

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Squalene synthase (Rattus norvegicus) | BDBM50029174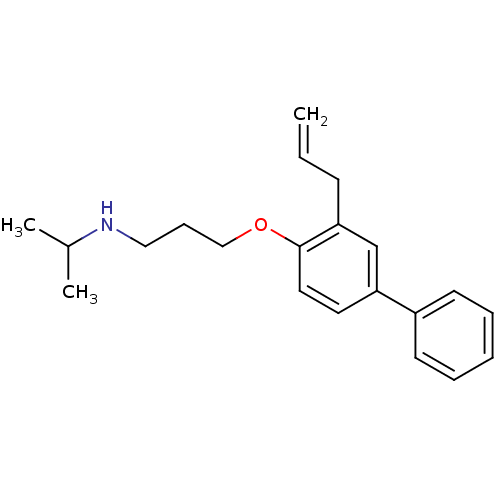 (CHEMBL131973 | N-(1-methylethyl)-3-[(3-prop-2-en-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PubMed | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029177 (CHEMBL134337 | Propionic acid 3-allyl-4-(3-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029166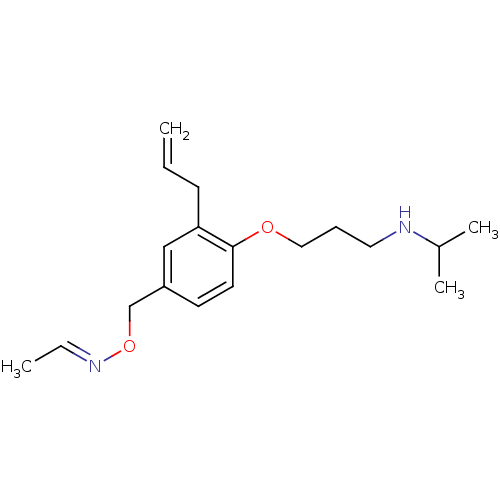 (Acetaldehyde O-[3-allyl-4-(3-isopropylamino-propox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029159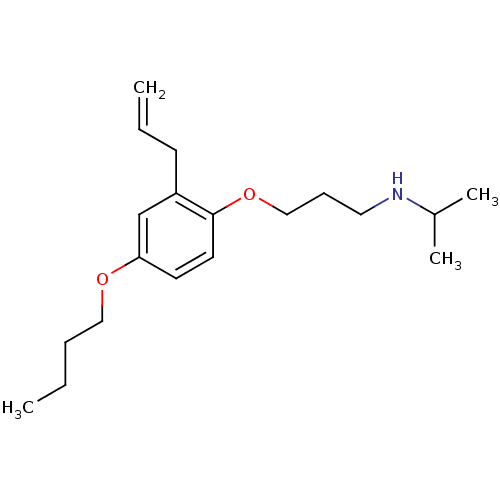 (CHEMBL132881 | [3-(2-Allyl-4-butoxy-phenoxy)-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029171 (CHEMBL341371 | N-[3-Benzyl-4-(3-isopropylamino-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012922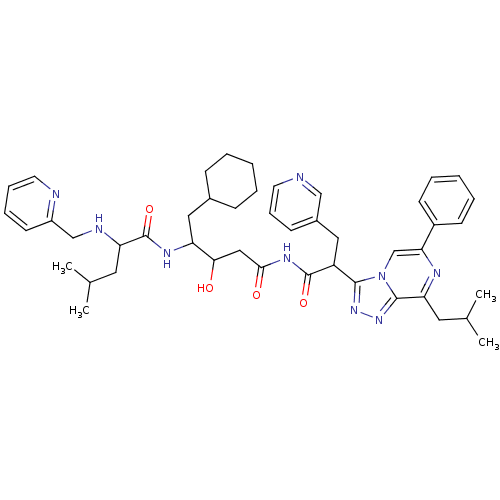 (4-Methyl-2-[(pyridin-2-ylmethyl)-amino]-pentanoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012922 (4-Methyl-2-[(pyridin-2-ylmethyl)-amino]-pentanoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029179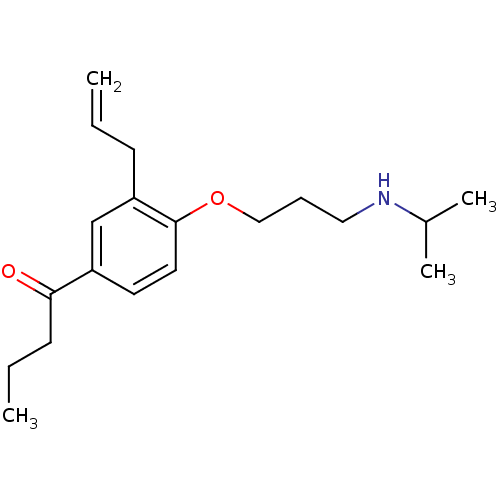 (1-[3-Allyl-4-(3-isopropylamino-propoxy)-phenyl]-bu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012928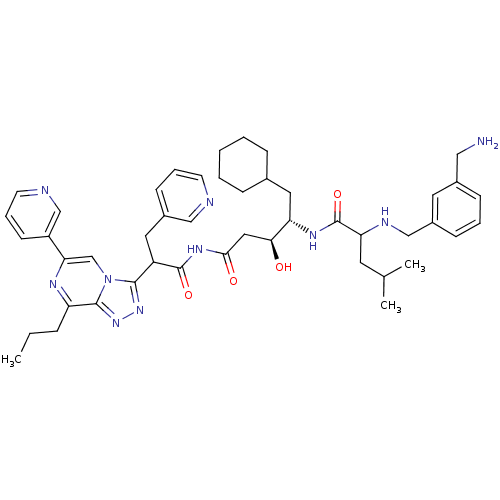 (2-(3-Aminomethyl-benzylamino)-4-methyl-pentanoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012923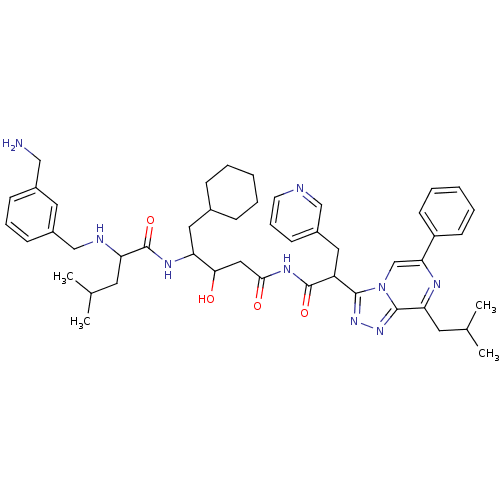 (2-(3-Aminomethyl-benzylamino)-4-methyl-pentanoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012923 (2-(3-Aminomethyl-benzylamino)-4-methyl-pentanoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012932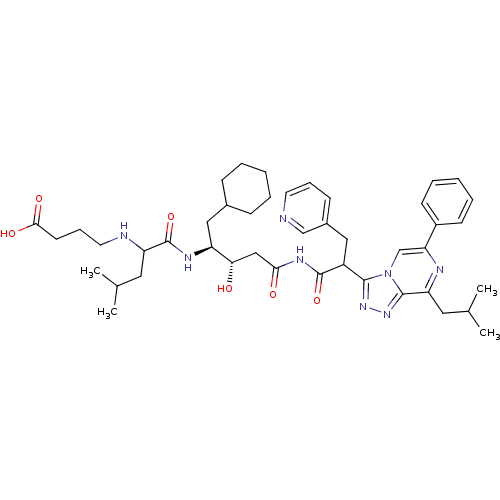 (4-(1-{1-Cyclohexylmethyl-2-hydroxy-4-[2-(8-isobuty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029163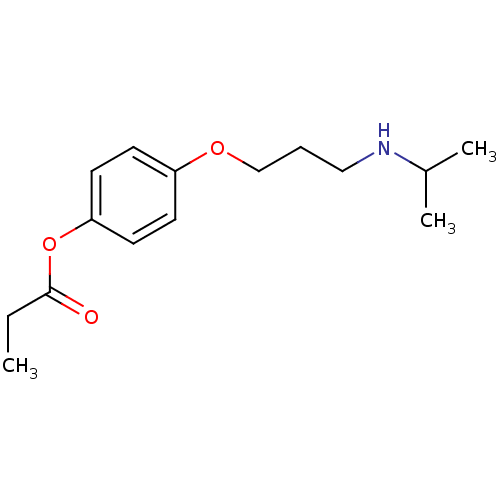 (CHEMBL340992 | Propionic acid 4-(3-isopropylamino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012925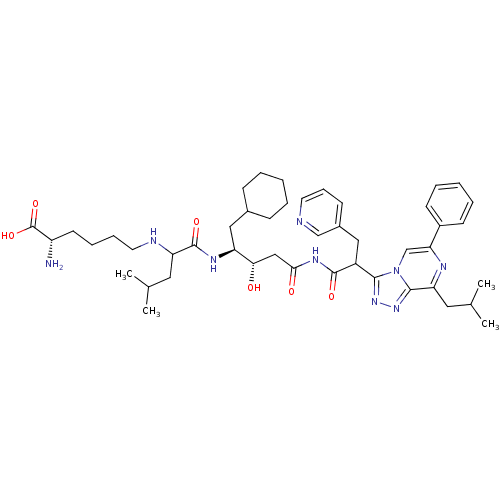 (2-Amino-6-(1-{1-cyclohexylmethyl-2-hydroxy-4-[2-(8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029170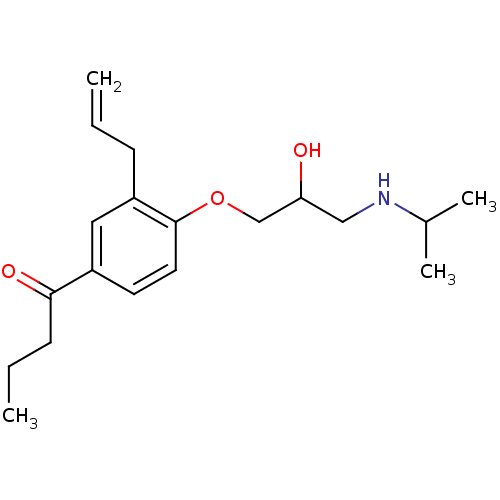 (1-[3-Allyl-4-(2-hydroxy-3-isopropylamino-propoxy)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029161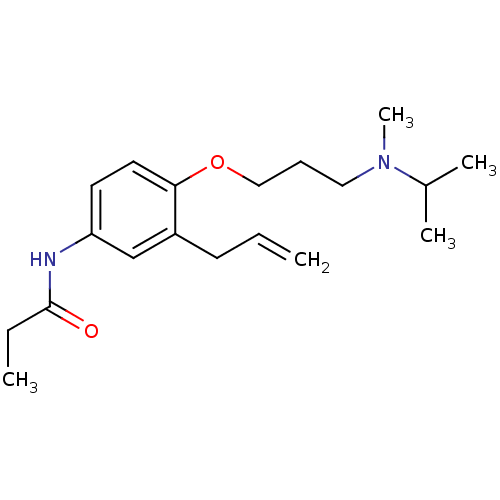 (CHEMBL433864 | N-{3-Allyl-4-[3-(isopropyl-methyl-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50075719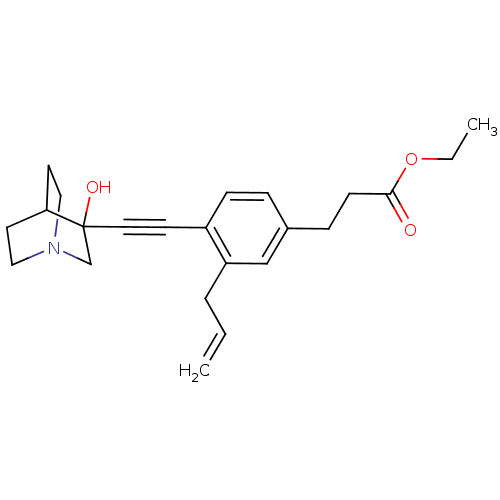 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Compound was measured for the inhibition of rat microsomal squalene synthase enzyme | J Med Chem 42: 1306-11 (1999) Article DOI: 10.1021/jm990038q BindingDB Entry DOI: 10.7270/Q23X85TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029172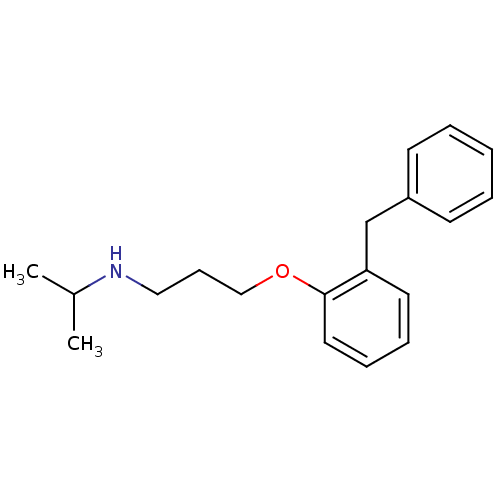 (CHEMBL131930 | [3-(2-Benzyl-phenoxy)-propyl]-isopr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052351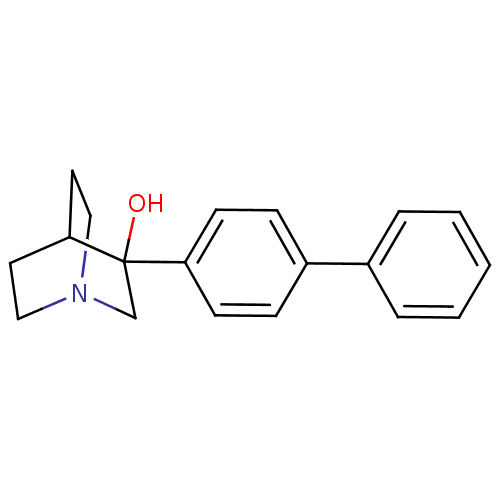 (3-Biphenyl-4-yl-1-aza-bicyclo[2.2.2]octan-3-ol | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Compound was measured for the inhibition of rat microsomal squalene synthase enzyme | J Med Chem 42: 1306-11 (1999) Article DOI: 10.1021/jm990038q BindingDB Entry DOI: 10.7270/Q23X85TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012935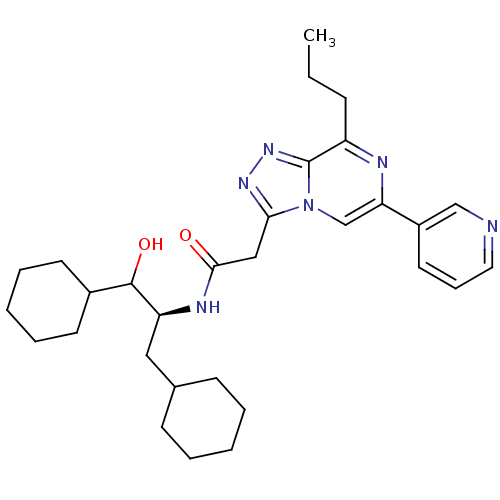 (CHEMBL431401 | N-(2-Cyclohexyl-1-cyclohexylmethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029181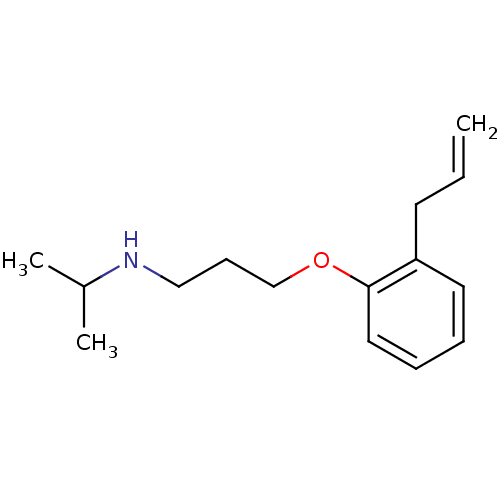 (CHEMBL134211 | [3-(2-Allyl-phenoxy)-propyl]-isopro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029165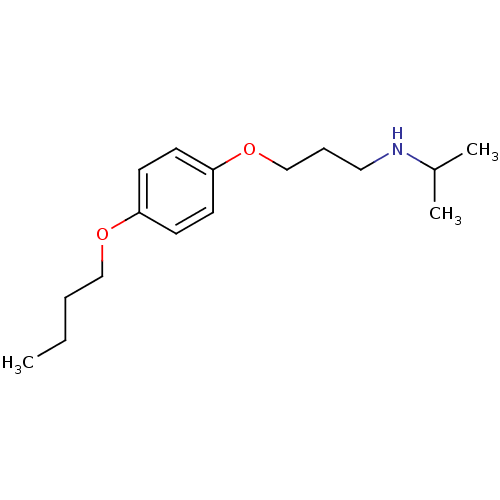 (CHEMBL132207 | [3-(4-Butoxy-phenoxy)-propyl]-isopr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012933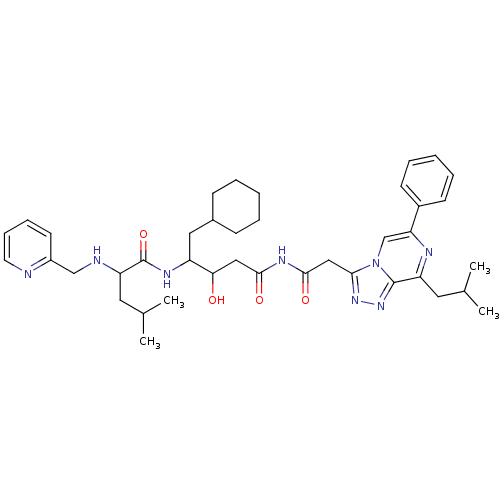 (4-Methyl-2-[(pyridin-2-ylmethyl)-amino]-pentanoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029168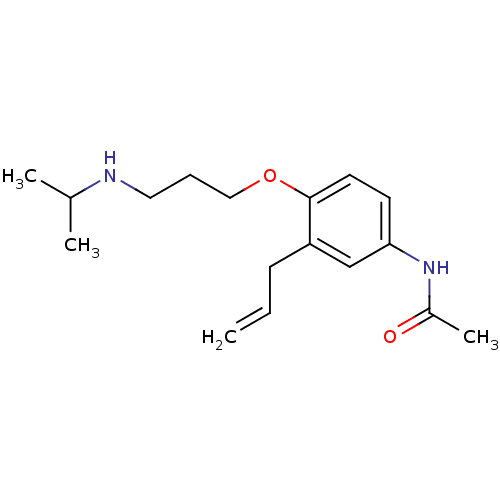 (CHEMBL133996 | N-[3-Allyl-4-(3-isopropylamino-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50075719 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of compound was measured on rat microsomal Oxidosqualene-lanosterol cyclase | J Med Chem 42: 1306-11 (1999) Article DOI: 10.1021/jm990038q BindingDB Entry DOI: 10.7270/Q23X85TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50052351 (3-Biphenyl-4-yl-1-aza-bicyclo[2.2.2]octan-3-ol | 3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of compound was measured on rat microsomal Oxidosqualene-lanosterol cyclase | J Med Chem 42: 1306-11 (1999) Article DOI: 10.1021/jm990038q BindingDB Entry DOI: 10.7270/Q23X85TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012929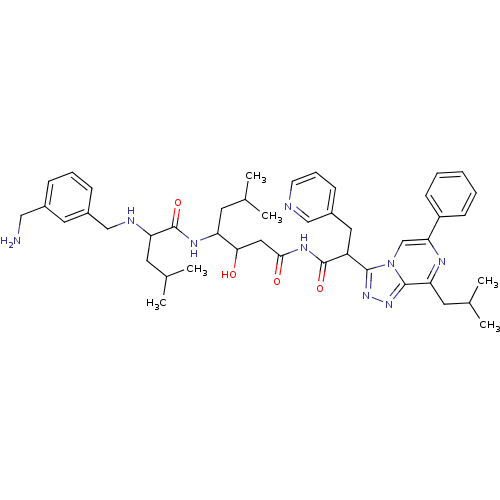 (2-(3-Aminomethyl-benzylamino)-4-methyl-pentanoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012929 (2-(3-Aminomethyl-benzylamino)-4-methyl-pentanoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029169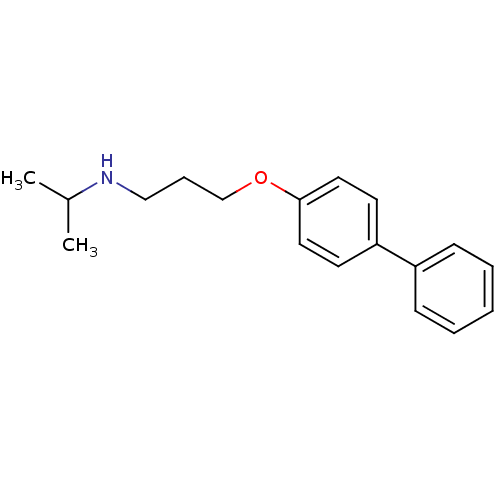 (CHEMBL134210 | [3-(Biphenyl-4-yloxy)-propyl]-isopr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029162 (3-Allyl-4-(3-isopropylamino-propoxy)-phenylamine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012924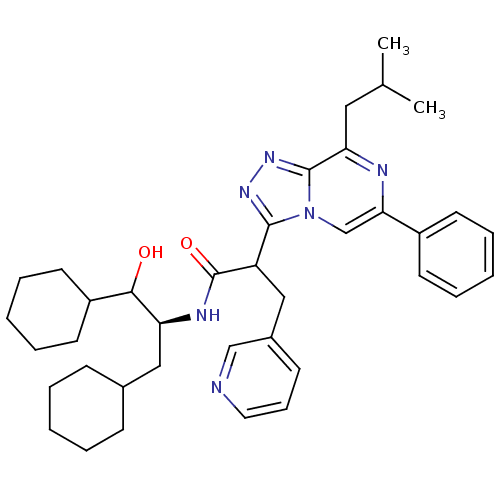 (CHEMBL78121 | N-(2-Cyclohexyl-1-cyclohexylmethyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012934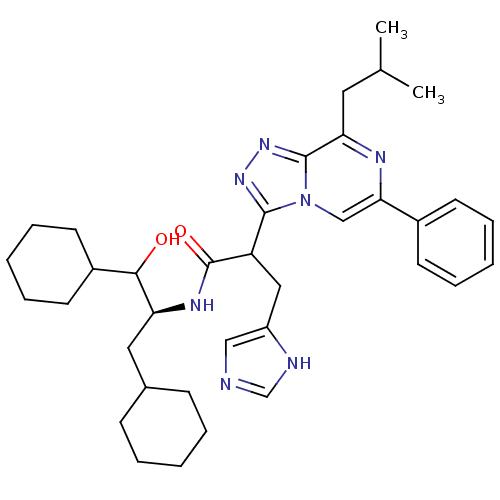 (CHEMBL307388 | N-(2-Cyclohexyl-1-cyclohexylmethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029175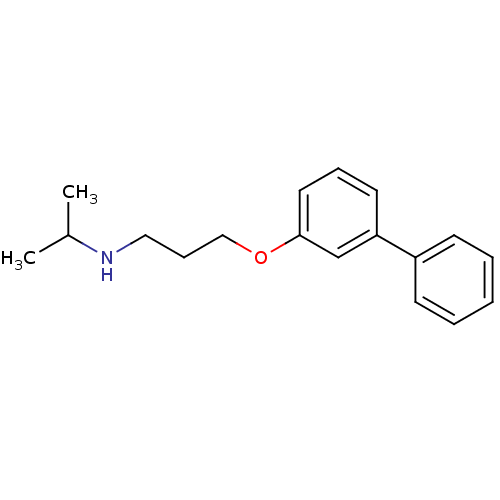 (CHEMBL337731 | [3-(Biphenyl-3-yloxy)-propyl]-isopr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029176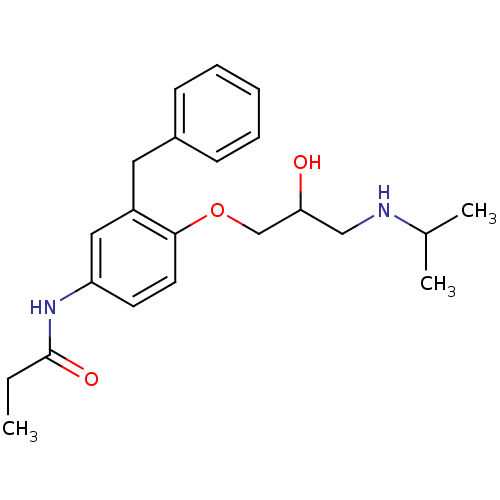 (CHEMBL334964 | N-[3-Benzyl-4-(2-hydroxy-3-isopropy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50406416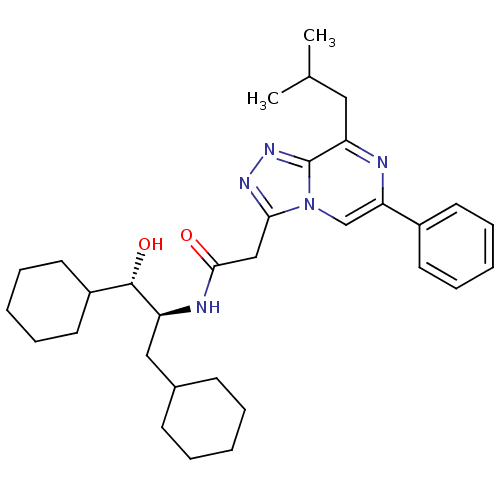 (CHEMBL2114143) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029182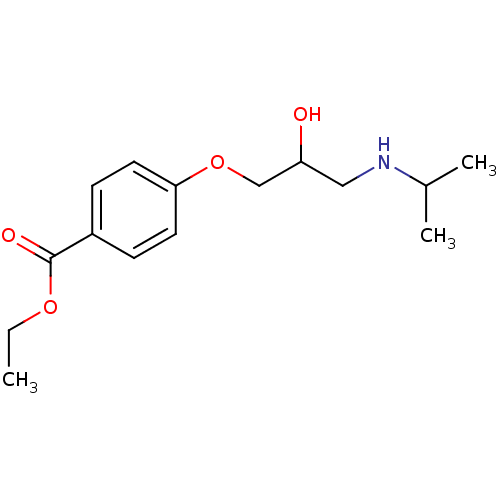 (4-(2-Hydroxy-3-isopropylamino-propoxy)-benzoic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012927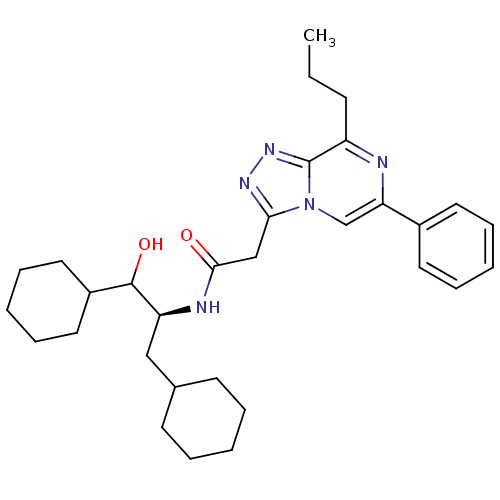 (CHEMBL76896 | N-(2-Cyclohexyl-1-cyclohexylmethyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029178 (CHEMBL133495 | Isopropyl-[3-(4-pyridin-3-yl-phenox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029180 (CHEMBL132078 | {3-[2-((E)-2,6-Dimethyl-hepta-1,5-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029164 (CHEMBL133161 | N-[4-(3-Isopropylamino-propoxy)-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029173 (1-(Biphenyl-4-yloxy)-3-isopropylamino-propan-2-ol ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029158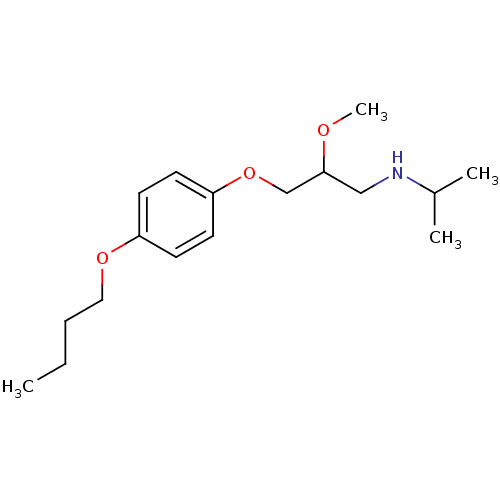 (CHEMBL423520 | [3-(4-Butoxy-phenoxy)-2-methoxy-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM25764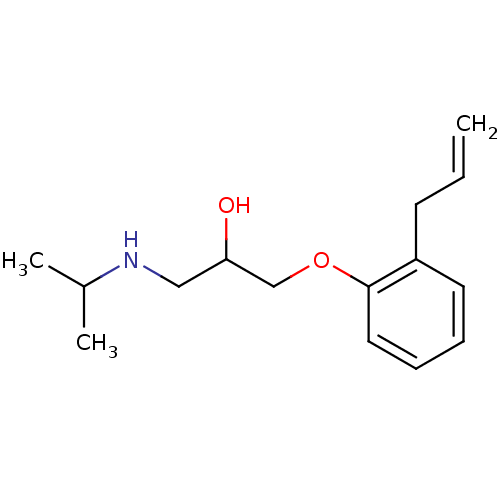 (ALPRENOLOL,(+) | ALPRENOLOL,(-) | Alfeprol | Alphe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029167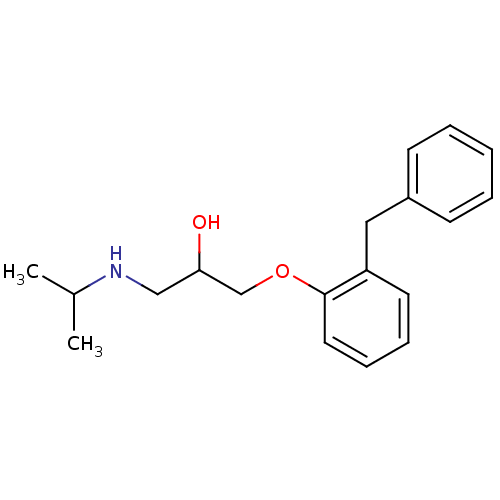 (1-(2-Benzyl-phenoxy)-3-isopropylamino-propan-2-ol ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029160 (CHEMBL55300 | Disodium salt of 13-[-2,6,10-trimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012926 (CHEMBL308335 | N-(2-Cyclohexyl-1-cyclohexylmethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50406415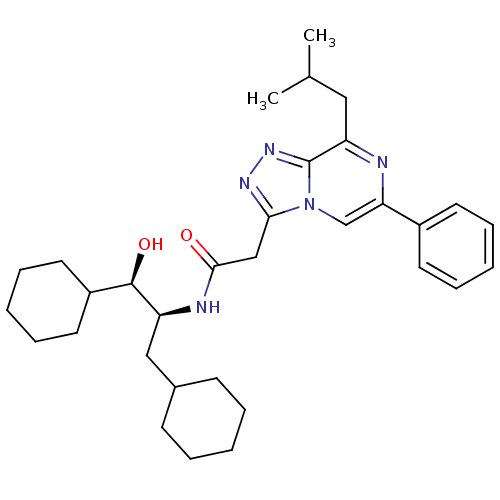 (CHEMBL2115170) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012931 (CHEMBL73980 | N-(1-Cyclohexylmethyl-2-hydroxy-hexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||