

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM163037 (US9061041, Compound C) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 30 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.3 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present ... | US Patent US9061041 (2015) BindingDB Entry DOI: 10.7270/Q2GM862K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM163034 (US9061041, 99) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 60 | -41.2 | n/a | n/a | n/a | n/a | n/a | 7.3 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present ... | US Patent US9061041 (2015) BindingDB Entry DOI: 10.7270/Q2GM862K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM163032 (US9061041, 26) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 70 | -40.8 | n/a | n/a | n/a | n/a | n/a | 7.3 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present ... | US Patent US9061041 (2015) BindingDB Entry DOI: 10.7270/Q2GM862K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM163036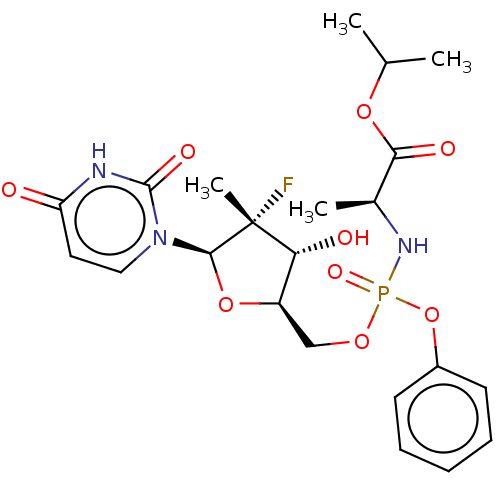 (US9061041, Compound B) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 1.50E+3 | -33.2 | n/a | n/a | n/a | n/a | n/a | 7.3 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present ... | US Patent US9061041 (2015) BindingDB Entry DOI: 10.7270/Q2GM862K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM163033 (US9061041, 24) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.70E+3 | -30.4 | n/a | n/a | n/a | n/a | n/a | 7.3 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present ... | US Patent US9061041 (2015) BindingDB Entry DOI: 10.7270/Q2GM862K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM163035 (US9061041, 93) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.00E+3 | -29.8 | n/a | n/a | n/a | n/a | n/a | 7.3 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present ... | US Patent US9061041 (2015) BindingDB Entry DOI: 10.7270/Q2GM862K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228403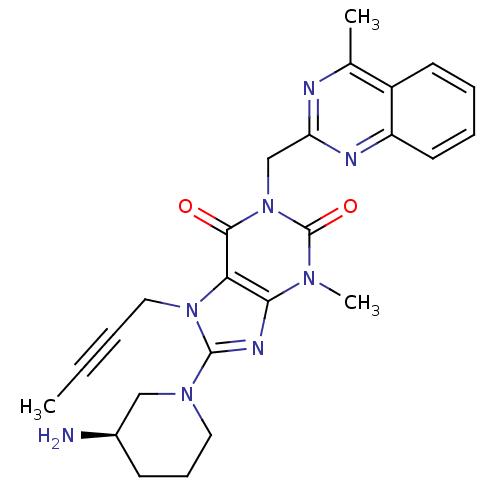 ((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 preincubated for 30 mins followed by Gly-Pro-AMC addition measured for 50 mins by continuous fluorescence assay | ACS Med Chem Lett 7: 498-501 (2016) Article DOI: 10.1021/acsmedchemlett.6b00027 BindingDB Entry DOI: 10.7270/Q2CN75SM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14776 (2-{2-ethoxy-5-[(4-ethylpiperazine-1-)sulfonyl]phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of human phosphodiesterase 5 | Bioorg Med Chem Lett 15: 2365-9 (2005) Article DOI: 10.1016/j.bmcl.2005.02.083 BindingDB Entry DOI: 10.7270/Q2TM79MQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120086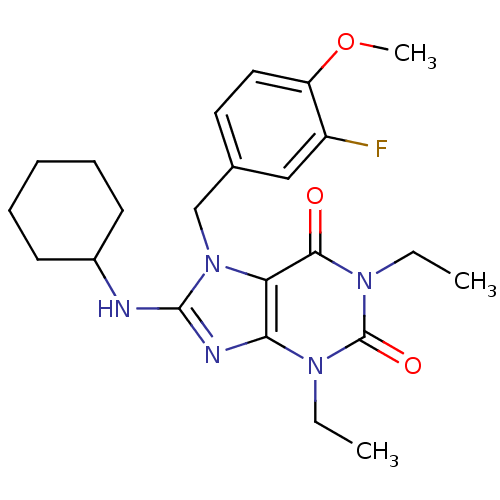 (8-Cyclohexylamino-1,3-diethyl-7-(3-fluoro-4-methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477384 (CHEMBL248276) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma-secretase mediated amyloid beta-40 production in HEK293 cell membranes | Bioorg Med Chem Lett 17: 511-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.011 BindingDB Entry DOI: 10.7270/Q26W9DTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50140583 (5-ethyl-3-(4-hydroxybenzyl)-2-(2-phenyl-1-ethynyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme | Bioorg Med Chem Lett 14: 1291-4 (2004) Article DOI: 10.1016/j.bmcl.2003.12.027 BindingDB Entry DOI: 10.7270/Q2BG2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50220297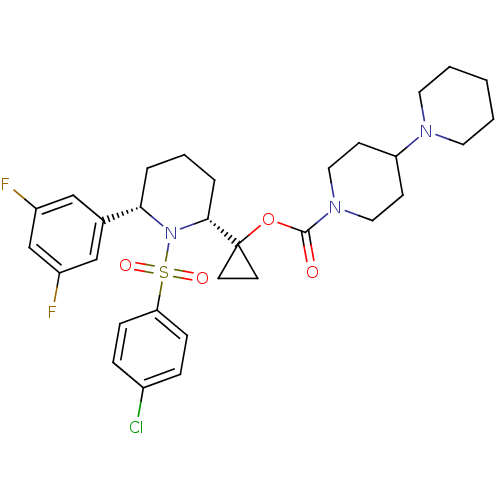 (1-((2R,6S)-1-(4-chlorophenylsulfonyl)-6-(3,5-diflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma-secretase assessed as reduction of membrane Abeta40 level | Bioorg Med Chem Lett 17: 5330-5 (2007) Article DOI: 10.1016/j.bmcl.2007.08.013 BindingDB Entry DOI: 10.7270/Q21G0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120069 (8-Cyclohexylamino-1,3-diethyl-7-(3-fluoro-4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119782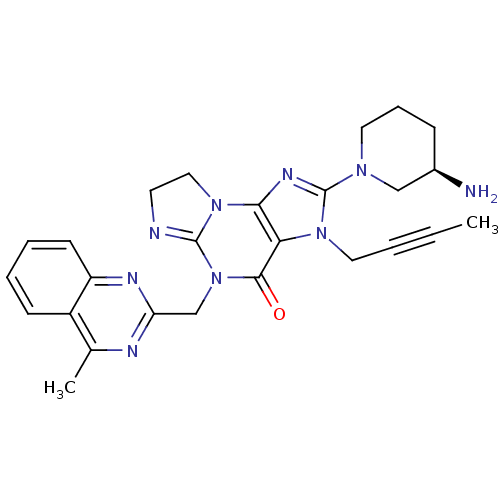 (US8691832, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description DPP4 activity was measured using a continuous fluorometric assay. The substrate, Gly-Pro-AMC, was cleaved by DPP4 to release the fluorescent AMC grou... | US Patent US8691832 (2014) BindingDB Entry DOI: 10.7270/Q2QV3K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119782 (US8691832, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120079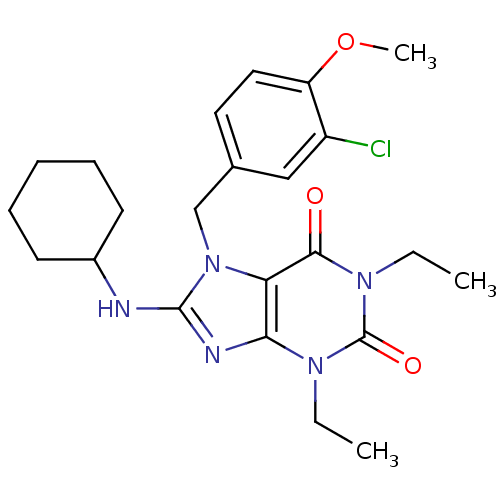 (7-(3-Chloro-4-methoxy-benzyl)-8-cyclohexylamino-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120074 (8-Cyclohexylamino-1,3-diethyl-7-(4-hydroxy-benzyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50140608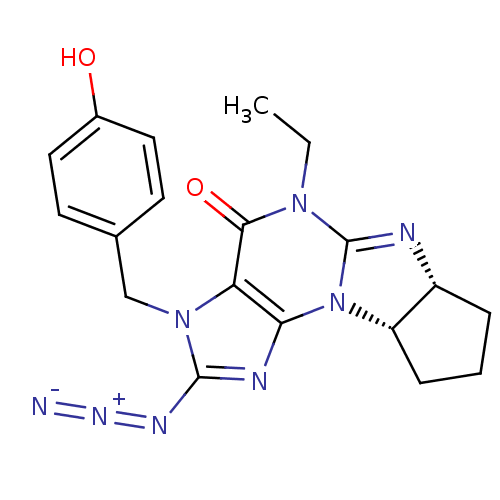 (2-azido-5-ethyl-3-(4-hydroxybenzyl)-(6aR,9aS)-3,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme | Bioorg Med Chem Lett 14: 1291-4 (2004) Article DOI: 10.1016/j.bmcl.2003.12.027 BindingDB Entry DOI: 10.7270/Q2BG2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120067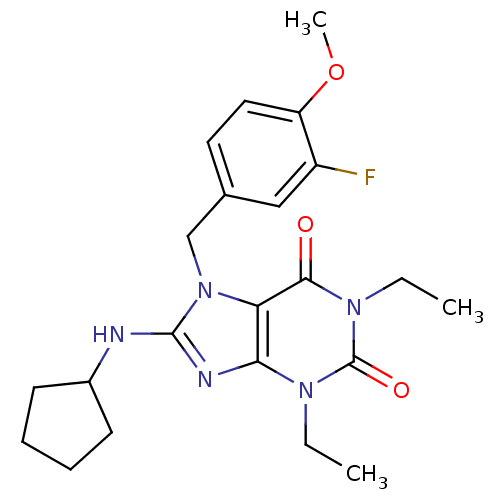 (8-Cyclopentylamino-1,3-diethyl-7-(3-fluoro-4-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477389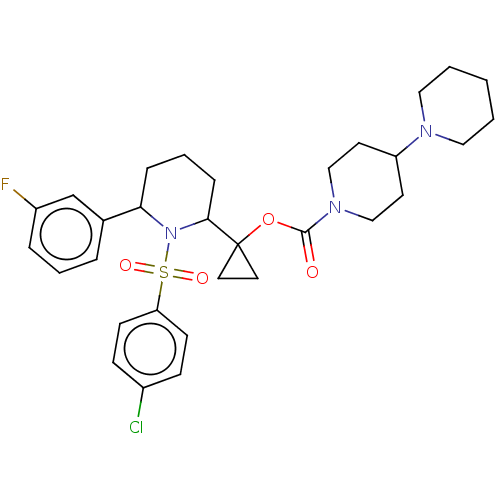 (CHEMBL248285) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma-secretase mediated amyloid beta-40 production in HEK293 cell membranes | Bioorg Med Chem Lett 17: 511-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.011 BindingDB Entry DOI: 10.7270/Q26W9DTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477366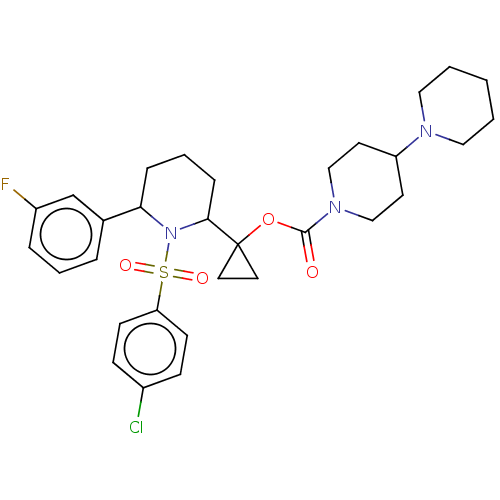 (CHEMBL537890) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma-secretase mediated amyloid beta-40 production in HEK293 cell membranes | Bioorg Med Chem Lett 17: 511-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.011 BindingDB Entry DOI: 10.7270/Q26W9DTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120072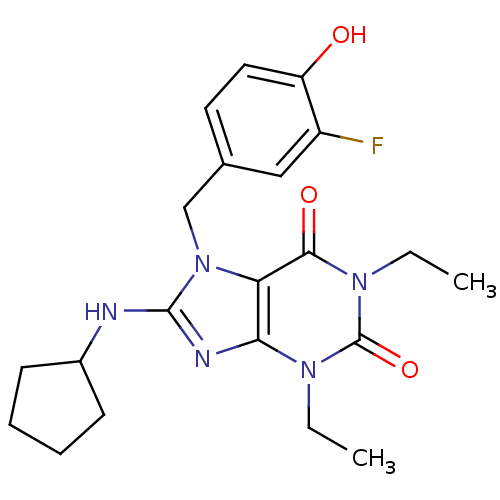 (8-Cyclopentylamino-1,3-diethyl-7-(3-fluoro-4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50140624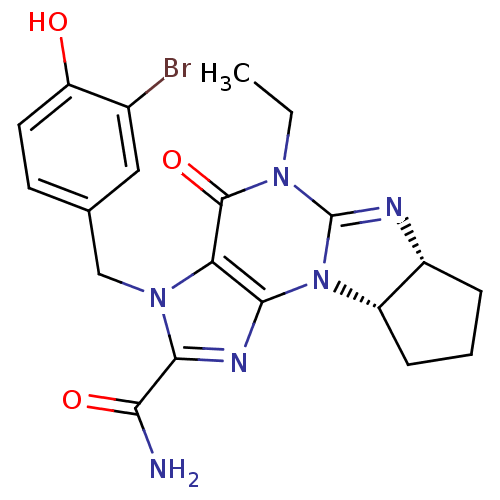 (3-(3-bromo-4-hydroxybenzyl)-5-ethyl-4-oxo-(6aR,9aS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme | Bioorg Med Chem Lett 14: 1291-4 (2004) Article DOI: 10.1016/j.bmcl.2003.12.027 BindingDB Entry DOI: 10.7270/Q2BG2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50140619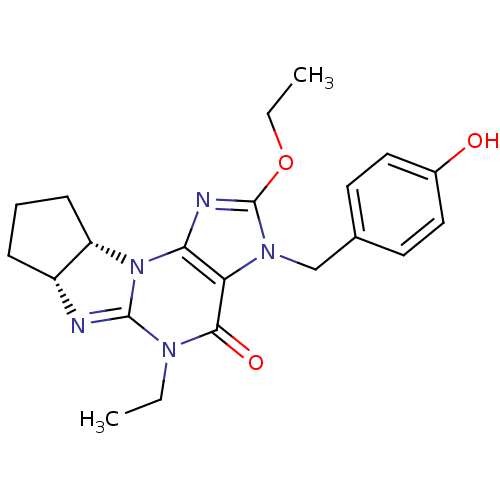 (2-ethoxy-5-ethyl-3-(4-hydroxybenzyl)-(6aR,9aS)-3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme | Bioorg Med Chem Lett 14: 1291-4 (2004) Article DOI: 10.1016/j.bmcl.2003.12.027 BindingDB Entry DOI: 10.7270/Q2BG2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477377 (CHEMBL248070) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma-secretase mediated amyloid beta-40 production in HEK293 cell membranes | Bioorg Med Chem Lett 17: 511-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.011 BindingDB Entry DOI: 10.7270/Q26W9DTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM103948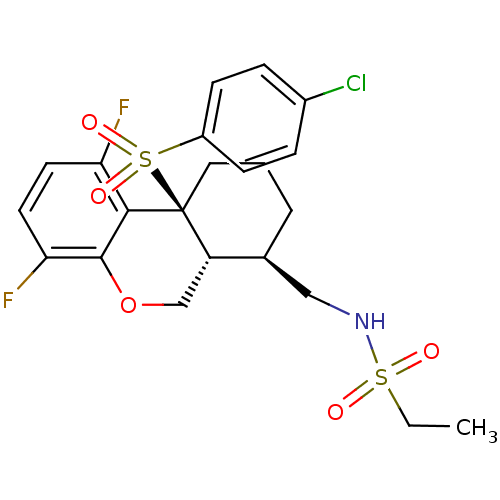 (US8569521, 144) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Gamma-secretase activity was determined as described by Zhang et al. (Biochemistry, 40(16), 5049-5055, 2001). | US Patent US8569521 (2013) BindingDB Entry DOI: 10.7270/Q2DR2T4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119801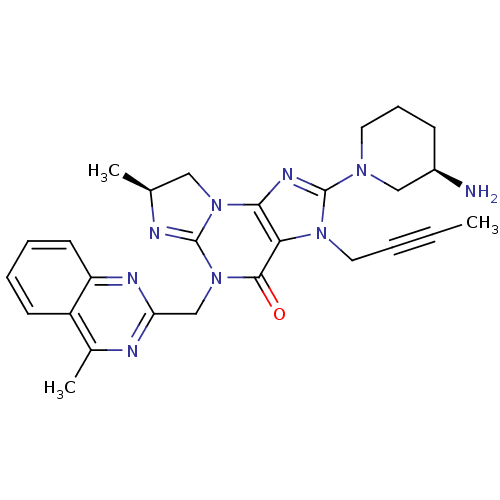 (US8691832, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description DPP4 activity was measured using a continuous fluorometric assay. The substrate, Gly-Pro-AMC, was cleaved by DPP4 to release the fluorescent AMC grou... | US Patent US8691832 (2014) BindingDB Entry DOI: 10.7270/Q2QV3K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477372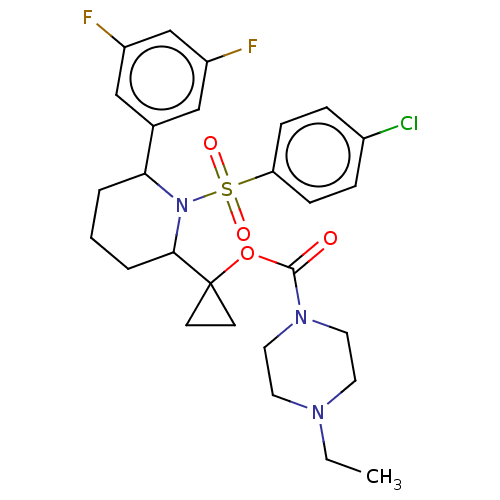 (CHEMBL245413) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma-secretase mediated amyloid beta-40 production in HEK293 cell membranes | Bioorg Med Chem Lett 17: 511-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.011 BindingDB Entry DOI: 10.7270/Q26W9DTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50140593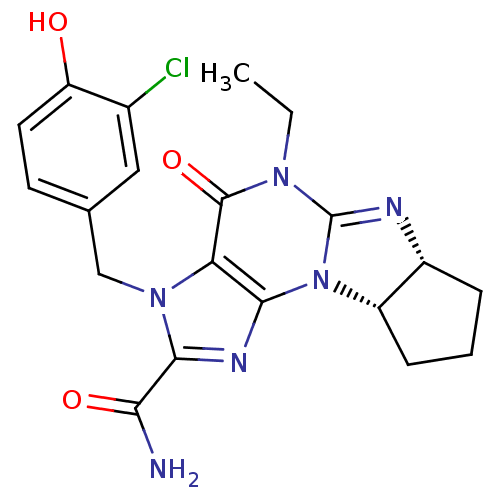 (3-(3-chloro-4-hydroxybenzyl)-5-ethyl-4-oxo-(6aR,9a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme | Bioorg Med Chem Lett 14: 1291-4 (2004) Article DOI: 10.1016/j.bmcl.2003.12.027 BindingDB Entry DOI: 10.7270/Q2BG2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120071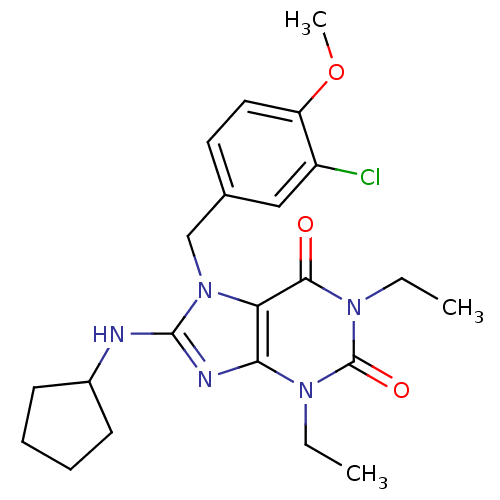 (7-(3-Chloro-4-methoxy-benzyl)-8-cyclopentylamino-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120080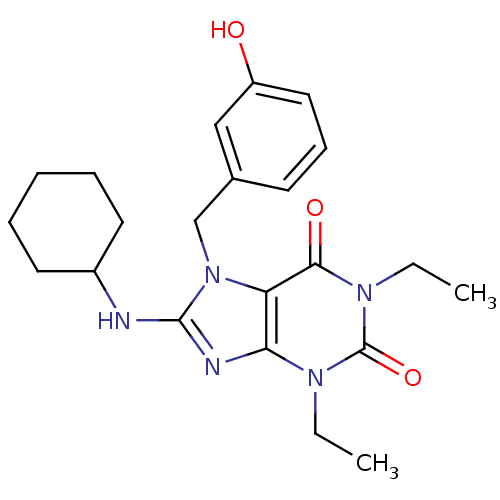 (8-Cyclohexylamino-1,3-diethyl-7-(3-hydroxy-benzyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50140590 (2N-methyl-3-(3-chloro-4-hydroxybenzyl)-5-ethyl-4-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme | Bioorg Med Chem Lett 14: 1291-4 (2004) Article DOI: 10.1016/j.bmcl.2003.12.027 BindingDB Entry DOI: 10.7270/Q2BG2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119786 (US8691832, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50140582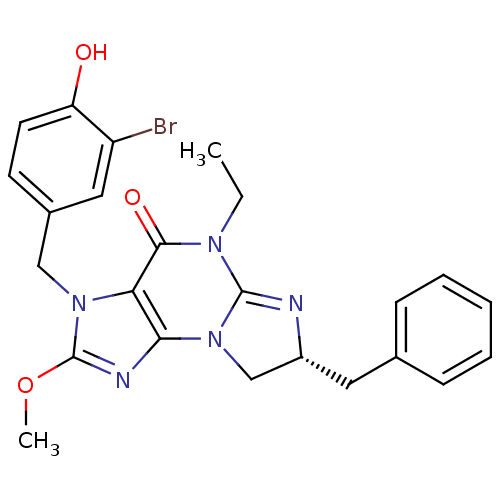 ((R)-3-(3-bromo-4-hydroxybenzyl)-7-benzyl-5-ethyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme | Bioorg Med Chem Lett 14: 1291-4 (2004) Article DOI: 10.1016/j.bmcl.2003.12.027 BindingDB Entry DOI: 10.7270/Q2BG2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119786 (US8691832, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description DPP4 activity was measured using a continuous fluorometric assay. The substrate, Gly-Pro-AMC, was cleaved by DPP4 to release the fluorescent AMC grou... | US Patent US8691832 (2014) BindingDB Entry DOI: 10.7270/Q2QV3K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50140580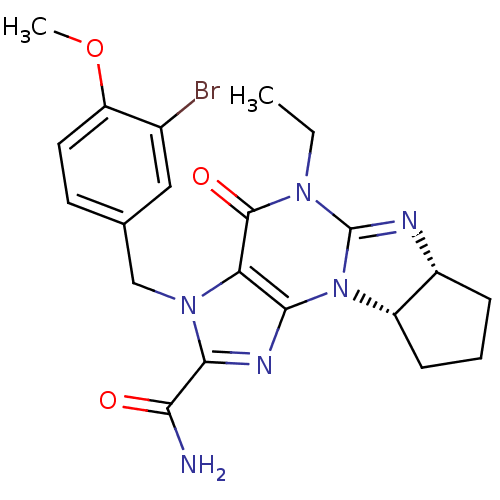 (3-(3-bromo-4-methoxybenzyl)-5-ethyl-4-oxo-(6aR,9aS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme | Bioorg Med Chem Lett 14: 1291-4 (2004) Article DOI: 10.1016/j.bmcl.2003.12.027 BindingDB Entry DOI: 10.7270/Q2BG2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120070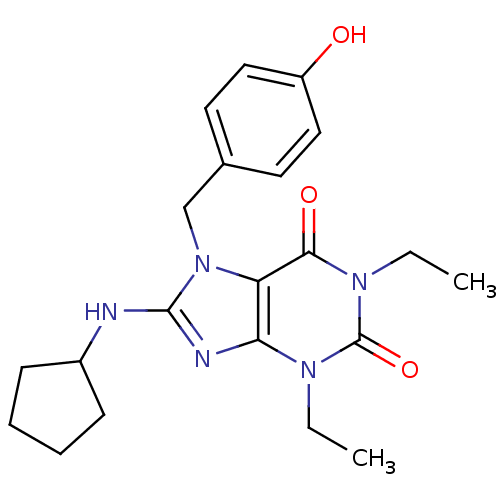 (8-Cyclopentylamino-1,3-diethyl-7-(4-methoxy-benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119788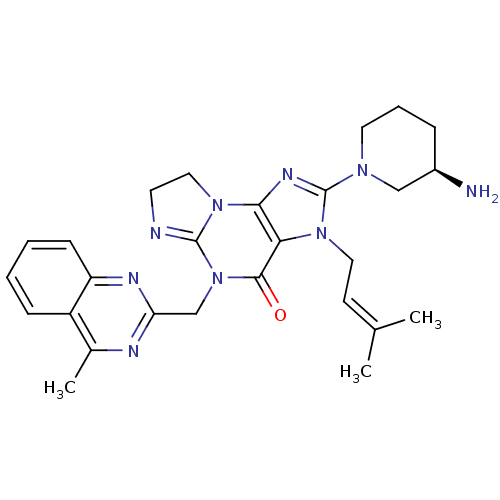 (US8691832, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50140603 (3-(3-bromo-4-hydroxybenzyl)-5-ethyl-2-methoxy-(6aR...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme | Bioorg Med Chem Lett 14: 1291-4 (2004) Article DOI: 10.1016/j.bmcl.2003.12.027 BindingDB Entry DOI: 10.7270/Q2BG2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50140620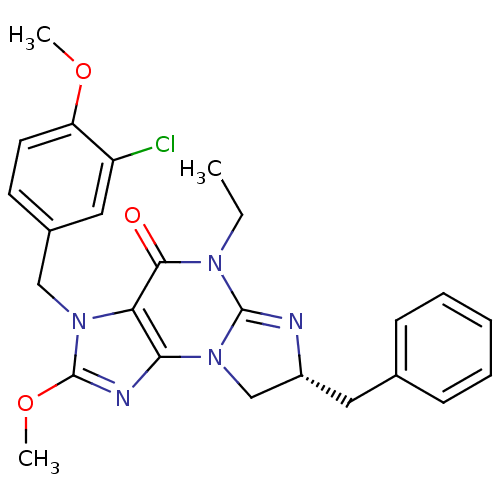 (7-benzyl-3-(3-chloro-4-methoxybenzyl)-5-ethyl-2-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme | Bioorg Med Chem Lett 14: 1291-4 (2004) Article DOI: 10.1016/j.bmcl.2003.12.027 BindingDB Entry DOI: 10.7270/Q2BG2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119788 (US8691832, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description DPP4 activity was measured using a continuous fluorometric assay. The substrate, Gly-Pro-AMC, was cleaved by DPP4 to release the fluorescent AMC grou... | US Patent US8691832 (2014) BindingDB Entry DOI: 10.7270/Q2QV3K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120081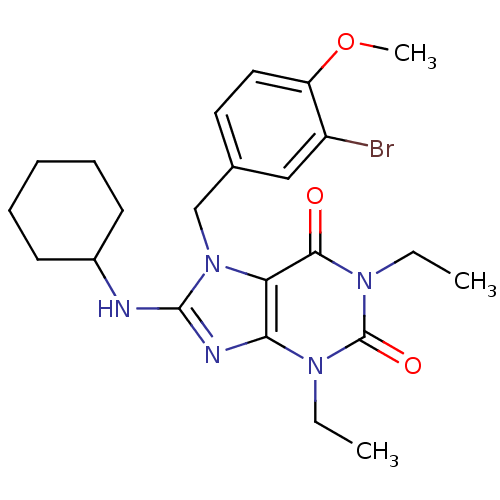 (7-(3-Bromo-4-methoxy-benzyl)-8-cyclohexylamino-1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM60417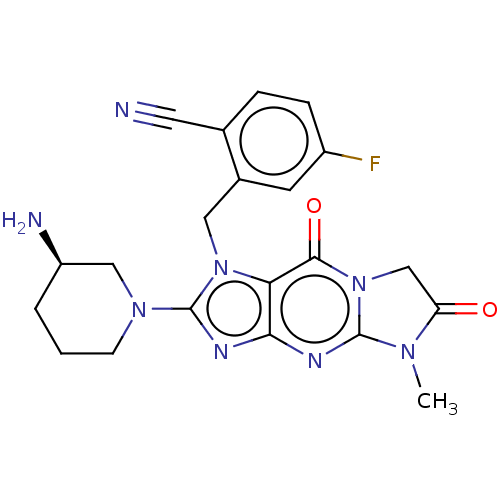 (US9051329, Example 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 preincubated for 30 mins followed by Gly-Pro-AMC addition measured for 50 mins by continuous fluorescence assay | ACS Med Chem Lett 7: 498-501 (2016) Article DOI: 10.1021/acsmedchemlett.6b00027 BindingDB Entry DOI: 10.7270/Q2CN75SM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50140598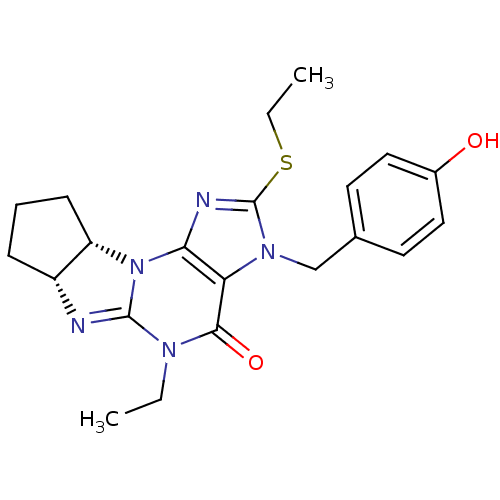 (5-ethyl-2-ethylsulfanyl-3-(4-hydroxybenzyl)-(6aR,9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme | Bioorg Med Chem Lett 14: 1291-4 (2004) Article DOI: 10.1016/j.bmcl.2003.12.027 BindingDB Entry DOI: 10.7270/Q2BG2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120078 (7-(3-Chloro-4-hydroxy-benzyl)-8-cyclopentylamino-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477363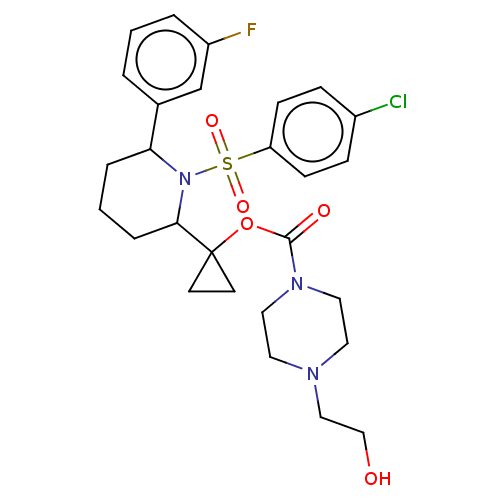 (CHEMBL248275) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma-secretase mediated amyloid beta-40 production in HEK293 cell membranes | Bioorg Med Chem Lett 17: 511-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.011 BindingDB Entry DOI: 10.7270/Q26W9DTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50140585 ((R)-3-(3-chloro-4-hydroxybenzyl)-7-benzyl-5-ethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme | Bioorg Med Chem Lett 14: 1291-4 (2004) Article DOI: 10.1016/j.bmcl.2003.12.027 BindingDB Entry DOI: 10.7270/Q2BG2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50140584 (3-(3-chloro-4-hydroxybenzyl)-5-ethyl-2-methoxy-(6a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme | Bioorg Med Chem Lett 14: 1291-4 (2004) Article DOI: 10.1016/j.bmcl.2003.12.027 BindingDB Entry DOI: 10.7270/Q2BG2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2 (Homo sapiens (Human)) | BDBM50129274 (CHEMBL3629745) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in HEK293 cells using human APP Swedish/London double mutant as substrate assessed as formation of amyloid beta 40 afte... | J Med Chem 58: 8806-17 (2015) Article DOI: 10.1021/acs.jmedchem.5b00774 BindingDB Entry DOI: 10.7270/Q2HQ41R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2 (Homo sapiens (Human)) | BDBM50129274 (CHEMBL3629745) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in HEK293 cells using human APP Swedish/London double mutant as substrate assessed as formation of amyloid beta 42 afte... | J Med Chem 58: 8806-17 (2015) Article DOI: 10.1021/acs.jmedchem.5b00774 BindingDB Entry DOI: 10.7270/Q2HQ41R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 755 total ) | Next | Last >> |