

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50403547 (ATROPEN | ATROPINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of carbachol-induced release of alpha-amylase from pancreatic acinar cells from that of rat ileum contained the M2 receptor subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50005685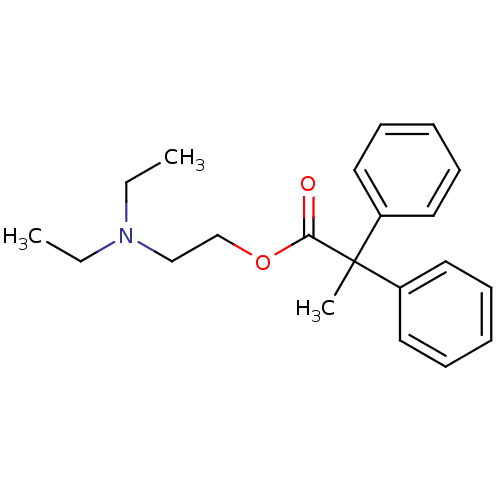 (2,2-Diphenyl-propionic acid 2-diethylamino-ethyl e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of carbachol-induced release of alpha-amylase from pancreatic acinar cells from that of rat ileum contained the M2 receptor subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50018230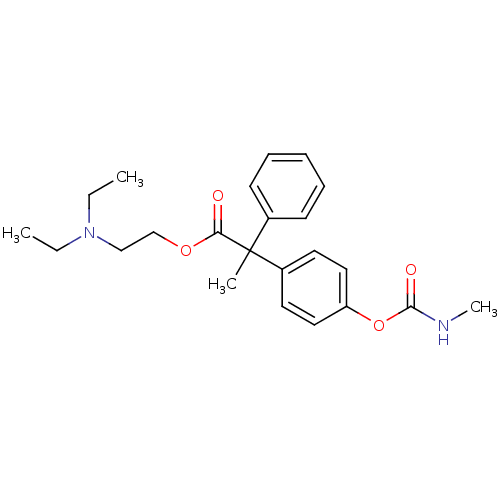 (2-(4-Methylcarbamoyloxy-phenyl)-2-phenyl-propionic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Binding affinity was determined from the inhibition of contraction of guinea pig ileum which has Muscarinic acetylcholine receptor M2 subtype. | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50403547 (ATROPEN | ATROPINE) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50018229 (2-(4-Hydroxy-phenyl)-2-phenyl-propionic acid 2-die...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of carbachol-induced release of alpha-amylase from pancreatic acinar cells from that of rat ileum contained the M2 receptor subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50018230 (2-(4-Methylcarbamoyloxy-phenyl)-2-phenyl-propionic...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50018225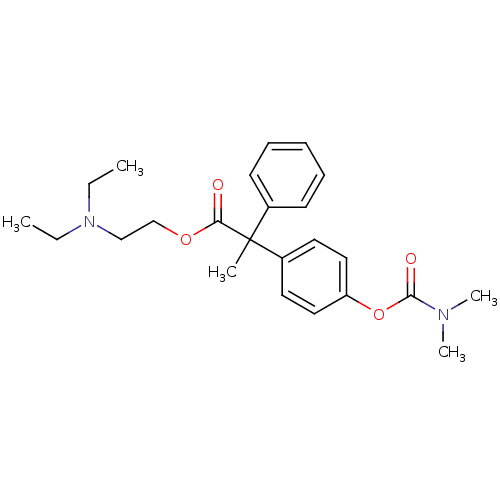 (2-(4-Dimethylcarbamoyloxy-phenyl)-2-phenyl-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Binding affinity was determined from the inhibition of contraction of guinea pig ileum which has Muscarinic acetylcholine receptor M2 subtype. | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50005685 (2,2-Diphenyl-propionic acid 2-diethylamino-ethyl e...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity choline transporter 1 (Homo sapiens (Human)) | BDBM50451447 (CHEMBL4217988) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]hemicholinium-3 from recombinant human choline transporter after 60 mins by scintillation counting method | Bioorg Med Chem Lett 27: 4805-4811 (2017) Article DOI: 10.1016/j.bmcl.2017.09.056 BindingDB Entry DOI: 10.7270/Q25Q4ZN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50018229 (2-(4-Hydroxy-phenyl)-2-phenyl-propionic acid 2-die...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM39341 (11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of carbachol-induced release of alpha-amylase from pancreatic acinar cells from that of rat ileum contained the muscarinic acetylcholine r... | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50018225 (2-(4-Dimethylcarbamoyloxy-phenyl)-2-phenyl-propion...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM39341 (11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the muscarinic acetylcholine receptor M1 | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50018226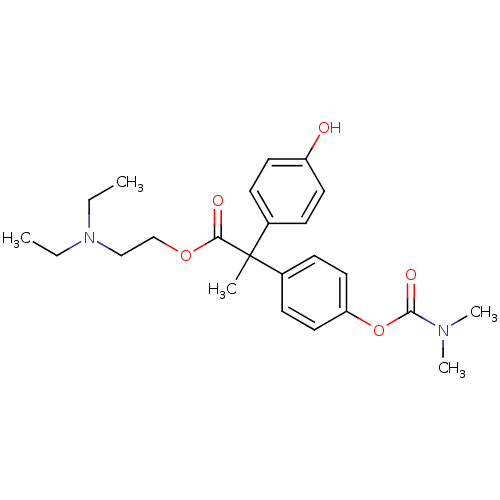 (2-(4-Dimethylcarbamoyloxy-phenyl)-2-(4-hydroxy-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of anticholinesterase activity by their ability to inactivate human serum butyrylcholinesterase | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50451447 (CHEMBL4217988) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]Dofetilide from recombinant human ERG after 60 mins by scintillation counting method | Bioorg Med Chem Lett 27: 4805-4811 (2017) Article DOI: 10.1016/j.bmcl.2017.09.056 BindingDB Entry DOI: 10.7270/Q25Q4ZN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM5485 (6-(cyclohexylmethoxy)-9H-purin-2-amine | CHEMBL269...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Inhibition of CDK2 | J Med Chem 49: 5141-53 (2006) Article DOI: 10.1021/jm060190+ BindingDB Entry DOI: 10.7270/Q27S7NDM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50018225 (2-(4-Dimethylcarbamoyloxy-phenyl)-2-phenyl-propion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of anticholinesterase activity by their ability to inactivate human serum butyrylcholinesterase | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50018230 (2-(4-Methylcarbamoyloxy-phenyl)-2-phenyl-propionic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Anticholinesterase activity by their ability to inactivate human serum butyrylcholinesterase | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50018227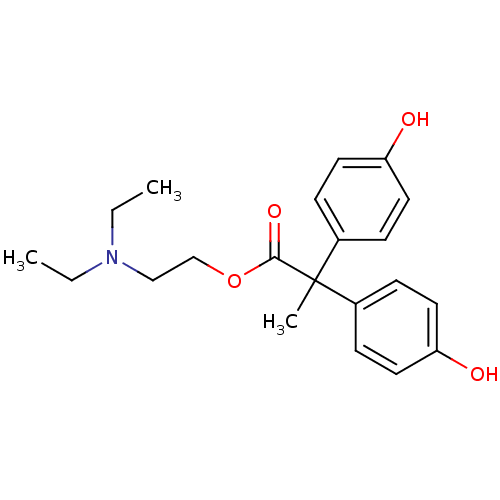 (2,2-Bis-(4-hydroxy-phenyl)-propionic acid 2-diethy...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50451447 (CHEMBL4217988) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]BTCP from recombinant human dopamine transporter after 120 mins by scintillation counting method | Bioorg Med Chem Lett 27: 4805-4811 (2017) Article DOI: 10.1016/j.bmcl.2017.09.056 BindingDB Entry DOI: 10.7270/Q25Q4ZN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM5594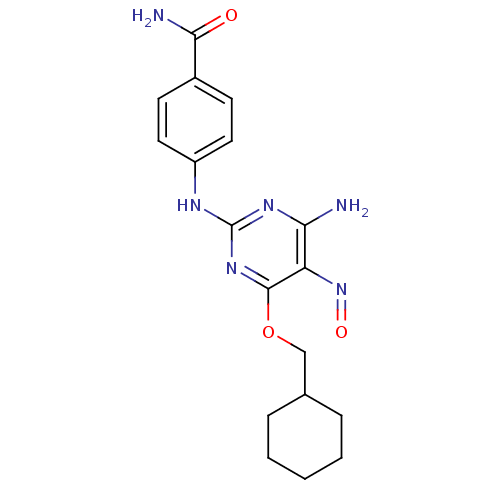 (2-arylamino-pyrimidine deriv. 9d | 4-{[4-amino-6-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Inhibition of CDK2 | J Med Chem 49: 5141-53 (2006) Article DOI: 10.1021/jm060190+ BindingDB Entry DOI: 10.7270/Q27S7NDM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50018226 (2-(4-Dimethylcarbamoyloxy-phenyl)-2-(4-hydroxy-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of carbachol-induced release of alpha-amylase from pancreatic acinar cells from that of rat ileum contained the M2 receptor subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50451447 (CHEMBL4217988) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from recombinant human norepinephrine transporter after 120 min by scintillation counting method | Bioorg Med Chem Lett 27: 4805-4811 (2017) Article DOI: 10.1016/j.bmcl.2017.09.056 BindingDB Entry DOI: 10.7270/Q25Q4ZN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50018226 (2-(4-Dimethylcarbamoyloxy-phenyl)-2-(4-hydroxy-phe...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to cerebral cortex membranes which contain predominantly the Muscarinic acetylcholine receptor M1 subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50313079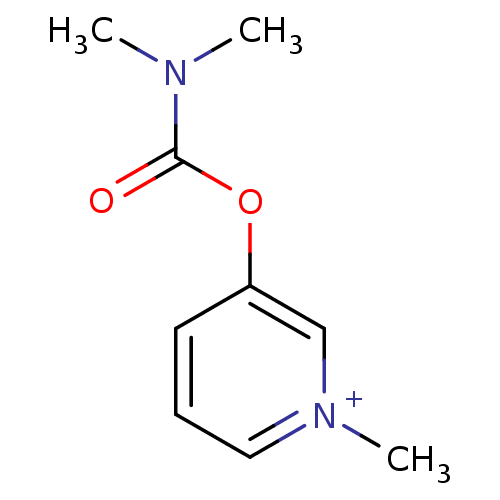 (3-Dimethylcarbamoyloxy-1-methyl-pyridinium; bromid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of anticholinesterase activity by their ability to inactivate human serum butyrylcholinesterase | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50018228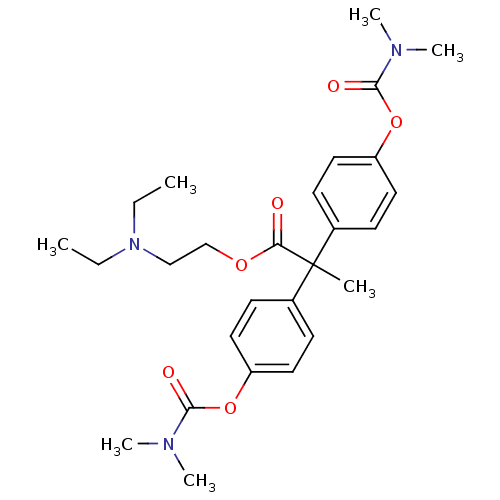 (2,2-Bis-(4-dimethylcarbamoyloxy-phenyl)-propionic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of anticholinesterase activity by their ability to inactivate human serum butyrylcholinesterase | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50018227 (2,2-Bis-(4-hydroxy-phenyl)-propionic acid 2-diethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of carbachol-induced release of alpha-amylase from pancreatic acinar cells from that of rat ileum contained the M2 receptor subtypes | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Research Curated by ChEMBL | Assay Description Inhibition of anticholinesterase activity by their ability to inactivate human serum butyrylcholinesterase | J Med Chem 32: 1522-8 (1989) BindingDB Entry DOI: 10.7270/Q2VQ3374 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50451447 (CHEMBL4217988) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK cells by patch clamp electrophysiology method | Bioorg Med Chem Lett 27: 4805-4811 (2017) Article DOI: 10.1016/j.bmcl.2017.09.056 BindingDB Entry DOI: 10.7270/Q25Q4ZN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50451447 (CHEMBL4217988) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK cells by automated patchXpress electrophysiology method | Bioorg Med Chem Lett 27: 4805-4811 (2017) Article DOI: 10.1016/j.bmcl.2017.09.056 BindingDB Entry DOI: 10.7270/Q25Q4ZN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50451457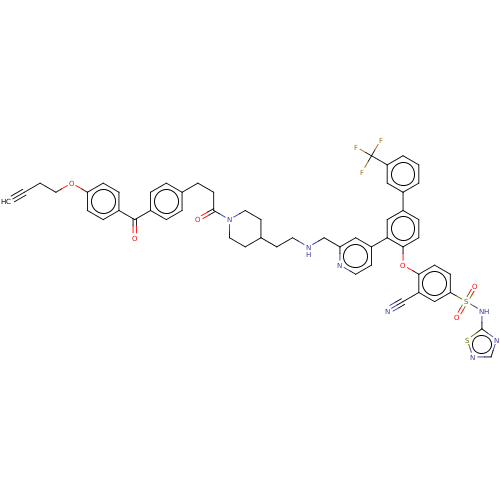 (CHEMBL4210135) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK cells by patch clamp electrophysiology method | Bioorg Med Chem Lett 27: 4805-4811 (2017) Article DOI: 10.1016/j.bmcl.2017.09.056 BindingDB Entry DOI: 10.7270/Q25Q4ZN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50451458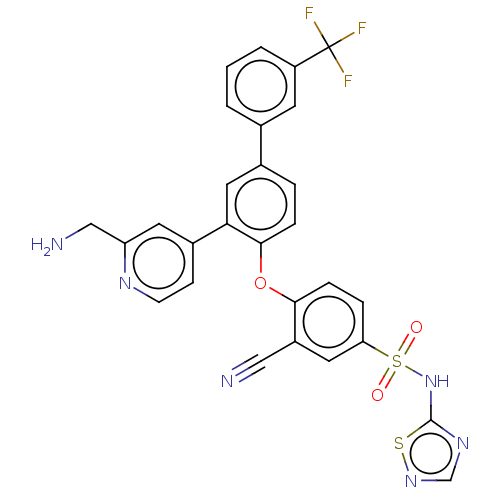 (CHEMBL4205773) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK cells by patch clamp electrophysiology method | Bioorg Med Chem Lett 27: 4805-4811 (2017) Article DOI: 10.1016/j.bmcl.2017.09.056 BindingDB Entry DOI: 10.7270/Q25Q4ZN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Mus musculus) | BDBM50451447 (CHEMBL4217988) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of mouse Nav1.7 expressed in HEK cells by patch clamp electrophysiology method | Bioorg Med Chem Lett 27: 4805-4811 (2017) Article DOI: 10.1016/j.bmcl.2017.09.056 BindingDB Entry DOI: 10.7270/Q25Q4ZN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50451456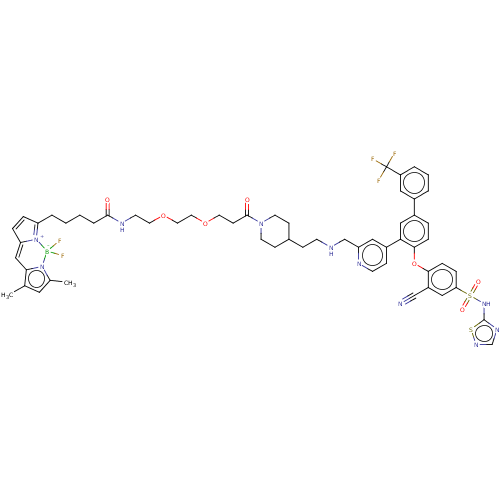 (CHEMBL4215058) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK cells by patch clamp electrophysiology method | Bioorg Med Chem Lett 27: 4805-4811 (2017) Article DOI: 10.1016/j.bmcl.2017.09.056 BindingDB Entry DOI: 10.7270/Q25Q4ZN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50451458 (CHEMBL4205773) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK cells by automated patchXpress electrophysiology method | Bioorg Med Chem Lett 27: 4805-4811 (2017) Article DOI: 10.1016/j.bmcl.2017.09.056 BindingDB Entry DOI: 10.7270/Q25Q4ZN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50451454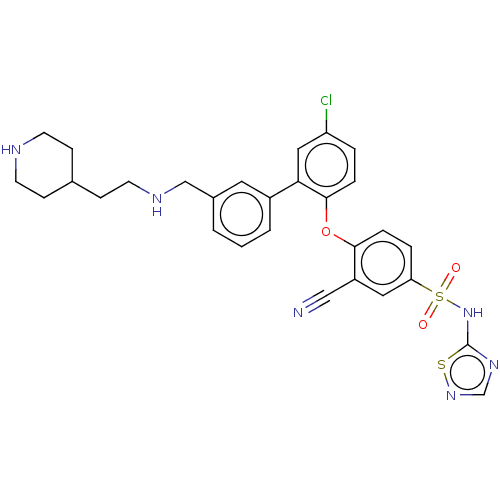 (CHEMBL4207534) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK cells by automated patchXpress electrophysiology method | Bioorg Med Chem Lett 27: 4805-4811 (2017) Article DOI: 10.1016/j.bmcl.2017.09.056 BindingDB Entry DOI: 10.7270/Q25Q4ZN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50451459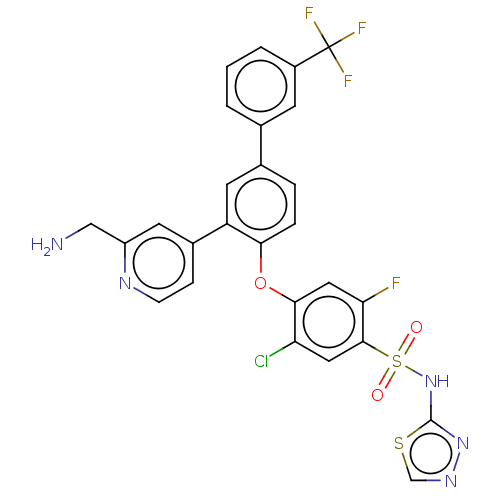 (CHEMBL4208664) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK cells by automated patchXpress electrophysiology method | Bioorg Med Chem Lett 27: 4805-4811 (2017) Article DOI: 10.1016/j.bmcl.2017.09.056 BindingDB Entry DOI: 10.7270/Q25Q4ZN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50451453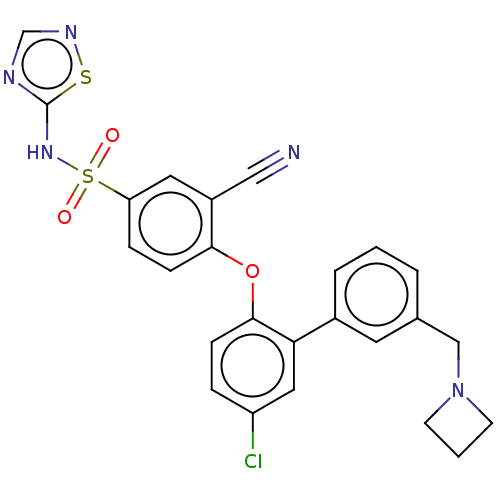 (CHEMBL2325603) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK cells by automated patchXpress electrophysiology method | Bioorg Med Chem Lett 27: 4805-4811 (2017) Article DOI: 10.1016/j.bmcl.2017.09.056 BindingDB Entry DOI: 10.7270/Q25Q4ZN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50507856 (CHEMBL4483603) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.8 expressed in HEK cells by patch-clamp electrophysiology method | Bioorg Med Chem 27: 230-239 (2019) Article DOI: 10.1016/j.bmc.2018.12.002 BindingDB Entry DOI: 10.7270/Q2ZK5KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Homo sapiens (Human)) | BDBM50451447 (CHEMBL4217988) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.2 expressed in HEK cells by patch clamp electrophysiology method | Bioorg Med Chem Lett 27: 4805-4811 (2017) Article DOI: 10.1016/j.bmcl.2017.09.056 BindingDB Entry DOI: 10.7270/Q25Q4ZN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50451448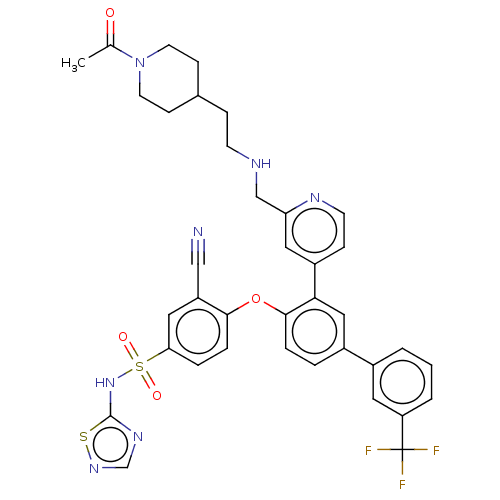 (CHEMBL4213464) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK cells by automated patchXpress electrophysiology method | Bioorg Med Chem Lett 27: 4805-4811 (2017) Article DOI: 10.1016/j.bmcl.2017.09.056 BindingDB Entry DOI: 10.7270/Q25Q4ZN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50451460 (CHEMBL4215917) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK cells by automated patchXpress electrophysiology method | Bioorg Med Chem Lett 27: 4805-4811 (2017) Article DOI: 10.1016/j.bmcl.2017.09.056 BindingDB Entry DOI: 10.7270/Q25Q4ZN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50530873 (CHEMBL4456215) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... | J Med Chem 62: 10402-10422 (2019) Article DOI: 10.1021/acs.jmedchem.9b01499 BindingDB Entry DOI: 10.7270/Q27H1P12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50530873 (CHEMBL4456215) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... | J Med Chem 62: 10402-10422 (2019) Article DOI: 10.1021/acs.jmedchem.9b01499 BindingDB Entry DOI: 10.7270/Q27H1P12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 8 subunit alpha (Rattus norvegicus) | BDBM50451447 (CHEMBL4217988) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat Nav1.6 expressed in HEK cells by patch clamp electrophysiology method | Bioorg Med Chem Lett 27: 4805-4811 (2017) Article DOI: 10.1016/j.bmcl.2017.09.056 BindingDB Entry DOI: 10.7270/Q25Q4ZN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 8 subunit alpha (Homo sapiens (Human)) | BDBM50451447 (CHEMBL4217988) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.6 expressed in HEK cells by patch clamp electrophysiology method | Bioorg Med Chem Lett 27: 4805-4811 (2017) Article DOI: 10.1016/j.bmcl.2017.09.056 BindingDB Entry DOI: 10.7270/Q25Q4ZN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50530894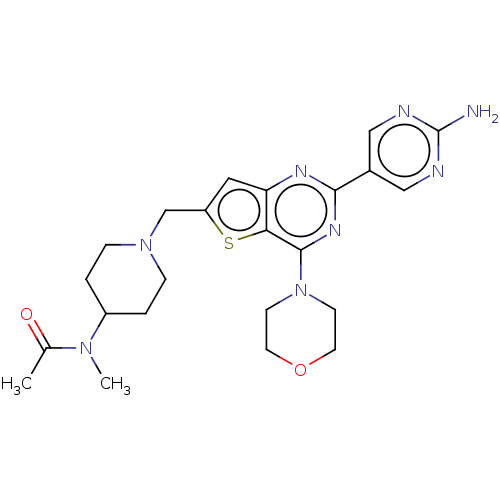 (CHEMBL4437468) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... | J Med Chem 62: 10402-10422 (2019) Article DOI: 10.1021/acs.jmedchem.9b01499 BindingDB Entry DOI: 10.7270/Q27H1P12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50530928 (CHEMBL4534127) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... | J Med Chem 62: 10402-10422 (2019) Article DOI: 10.1021/acs.jmedchem.9b01499 BindingDB Entry DOI: 10.7270/Q27H1P12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50530894 (CHEMBL4437468) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... | J Med Chem 62: 10402-10422 (2019) Article DOI: 10.1021/acs.jmedchem.9b01499 BindingDB Entry DOI: 10.7270/Q27H1P12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50530928 (CHEMBL4534127) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... | J Med Chem 62: 10402-10422 (2019) Article DOI: 10.1021/acs.jmedchem.9b01499 BindingDB Entry DOI: 10.7270/Q27H1P12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 744 total ) | Next | Last >> |