4434688)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc(cc1N1CCC(CN)CC1)-c1ccncc1 |r| Show InChI InChI=1S/C27H32N4O2/c1-19(22-4-3-5-24(16-22)33-2)30-27(32)25-7-6-23(21-8-12-29-13-9-21)17-26(25)31-14-10-20(18-28)11-15-31/h3-9,12-13,16-17,19-20H,10-11,14-15,18,28H2,1-2H3,(H,30,32)/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... |
J Med Chem 61: 11074-11100 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01098
BindingDB Entry DOI: 10.7270/Q2RB77XJ |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306157
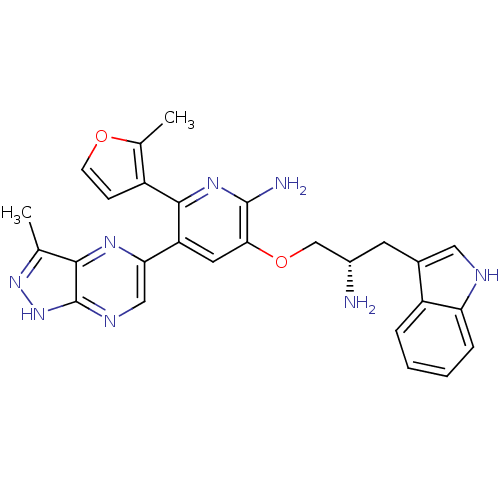
(3-((S)-2-amino-3-(1H-indol-3-yl)propoxy)-5-(3-meth...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C27H26N8O2/c1-14-24-27(35-34-14)31-12-22(32-24)20-10-23(26(29)33-25(20)18-7-8-36-15(18)2)37-13-17(28)9-16-11-30-21-6-4-3-5-19(16)21/h3-8,10-12,17,30H,9,13,28H2,1-2H3,(H2,29,33)(H,31,34,35)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306158
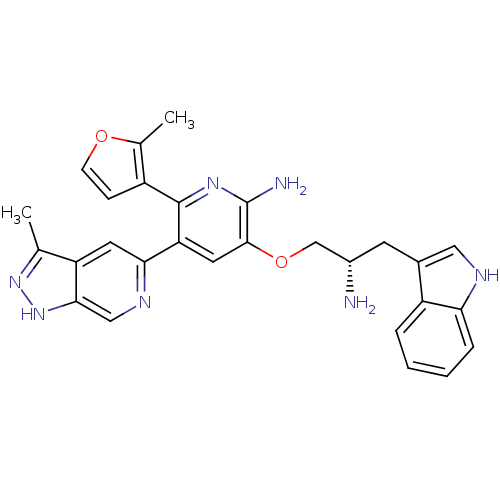
(3-((S)-2-amino-3-(1H-indol-3-yl)propoxy)-5-(3-meth...)Show SMILES Cc1n[nH]c2cnc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C28H27N7O2/c1-15-21-10-24(32-13-25(21)35-34-15)22-11-26(28(30)33-27(22)19-7-8-36-16(19)2)37-14-18(29)9-17-12-31-23-6-4-3-5-20(17)23/h3-8,10-13,18,31H,9,14,29H2,1-2H3,(H2,30,33)(H,34,35)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306163
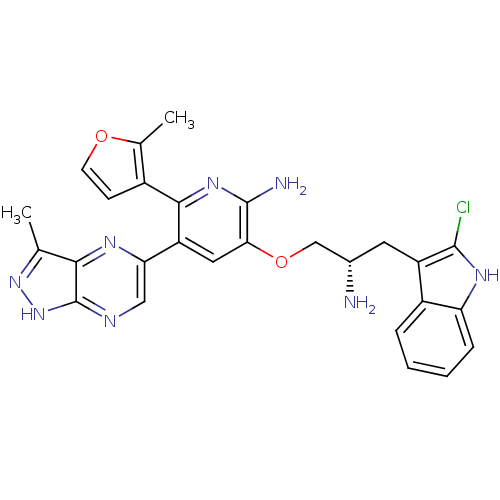
(3-((S)-2-amino-3-(2-chloro-1H-indol-3-yl)propoxy)-...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c(Cl)[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C27H25ClN8O2/c1-13-23-27(36-35-13)31-11-21(32-23)19-10-22(26(30)34-24(19)16-7-8-37-14(16)2)38-12-15(29)9-18-17-5-3-4-6-20(17)33-25(18)28/h3-8,10-11,15,33H,9,12,29H2,1-2H3,(H2,30,34)(H,31,35,36)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306164
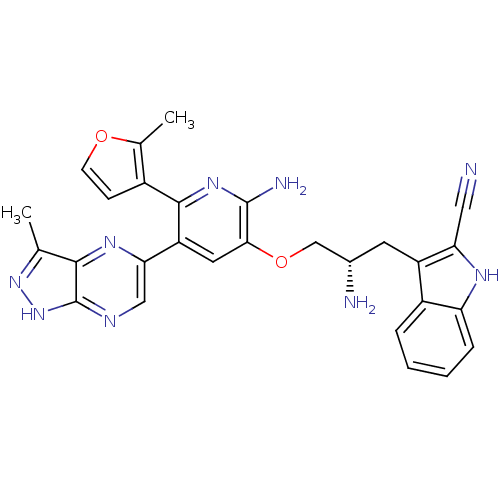
(3-((2S)-2-amino-3-(2-amino-5-(3-methyl-1H-pyrazolo...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c([nH]c3ccccc23)C#N)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C28H25N9O2/c1-14-25-28(37-36-14)32-12-23(34-25)20-10-24(27(31)35-26(20)17-7-8-38-15(17)2)39-13-16(30)9-19-18-5-3-4-6-21(18)33-22(19)11-29/h3-8,10,12,16,33H,9,13,30H2,1-2H3,(H2,31,35)(H,32,36,37)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306164

(3-((2S)-2-amino-3-(2-amino-5-(3-methyl-1H-pyrazolo...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c([nH]c3ccccc23)C#N)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C28H25N9O2/c1-14-25-28(37-36-14)32-12-23(34-25)20-10-24(27(31)35-26(20)17-7-8-38-15(17)2)39-13-16(30)9-19-18-5-3-4-6-21(18)33-22(19)11-29/h3-8,10,12,16,33H,9,13,30H2,1-2H3,(H2,31,35)(H,32,36,37)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306165
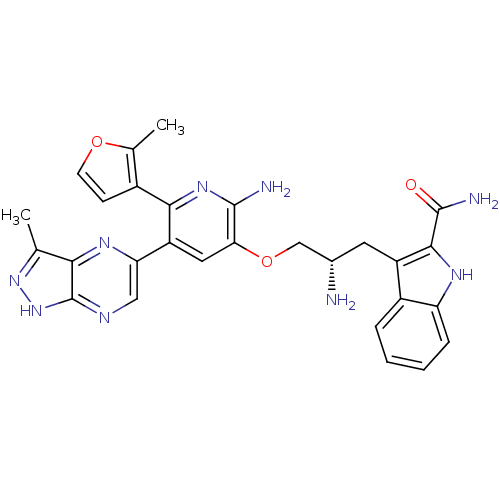
(3-((2S)-2-amino-3-(2-amino-5-(3-methyl-1H-pyrazolo...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c([nH]c3ccccc23)C(N)=O)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C28H27N9O3/c1-13-23-28(37-36-13)32-11-21(34-23)19-10-22(26(30)35-24(19)16-7-8-39-14(16)2)40-12-15(29)9-18-17-5-3-4-6-20(17)33-25(18)27(31)38/h3-8,10-11,15,33H,9,12,29H2,1-2H3,(H2,30,35)(H2,31,38)(H,32,36,37)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306165

(3-((2S)-2-amino-3-(2-amino-5-(3-methyl-1H-pyrazolo...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c([nH]c3ccccc23)C(N)=O)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C28H27N9O3/c1-13-23-28(37-36-13)32-11-21(34-23)19-10-22(26(30)35-24(19)16-7-8-39-14(16)2)40-12-15(29)9-18-17-5-3-4-6-20(17)33-25(18)27(31)38/h3-8,10-11,15,33H,9,12,29H2,1-2H3,(H2,30,35)(H2,31,38)(H,32,36,37)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306154

((2S)-1-(1H-indol-3-yl)-3-(5-(3-methyl-1H-pyrazolo[...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)cnc1-c1ccoc1C |r| Show InChI InChI=1S/C27H25N7O2/c1-15-25-27(34-33-15)31-13-24(32-25)22-10-19(12-30-26(22)20-7-8-35-16(20)2)36-14-18(28)9-17-11-29-23-6-4-3-5-21(17)23/h3-8,10-13,18,29H,9,14,28H2,1-2H3,(H,31,33,34)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306155
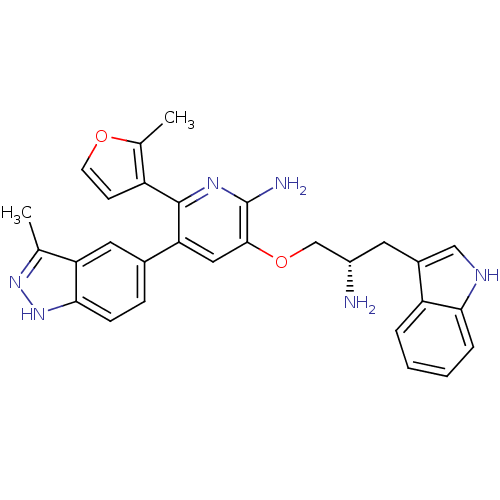
(3-((S)-2-amino-3-(1H-indol-3-yl)propoxy)-5-(3-meth...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C29H28N6O2/c1-16-23-12-18(7-8-26(23)35-34-16)24-13-27(29(31)33-28(24)21-9-10-36-17(21)2)37-15-20(30)11-19-14-32-25-6-4-3-5-22(19)25/h3-10,12-14,20,32H,11,15,30H2,1-2H3,(H2,31,33)(H,34,35)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306156
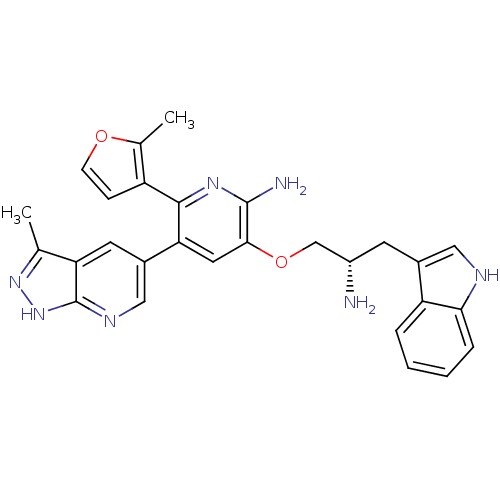
(3-((S)-2-amino-3-(1H-indol-3-yl)propoxy)-5-(3-meth...)Show SMILES Cc1n[nH]c2ncc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C28H27N7O2/c1-15-22-10-18(13-32-28(22)35-34-15)23-11-25(27(30)33-26(23)20-7-8-36-16(20)2)37-14-19(29)9-17-12-31-24-6-4-3-5-21(17)24/h3-8,10-13,19,31H,9,14,29H2,1-2H3,(H2,30,33)(H,32,34,35)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50467984

(CHEMBL4293287)Show SMILES COc1cc2nccc(Nc3n[nH]c(C)c3C)c2cc1S(=O)(=O)C(C)(C)C Show InChI InChI=1S/C19H24N4O3S/c1-11-12(2)22-23-18(11)21-14-7-8-20-15-10-16(26-6)17(9-13(14)15)27(24,25)19(3,4)5/h7-10H,1-6H3,(H2,20,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full length His/flag-tagged RIPK2 (unknown origin) expressed in baculovirus expression system using fluorescently labeled substrate inc... |
ACS Med Chem Lett 9: 1039-1044 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00344
BindingDB Entry DOI: 10.7270/Q25D8VJV |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50467989
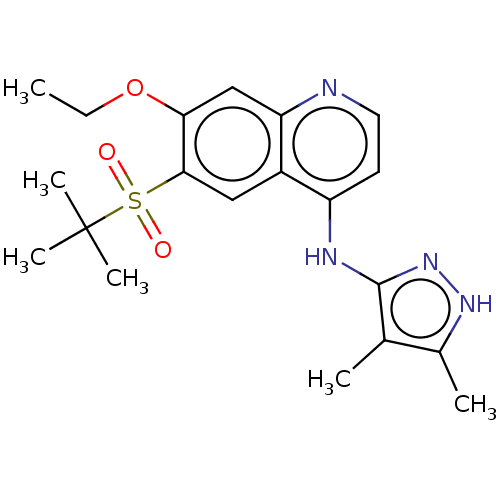
(CHEMBL4282034)Show SMILES CCOc1cc2nccc(Nc3n[nH]c(C)c3C)c2cc1S(=O)(=O)C(C)(C)C Show InChI InChI=1S/C20H26N4O3S/c1-7-27-17-11-16-14(10-18(17)28(25,26)20(4,5)6)15(8-9-21-16)22-19-12(2)13(3)23-24-19/h8-11H,7H2,1-6H3,(H2,21,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full length His/flag-tagged RIPK2 (unknown origin) expressed in baculovirus expression system using fluorescently labeled substrate inc... |
ACS Med Chem Lett 9: 1039-1044 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00344
BindingDB Entry DOI: 10.7270/Q25D8VJV |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50506948

(CHEMBL4448806)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccncc1 |r| Show InChI InChI=1S/C21H20N2O2/c1-15(19-4-3-5-20(14-19)25-2)23-21(24)18-8-6-16(7-9-18)17-10-12-22-13-11-17/h3-15H,1-2H3,(H,23,24)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... |
J Med Chem 61: 11074-11100 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01098
BindingDB Entry DOI: 10.7270/Q2RB77XJ |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306156

(3-((S)-2-amino-3-(1H-indol-3-yl)propoxy)-5-(3-meth...)Show SMILES Cc1n[nH]c2ncc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C28H27N7O2/c1-15-22-10-18(13-32-28(22)35-34-15)23-11-25(27(30)33-26(23)20-7-8-36-16(20)2)37-14-19(29)9-17-12-31-24-6-4-3-5-21(17)24/h3-8,10-13,19,31H,9,14,29H2,1-2H3,(H2,30,33)(H,32,34,35)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306157

(3-((S)-2-amino-3-(1H-indol-3-yl)propoxy)-5-(3-meth...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C27H26N8O2/c1-14-24-27(35-34-14)31-12-22(32-24)20-10-23(26(29)33-25(20)18-7-8-36-15(18)2)37-13-17(28)9-16-11-30-21-6-4-3-5-19(16)21/h3-8,10-12,17,30H,9,13,28H2,1-2H3,(H2,29,33)(H,31,34,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306163

(3-((S)-2-amino-3-(2-chloro-1H-indol-3-yl)propoxy)-...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c(Cl)[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C27H25ClN8O2/c1-13-23-27(36-35-13)31-11-21(32-23)19-10-22(26(30)34-24(19)16-7-8-37-14(16)2)38-12-15(29)9-18-17-5-3-4-6-20(17)33-25(18)28/h3-8,10-11,15,33H,9,12,29H2,1-2H3,(H2,30,34)(H,31,35,36)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50467986

(CHEMBL4289904)Show SMILES Cc1[nH]nc(Nc2ccnc3cc(OCCO)c(cc23)S(=O)(=O)C(C)(C)C)c1C Show InChI InChI=1S/C20H26N4O4S/c1-12-13(2)23-24-19(12)22-15-6-7-21-16-11-17(28-9-8-25)18(10-14(15)16)29(26,27)20(3,4)5/h6-7,10-11,25H,8-9H2,1-5H3,(H2,21,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full length His/flag-tagged RIPK2 (unknown origin) expressed in baculovirus expression system using fluorescently labeled substrate inc... |
ACS Med Chem Lett 9: 1039-1044 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00344
BindingDB Entry DOI: 10.7270/Q25D8VJV |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50467980

(CHEMBL4292228)Show SMILES COCCOc1cc2nccc(Nc3n[nH]c(C)c3C)c2cc1S(=O)(=O)C(C)(C)C Show InChI InChI=1S/C21H28N4O4S/c1-13-14(2)24-25-20(13)23-16-7-8-22-17-12-18(29-10-9-28-6)19(11-15(16)17)30(26,27)21(3,4)5/h7-8,11-12H,9-10H2,1-6H3,(H2,22,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full length His/flag-tagged RIPK2 (unknown origin) expressed in baculovirus expression system using fluorescently labeled substrate inc... |
ACS Med Chem Lett 9: 1039-1044 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00344
BindingDB Entry DOI: 10.7270/Q25D8VJV |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50467979
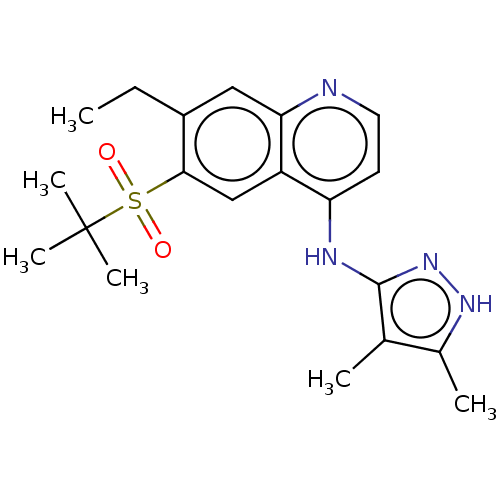
(CHEMBL4285837)Show SMILES CCc1cc2nccc(Nc3n[nH]c(C)c3C)c2cc1S(=O)(=O)C(C)(C)C Show InChI InChI=1S/C20H26N4O2S/c1-7-14-10-17-15(11-18(14)27(25,26)20(4,5)6)16(8-9-21-17)22-19-12(2)13(3)23-24-19/h8-11H,7H2,1-6H3,(H2,21,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full length His/flag-tagged RIPK2 (unknown origin) expressed in baculovirus expression system using fluorescently labeled substrate inc... |
ACS Med Chem Lett 9: 1039-1044 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00344
BindingDB Entry DOI: 10.7270/Q25D8VJV |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50506933
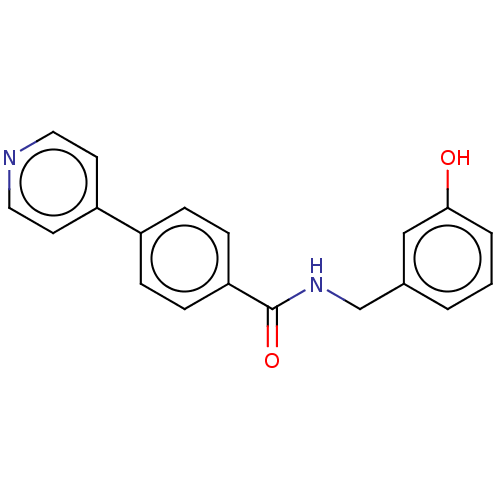
(CHEMBL4536833)Show InChI InChI=1S/C19H16N2O2/c22-18-3-1-2-14(12-18)13-21-19(23)17-6-4-15(5-7-17)16-8-10-20-11-9-16/h1-12,22H,13H2,(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... |
J Med Chem 61: 11074-11100 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01098
BindingDB Entry DOI: 10.7270/Q2RB77XJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50506954
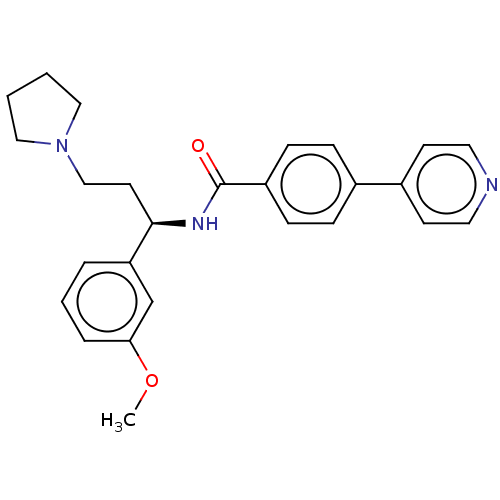
(CHEMBL4583341)Show SMILES COc1cccc(c1)[C@@H](CCN1CCCC1)NC(=O)c1ccc(cc1)-c1ccncc1 |r| Show InChI InChI=1S/C26H29N3O2/c1-31-24-6-4-5-23(19-24)25(13-18-29-16-2-3-17-29)28-26(30)22-9-7-20(8-10-22)21-11-14-27-15-12-21/h4-12,14-15,19,25H,2-3,13,16-18H2,1H3,(H,28,30)/t25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... |
J Med Chem 61: 11074-11100 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01098
BindingDB Entry DOI: 10.7270/Q2RB77XJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50506950

(CHEMBL4434688)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc(cc1N1CCC(CN)CC1)-c1ccncc1 |r| Show InChI InChI=1S/C27H32N4O2/c1-19(22-4-3-5-24(16-22)33-2)30-27(32)25-7-6-23(21-8-12-29-13-9-21)17-26(25)31-14-10-20(18-28)11-15-31/h3-9,12-13,16-17,19-20H,10-11,14-15,18,28H2,1-2H3,(H,30,32)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of human GST-tagged catalytic ROCK1 expressed in baculovirus system using STK S2 peptide substrate and and 33P-ATP after 60 mins by HTRF a... |
J Med Chem 61: 11074-11100 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01098
BindingDB Entry DOI: 10.7270/Q2RB77XJ |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306158

(3-((S)-2-amino-3-(1H-indol-3-yl)propoxy)-5-(3-meth...)Show SMILES Cc1n[nH]c2cnc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C28H27N7O2/c1-15-21-10-24(32-13-25(21)35-34-15)22-11-26(28(30)33-27(22)19-7-8-36-16(19)2)37-14-18(29)9-17-12-31-23-6-4-3-5-20(17)23/h3-8,10-13,18,31H,9,14,29H2,1-2H3,(H2,30,33)(H,34,35)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306160
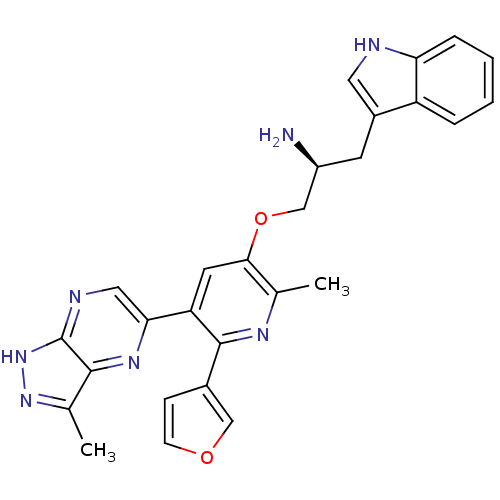
((S)-1-(6-(furan-3-yl)-2-methyl-5-(3-methyl-1H-pyra...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(C)nc1-c1ccoc1 |r| Show InChI InChI=1S/C27H25N7O2/c1-15-24(36-14-19(28)9-18-11-29-22-6-4-3-5-20(18)22)10-21(26(31-15)17-7-8-35-13-17)23-12-30-27-25(32-23)16(2)33-34-27/h3-8,10-13,19,29H,9,14,28H2,1-2H3,(H,30,33,34)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306155

(3-((S)-2-amino-3-(1H-indol-3-yl)propoxy)-5-(3-meth...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C29H28N6O2/c1-16-23-12-18(7-8-26(23)35-34-16)24-13-27(29(31)33-28(24)21-9-10-36-17(21)2)37-15-20(30)11-19-14-32-25-6-4-3-5-22(19)25/h3-10,12-14,20,32H,11,15,30H2,1-2H3,(H2,31,33)(H,34,35)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319456
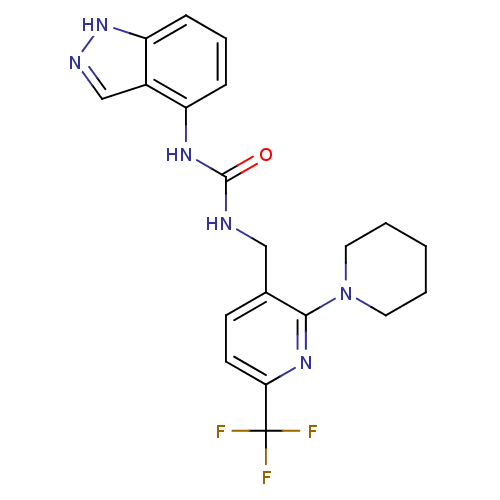
(1-(1H-indazol-4-yl)-3-((2-(piperidin-1-yl)-6-(trif...)Show SMILES FC(F)(F)c1ccc(CNC(=O)Nc2cccc3[nH]ncc23)c(n1)N1CCCCC1 Show InChI InChI=1S/C20H21F3N6O/c21-20(22,23)17-8-7-13(18(27-17)29-9-2-1-3-10-29)11-24-19(30)26-15-5-4-6-16-14(15)12-25-28-16/h4-8,12H,1-3,9-11H2,(H,25,28)(H2,24,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319471

(1-(2-(3,3-dimethylbutyl)-4-(trifluoromethyl)benzyl...)Show SMILES CC(C)(C)CCc1cc(ccc1CNC(=O)Nc1cccc2[nH]ncc12)C(F)(F)F Show InChI InChI=1S/C22H25F3N4O/c1-21(2,3)10-9-14-11-16(22(23,24)25)8-7-15(14)12-26-20(30)28-18-5-4-6-19-17(18)13-27-29-19/h4-8,11,13H,9-10,12H2,1-3H3,(H,27,29)(H2,26,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50467980

(CHEMBL4292228)Show SMILES COCCOc1cc2nccc(Nc3n[nH]c(C)c3C)c2cc1S(=O)(=O)C(C)(C)C Show InChI InChI=1S/C21H28N4O4S/c1-13-14(2)24-25-20(13)23-16-7-8-22-17-12-18(29-10-9-28-6)19(11-15(16)17)30(26,27)21(3,4)5/h7-8,11-12H,9-10H2,1-6H3,(H2,22,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RIPK2 in human whole blood assessed as reduction in MDP-stimulated TNFalpha levels incubated for 30 mins followed by MDP addition measu... |
ACS Med Chem Lett 9: 1039-1044 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00344
BindingDB Entry DOI: 10.7270/Q25D8VJV |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50184765
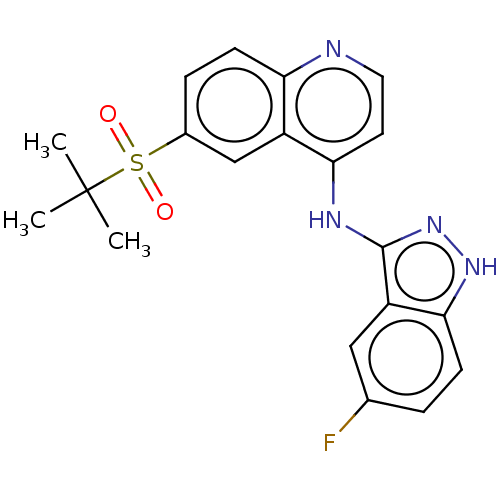
(CHEMBL3823499)Show SMILES CC(C)(C)S(=O)(=O)c1ccc2nccc(Nc3n[nH]c4ccc(F)cc34)c2c1 Show InChI InChI=1S/C20H19FN4O2S/c1-20(2,3)28(26,27)13-5-7-16-14(11-13)17(8-9-22-16)23-19-15-10-12(21)4-6-18(15)24-25-19/h4-11H,1-3H3,(H2,22,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full length His/flag-tagged RIPK2 (unknown origin) expressed in baculovirus expression system using fluorescently labeled substrate inc... |
ACS Med Chem Lett 9: 1039-1044 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00344
BindingDB Entry DOI: 10.7270/Q25D8VJV |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50133817
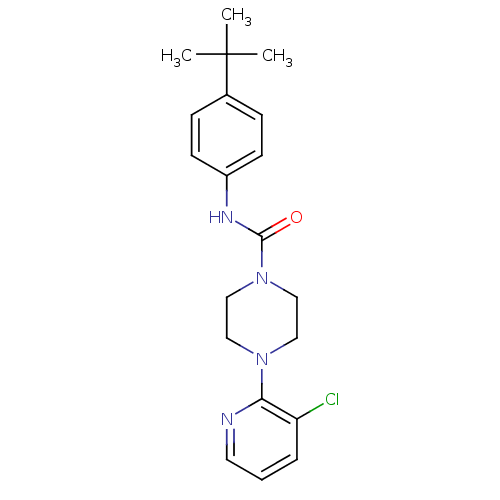
(4-(3-Chloro-pyridin-2-yl)-piperazine-1-carboxylic ...)Show SMILES CC(C)(C)c1ccc(NC(=O)N2CCN(CC2)c2ncccc2Cl)cc1 Show InChI InChI=1S/C20H25ClN4O/c1-20(2,3)15-6-8-16(9-7-15)23-19(26)25-13-11-24(12-14-25)18-17(21)5-4-10-22-18/h4-10H,11-14H2,1-3H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in human 1321N1 cells assessed as inhibition of capsaicin-induced calcium influx by FLIPR as... |
Bioorg Med Chem 16: 8516-25 (2008)
Article DOI: 10.1016/j.bmc.2008.08.005
BindingDB Entry DOI: 10.7270/Q2FX798X |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319465
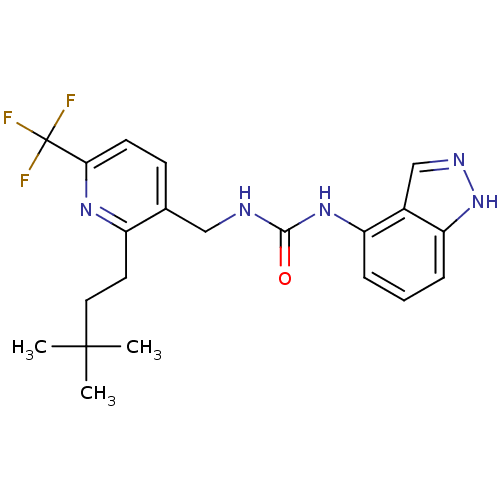
(1-((2-(3,3-dimethylbutyl)-6-(trifluoromethyl)pyrid...)Show SMILES CC(C)(C)CCc1nc(ccc1CNC(=O)Nc1cccc2[nH]ncc12)C(F)(F)F Show InChI InChI=1S/C21H24F3N5O/c1-20(2,3)10-9-15-13(7-8-18(27-15)21(22,23)24)11-25-19(30)28-16-5-4-6-17-14(16)12-26-29-17/h4-8,12H,9-11H2,1-3H3,(H,26,29)(H2,25,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50506925
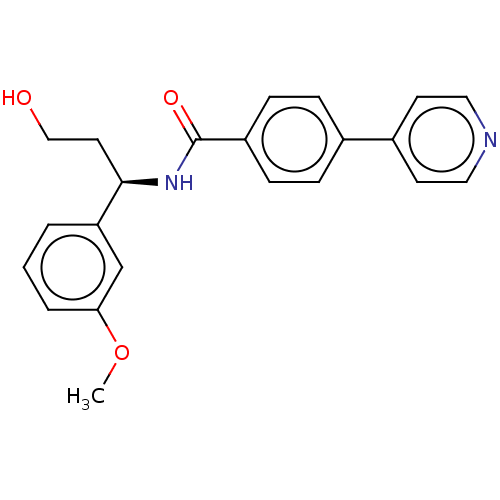
(CHEMBL4469316)Show SMILES COc1cccc(c1)[C@@H](CCO)NC(=O)c1ccc(cc1)-c1ccncc1 |r| Show InChI InChI=1S/C22H22N2O3/c1-27-20-4-2-3-19(15-20)21(11-14-25)24-22(26)18-7-5-16(6-8-18)17-9-12-23-13-10-17/h2-10,12-13,15,21,25H,11,14H2,1H3,(H,24,26)/t21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... |
J Med Chem 61: 11074-11100 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01098
BindingDB Entry DOI: 10.7270/Q2RB77XJ |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306161

(3-((S)-2-amino-3-phenylpropoxy)-5-(3-methyl-1H-pyr...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2ccccc2)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C25H25N7O2/c1-14-22-25(32-31-14)28-12-20(29-22)19-11-21(24(27)30-23(19)18-8-9-33-15(18)2)34-13-17(26)10-16-6-4-3-5-7-16/h3-9,11-12,17H,10,13,26H2,1-2H3,(H2,27,30)(H,28,31,32)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306161

(3-((S)-2-amino-3-phenylpropoxy)-5-(3-methyl-1H-pyr...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2ccccc2)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C25H25N7O2/c1-14-22-25(32-31-14)28-12-20(29-22)19-11-21(24(27)30-23(19)18-8-9-33-15(18)2)34-13-17(26)10-16-6-4-3-5-7-16/h3-9,11-12,17H,10,13,26H2,1-2H3,(H2,27,30)(H,28,31,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data