 Found 1249 hits with Last Name = 'holt' and Initial = 'da'
Found 1249 hits with Last Name = 'holt' and Initial = 'da' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50458766
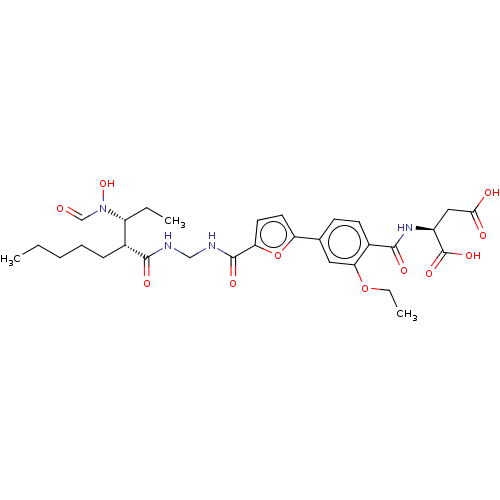
(CHEMBL4212386)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1ccc(C(=O)N[C@@H](CC(O)=O)C(O)=O)c(OCC)c1 |r| Show InChI InChI=1S/C30H40N4O11/c1-4-7-8-9-19(22(5-2)34(43)17-35)27(38)31-16-32-29(40)24-13-12-23(45-24)18-10-11-20(25(14-18)44-6-3)28(39)33-21(30(41)42)15-26(36)37/h10-14,17,19,21-22,43H,4-9,15-16H2,1-3H3,(H,31,38)(H,32,40)(H,33,39)(H,36,37)(H,41,42)/t19-,21+,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tolloid-like protein 1
(Homo sapiens) | BDBM50458766

(CHEMBL4212386)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1ccc(C(=O)N[C@@H](CC(O)=O)C(O)=O)c(OCC)c1 |r| Show InChI InChI=1S/C30H40N4O11/c1-4-7-8-9-19(22(5-2)34(43)17-35)27(38)31-16-32-29(40)24-13-12-23(45-24)18-10-11-20(25(14-18)44-6-3)28(39)33-21(30(41)42)15-26(36)37/h10-14,17,19,21-22,43H,4-9,15-16H2,1-3H3,(H,31,38)(H,32,40)(H,33,39)(H,36,37)(H,41,42)/t19-,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tolloid-like protein 2
(Homo sapiens) | BDBM50458766

(CHEMBL4212386)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1ccc(C(=O)N[C@@H](CC(O)=O)C(O)=O)c(OCC)c1 |r| Show InChI InChI=1S/C30H40N4O11/c1-4-7-8-9-19(22(5-2)34(43)17-35)27(38)31-16-32-29(40)24-13-12-23(45-24)18-10-11-20(25(14-18)44-6-3)28(39)33-21(30(41)42)15-26(36)37/h10-14,17,19,21-22,43H,4-9,15-16H2,1-3H3,(H,31,38)(H,32,40)(H,33,39)(H,36,37)(H,41,42)/t19-,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50458771
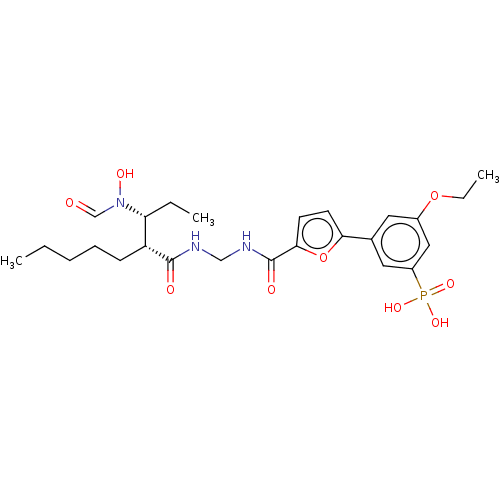
(CHEMBL4214046)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1cc(OCC)cc(c1)P(O)(O)=O |r| Show InChI InChI=1S/C25H36N3O9P/c1-4-7-8-9-20(21(5-2)28(32)16-29)24(30)26-15-27-25(31)23-11-10-22(37-23)17-12-18(36-6-3)14-19(13-17)38(33,34)35/h10-14,16,20-21,32H,4-9,15H2,1-3H3,(H,26,30)(H,27,31)(H2,33,34,35)/t20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50039257
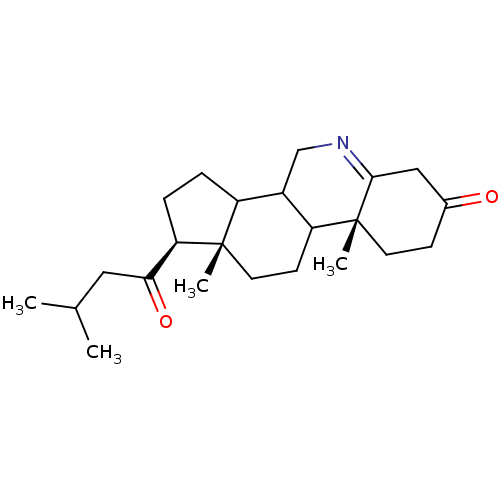
((1S,9aR,11aS)-9a,11a-Dimethyl-1-(3-methyl-butyryl)...)Show SMILES CC(C)CC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:12| Show InChI InChI=1S/C23H35NO2/c1-14(2)11-20(26)19-6-5-17-16-13-24-21-12-15(25)7-9-23(21,4)18(16)8-10-22(17,19)3/h14,16-19H,5-13H2,1-4H3/t16?,17?,18?,19-,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50043604
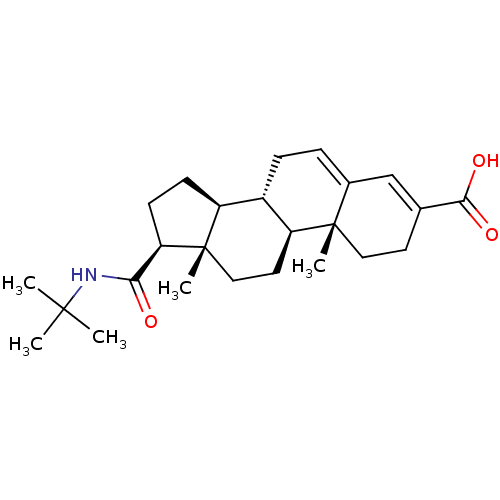
((8S,9S,10R,13S,14S,17S)-17-(tert-butylcarbamoyl)-1...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC=C4C=C(CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O |c:15,t:13| Show InChI InChI=1S/C25H37NO3/c1-23(2,3)26-21(27)20-9-8-18-17-7-6-16-14-15(22(28)29)10-12-24(16,4)19(17)11-13-25(18,20)5/h6,14,17-20H,7-13H2,1-5H3,(H,26,27)(H,28,29)/t17-,18-,19-,20+,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50043604

((8S,9S,10R,13S,14S,17S)-17-(tert-butylcarbamoyl)-1...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC=C4C=C(CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O |c:15,t:13| Show InChI InChI=1S/C25H37NO3/c1-23(2,3)26-21(27)20-9-8-18-17-7-6-16-14-15(22(28)29)10-12-24(16,4)19(17)11-13-25(18,20)5/h6,14,17-20H,7-13H2,1-5H3,(H,26,27)(H,28,29)/t17-,18-,19-,20+,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Apparent inhibition constant towards human Steroid 5-alpha-reductase type 2 |
Bioorg Med Chem Lett 4: 2327-2330 (1994)
Article DOI: 10.1016/0960-894X(94)85034-8
BindingDB Entry DOI: 10.7270/Q25Q4X8P |
More data for this
Ligand-Target Pair | |
Tolloid-like protein 1
(Homo sapiens) | BDBM50458771

(CHEMBL4214046)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1cc(OCC)cc(c1)P(O)(O)=O |r| Show InChI InChI=1S/C25H36N3O9P/c1-4-7-8-9-20(21(5-2)28(32)16-29)24(30)26-15-27-25(31)23-11-10-22(37-23)17-12-18(36-6-3)14-19(13-17)38(33,34)35/h10-14,16,20-21,32H,4-9,15H2,1-3H3,(H,26,30)(H,27,31)(H2,33,34,35)/t20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | |
Tolloid-like protein 2
(Homo sapiens) | BDBM50458771

(CHEMBL4214046)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1cc(OCC)cc(c1)P(O)(O)=O |r| Show InChI InChI=1S/C25H36N3O9P/c1-4-7-8-9-20(21(5-2)28(32)16-29)24(30)26-15-27-25(31)23-11-10-22(37-23)17-12-18(36-6-3)14-19(13-17)38(33,34)35/h10-14,16,20-21,32H,4-9,15H2,1-3H3,(H,26,30)(H,27,31)(H2,33,34,35)/t20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50403606
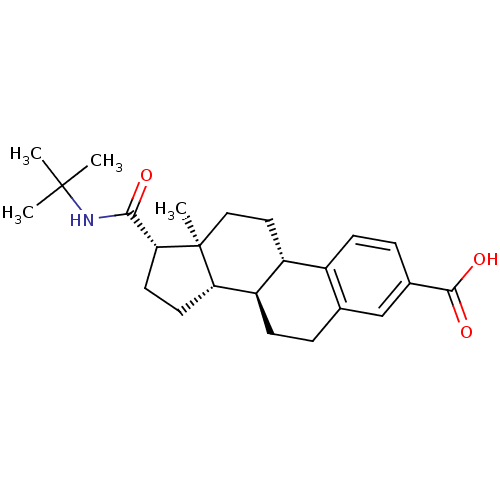
(CHEMBL1627951)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CCc4cc(ccc4[C@H]3CC[C@]12C)C(O)=O Show InChI InChI=1S/C24H33NO3/c1-23(2,3)25-21(26)20-10-9-19-18-8-5-14-13-15(22(27)28)6-7-16(14)17(18)11-12-24(19,20)4/h6-7,13,17-20H,5,8-12H2,1-4H3,(H,25,26)(H,27,28)/t17-,18-,19+,20-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TNNI3K
(Homo sapiens (Human)) | BDBM50578225
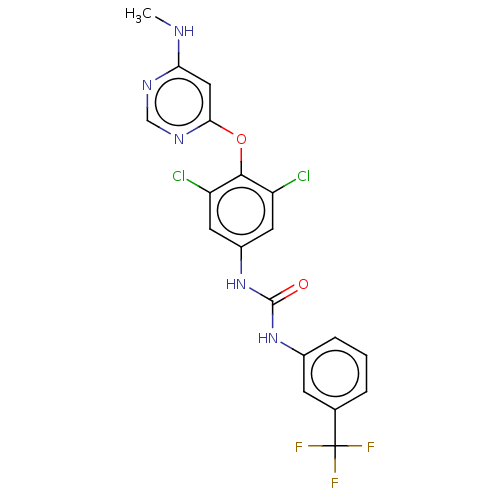
(CHEMBL4869303)Show SMILES CNc1cc(Oc2c(Cl)cc(NC(=O)Nc3cccc(c3)C(F)(F)F)cc2Cl)ncn1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to full length human His-MBP-TNNI3K assessed as off-rate constant in presence of rhodamine green labeled GW805818X by fluorescence c... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50403324
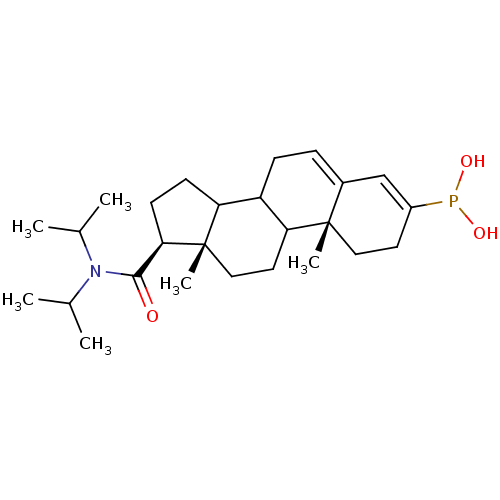
(CHEMBL78060)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C=C(CC[C@]4(C)C3CC[C@]12C)P(O)O |c:17,t:15| Show InChI InChI=1S/C26H42NO3P/c1-16(2)27(17(3)4)24(28)23-10-9-21-20-8-7-18-15-19(31(29)30)11-13-25(18,5)22(20)12-14-26(21,23)6/h7,15-17,20-23,29-30H,8-14H2,1-6H3/t20?,21?,22?,23-,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Apparent inhibition constant towards human Steroid 5-alpha-reductase type 2 |
Bioorg Med Chem Lett 4: 2327-2330 (1994)
Article DOI: 10.1016/0960-894X(94)85034-8
BindingDB Entry DOI: 10.7270/Q25Q4X8P |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50039285
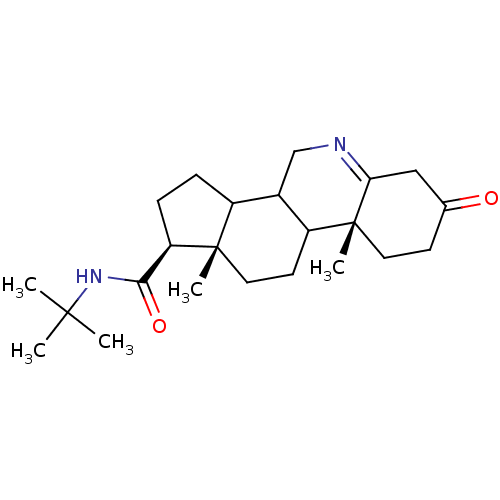
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:13| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)18-7-6-16-15-13-24-19-12-14(26)8-10-23(19,5)17(15)9-11-22(16,18)4/h15-18H,6-13H2,1-5H3,(H,25,27)/t15?,16?,17?,18-,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50403610
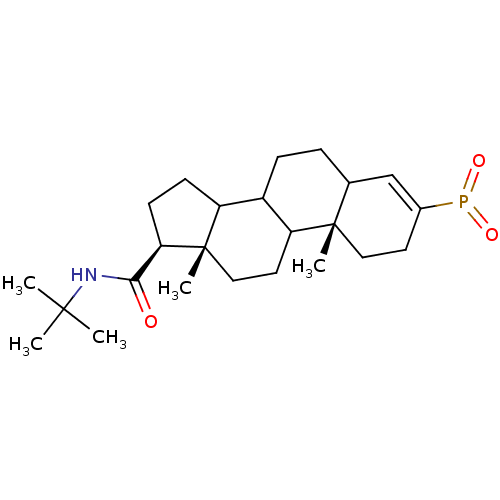
(CHEMBL143220)Show SMILES CC(C)(C)NC(=O)[C@H]1CCC2C3CCC4C=C(CC[C@]4(C)C3CC[C@]12C)P(=O)=O |c:15| Show InChI InChI=1S/C24H38NO3P/c1-22(2,3)25-21(26)20-9-8-18-17-7-6-15-14-16(29(27)28)10-12-23(15,4)19(17)11-13-24(18,20)5/h14-15,17-20H,6-13H2,1-5H3,(H,25,26)/t15?,17?,18?,19?,20-,23+,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50334788

((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4NC(=O)C=C[C@]4(C)[C@H]3CC[C@]12C |r,c:18| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27)/t14-,15-,16-,17+,18+,22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Apparent inhibition constant towards human Steroid 5-alpha-reductase type 2 |
Bioorg Med Chem Lett 4: 2327-2330 (1994)
Article DOI: 10.1016/0960-894X(94)85034-8
BindingDB Entry DOI: 10.7270/Q25Q4X8P |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50213061
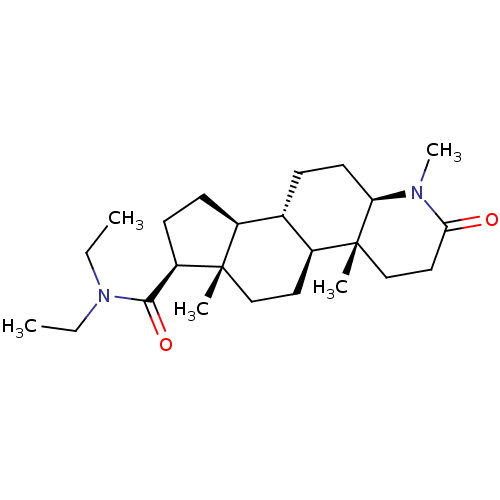
(CHEMBL2298601)Show SMILES CCN(CC)C(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4N(C)C(=O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C24H40N2O2/c1-6-26(7-2)22(28)19-10-9-17-16-8-11-20-24(4,15-13-21(27)25(20)5)18(16)12-14-23(17,19)3/h16-20H,6-15H2,1-5H3/t16-,17-,18-,19+,20+,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50213061

(CHEMBL2298601)Show SMILES CCN(CC)C(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4N(C)C(=O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C24H40N2O2/c1-6-26(7-2)22(28)19-10-9-17-16-8-11-20-24(4,15-13-21(27)25(20)5)18(16)12-14-23(17,19)3/h16-20H,6-15H2,1-5H3/t16-,17-,18-,19+,20+,23-,24+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50368782
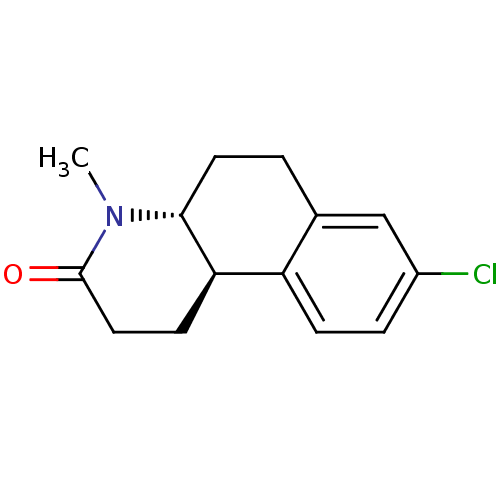
(Bexlosteride | CHEMBL24955 | LY-191704)Show InChI InChI=1S/C14H16ClNO/c1-16-13-6-2-9-8-10(15)3-4-11(9)12(13)5-7-14(16)17/h3-4,8,12-13H,2,5-7H2,1H3/t12-,13-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human Steroid 5-alpha-reductase type I |
Bioorg Med Chem Lett 4: 1365-1368 (1994)
Article DOI: 10.1016/S0960-894X(01)80363-2
BindingDB Entry DOI: 10.7270/Q26M381K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50368782

(Bexlosteride | CHEMBL24955 | LY-191704)Show InChI InChI=1S/C14H16ClNO/c1-16-13-6-2-9-8-10(15)3-4-11(9)12(13)5-7-14(16)17/h3-4,8,12-13H,2,5-7H2,1H3/t12-,13-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human Steroid 5-alpha-reductase type I |
Bioorg Med Chem Lett 4: 1365-1368 (1994)
Article DOI: 10.1016/S0960-894X(01)80363-2
BindingDB Entry DOI: 10.7270/Q26M381K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50180894
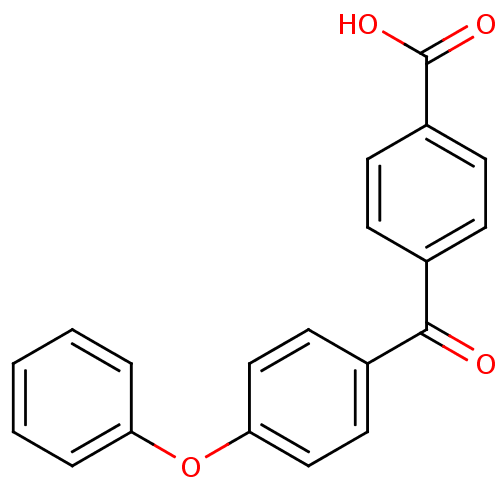
(4-(4-Phenoxy-benzoyl)-benzoic acid | 4-(4-phenoxyb...)Show InChI InChI=1S/C20H14O4/c21-19(14-6-8-16(9-7-14)20(22)23)15-10-12-18(13-11-15)24-17-4-2-1-3-5-17/h1-13H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403336
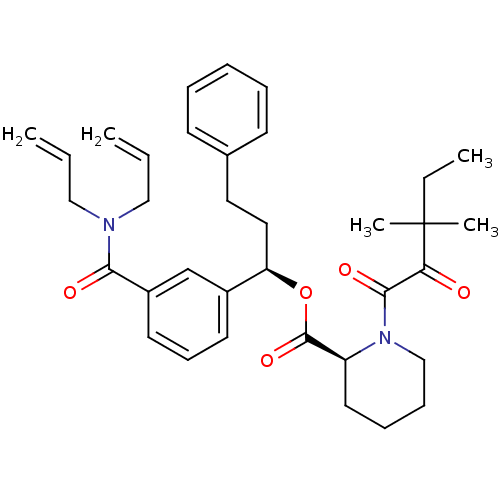
(CHEMBL326881)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccccc1)c1cccc(c1)C(=O)N(CC=C)CC=C Show InChI InChI=1S/C35H44N2O5/c1-6-22-36(23-7-2)32(39)28-18-14-17-27(25-28)30(21-20-26-15-10-9-11-16-26)42-34(41)29-19-12-13-24-37(29)33(40)31(38)35(4,5)8-3/h6-7,9-11,14-18,25,29-30H,1-2,8,12-13,19-24H2,3-5H3/t29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12) |
Bioorg Med Chem Lett 4: 325-328 (1994)
Article DOI: 10.1016/S0960-894X(01)80137-2
BindingDB Entry DOI: 10.7270/Q21Z45M7 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403339
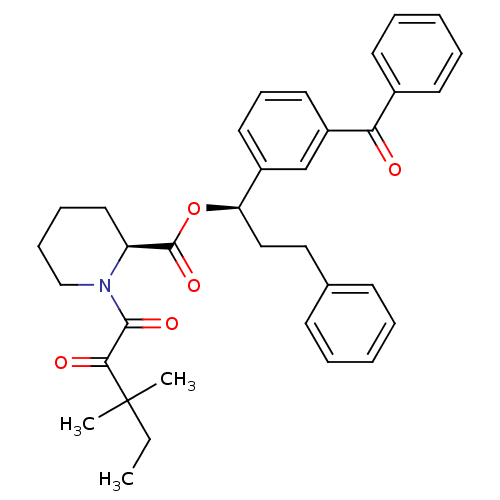
(CHEMBL109950)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccccc1)c1cccc(c1)C(=O)c1ccccc1 Show InChI InChI=1S/C35H39NO5/c1-4-35(2,3)32(38)33(39)36-23-12-11-20-29(36)34(40)41-30(22-21-25-14-7-5-8-15-25)27-18-13-19-28(24-27)31(37)26-16-9-6-10-17-26/h5-10,13-19,24,29-30H,4,11-12,20-23H2,1-3H3/t29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12) |
Bioorg Med Chem Lett 4: 325-328 (1994)
Article DOI: 10.1016/S0960-894X(01)80137-2
BindingDB Entry DOI: 10.7270/Q21Z45M7 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057468

((10R,13S,17S)-17-Diisopropylcarbamoyl-10,13-dimeth...)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3C=CC4=CC(=CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:14,18,t:16| Show InChI InChI=1S/C27H39NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h7-8,11,15-17,20-23H,9-10,12-14H2,1-6H3,(H,30,31)/t20?,21?,22?,23-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Steroid 5-alpha-reductase was determined in human prostatic tissue expressed as apparent inhibition constant; Ra... |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50406356

(CHEMBL426217)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C=C(CC[C@]4(C)C3C=C[C@]12C)C(O)=O |c:17,26,t:15| Show InChI InChI=1S/C27H39NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h7,12,14-17,20-23H,8-11,13H2,1-6H3,(H,30,31)/t20?,21?,22?,23-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057448

((10R,13S,17S)-17-Diisopropylcarbamoyl-10,13-dimeth...)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:17,t:15| Show InChI InChI=1S/C27H41NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h7,15-17,20-23H,8-14H2,1-6H3,(H,30,31)/t20?,21?,22?,23-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Steroid 5-alpha-reductase was determined in human prostatic tissue expressed as apparent inhibition constant; Ra... |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057448

((10R,13S,17S)-17-Diisopropylcarbamoyl-10,13-dimeth...)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:17,t:15| Show InChI InChI=1S/C27H41NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h7,15-17,20-23H,8-14H2,1-6H3,(H,30,31)/t20?,21?,22?,23-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against human prostatic Steroid 5-alpha-reductase |
Bioorg Med Chem Lett 1: 27-32 (1991)
Article DOI: 10.1016/S0960-894X(01)81084-2
BindingDB Entry DOI: 10.7270/Q29P31J1 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50044879

((4aR,10bR)-8-Chloro-4,10b-dimethyl-1,4,4a,5,6,10b-...)Show InChI InChI=1S/C15H18ClNO/c1-15-8-7-14(18)17(2)13(15)6-3-10-9-11(16)4-5-12(10)15/h4-5,9,13H,3,6-8H2,1-2H3/t13-,15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human Steroid 5-alpha-reductase type I |
Bioorg Med Chem Lett 4: 1365-1368 (1994)
Article DOI: 10.1016/S0960-894X(01)80363-2
BindingDB Entry DOI: 10.7270/Q26M381K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50039257

((1S,9aR,11aS)-9a,11a-Dimethyl-1-(3-methyl-butyryl)...)Show SMILES CC(C)CC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:12| Show InChI InChI=1S/C23H35NO2/c1-14(2)11-20(26)19-6-5-17-16-13-24-21-12-15(25)7-9-23(21,4)18(16)8-10-22(17,19)3/h14,16-19H,5-13H2,1-4H3/t16?,17?,18?,19-,22+,23-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407305
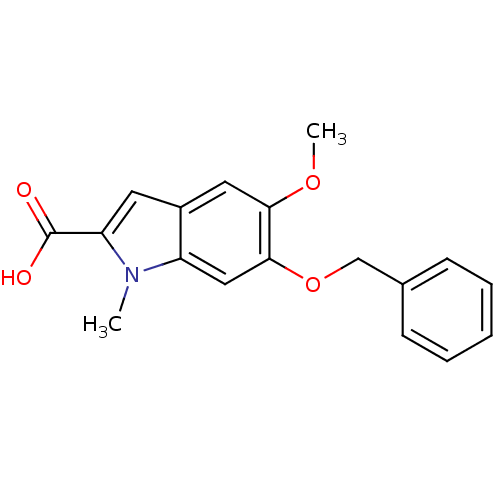
(CHEMBL36772)Show InChI InChI=1S/C18H17NO4/c1-19-14-10-17(23-11-12-6-4-3-5-7-12)16(22-2)9-13(14)8-15(19)18(20)21/h3-10H,11H2,1-2H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50009101
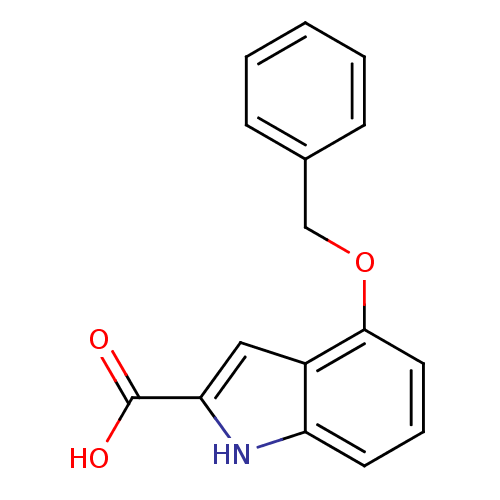
(4-Benzyloxy-1H-indole-2-carboxylic acid | CHEMBL23...)Show InChI InChI=1S/C16H13NO3/c18-16(19)14-9-12-13(17-14)7-4-8-15(12)20-10-11-5-2-1-3-6-11/h1-9,17H,10H2,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50044879

((4aR,10bR)-8-Chloro-4,10b-dimethyl-1,4,4a,5,6,10b-...)Show InChI InChI=1S/C15H18ClNO/c1-15-8-7-14(18)17(2)13(15)6-3-10-9-11(16)4-5-12(10)15/h4-5,9,13H,3,6-8H2,1-2H3/t13-,15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057467
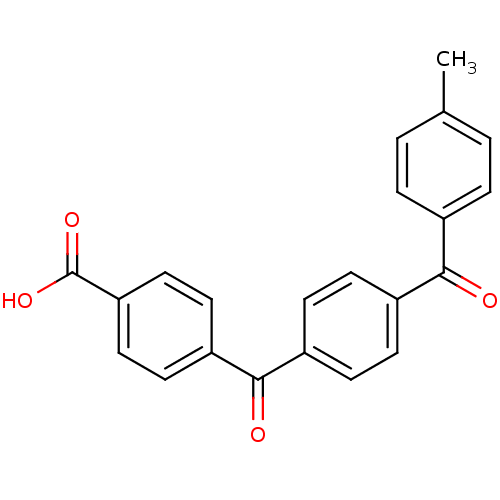
(4-[4-(4-Methyl-benzoyl)-benzoyl]-benzoic acid | CH...)Show SMILES Cc1ccc(cc1)C(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H16O4/c1-14-2-4-15(5-3-14)20(23)16-6-8-17(9-7-16)21(24)18-10-12-19(13-11-18)22(25)26/h2-13H,1H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50044879

((4aR,10bR)-8-Chloro-4,10b-dimethyl-1,4,4a,5,6,10b-...)Show InChI InChI=1S/C15H18ClNO/c1-15-8-7-14(18)17(2)13(15)6-3-10-9-11(16)4-5-12(10)15/h4-5,9,13H,3,6-8H2,1-2H3/t13-,15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human Steroid 5-alpha-reductase type I |
Bioorg Med Chem Lett 4: 1365-1368 (1994)
Article DOI: 10.1016/S0960-894X(01)80363-2
BindingDB Entry DOI: 10.7270/Q26M381K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50044879

((4aR,10bR)-8-Chloro-4,10b-dimethyl-1,4,4a,5,6,10b-...)Show InChI InChI=1S/C15H18ClNO/c1-15-8-7-14(18)17(2)13(15)6-3-10-9-11(16)4-5-12(10)15/h4-5,9,13H,3,6-8H2,1-2H3/t13-,15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50452771

(CHEMBL2311126)Show SMILES [H][C@@]12CC[C@H](C(=O)NC(C)(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2(C)C=C(CC[C@]12C)C(O)=O |c:26| Show InChI InChI=1S/C26H41NO3/c1-23(2,3)27-21(28)20-8-7-18-17-10-12-24(4)15-16(22(29)30)9-14-26(24,6)19(17)11-13-25(18,20)5/h15,17-20H,7-14H2,1-6H3,(H,27,28)(H,29,30)/t17-,18-,19-,20+,24-,25-,26+/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in rat ventral prostates. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TNNI3K
(Mus musculus) | BDBM50578225

(CHEMBL4869303)Show SMILES CNc1cc(Oc2c(Cl)cc(NC(=O)Nc3cccc(c3)C(F)(F)F)cc2Cl)ncn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to full length mouse His-MBP-TNNI3K assessed as off-rate constant in presence of rhodamine green labeled GW805818X by fluorescence c... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50044885
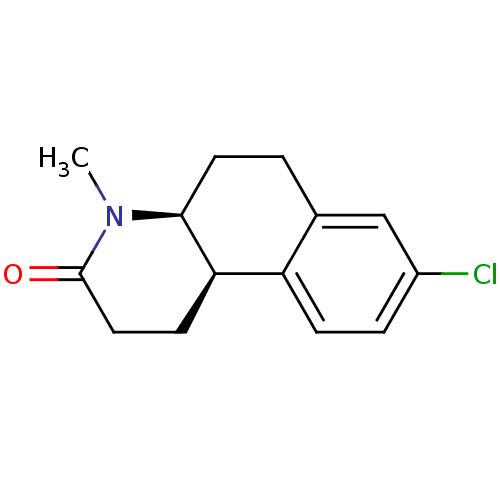
((4aS,10bR)-8-Chloro-4-methyl-1,4,4a,5,6,10b-hexahy...)Show InChI InChI=1S/C14H16ClNO/c1-16-13-6-2-9-8-10(15)3-4-11(9)12(13)5-7-14(16)17/h3-4,8,12-13H,2,5-7H2,1H3/t12-,13+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human Steroid 5-alpha-reductase type I |
Bioorg Med Chem Lett 4: 1365-1368 (1994)
Article DOI: 10.1016/S0960-894X(01)80363-2
BindingDB Entry DOI: 10.7270/Q26M381K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50044885

((4aS,10bR)-8-Chloro-4-methyl-1,4,4a,5,6,10b-hexahy...)Show InChI InChI=1S/C14H16ClNO/c1-16-13-6-2-9-8-10(15)3-4-11(9)12(13)5-7-14(16)17/h3-4,8,12-13H,2,5-7H2,1H3/t12-,13+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human Steroid 5-alpha-reductase type I |
Bioorg Med Chem Lett 4: 1365-1368 (1994)
Article DOI: 10.1016/S0960-894X(01)80363-2
BindingDB Entry DOI: 10.7270/Q26M381K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407304
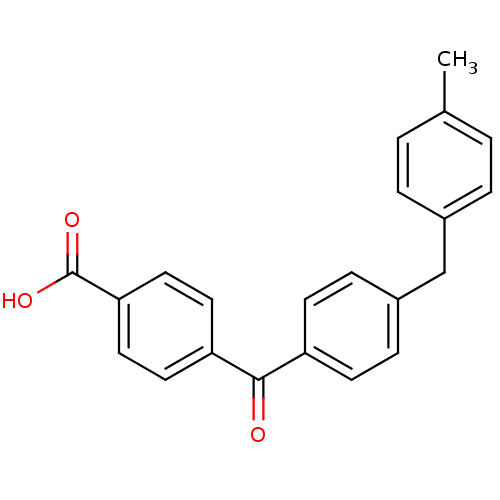
(CHEMBL289822)Show SMILES Cc1ccc(Cc2ccc(cc2)C(=O)c2ccc(cc2)C(O)=O)cc1 Show InChI InChI=1S/C22H18O3/c1-15-2-4-16(5-3-15)14-17-6-8-18(9-7-17)21(23)19-10-12-20(13-11-19)22(24)25/h2-13H,14H2,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50044881
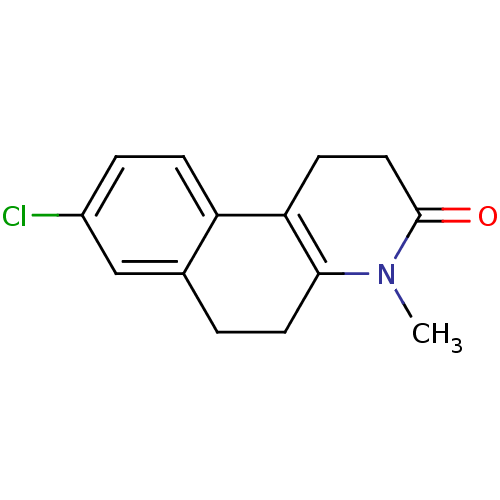
(8-Chloro-4-methyl-1,4,5,6-tetrahydro-2H-benzo[f]qu...)Show InChI InChI=1S/C14H14ClNO/c1-16-13-6-2-9-8-10(15)3-4-11(9)12(13)5-7-14(16)17/h3-4,8H,2,5-7H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human Steroid 5-alpha-reductase type I |
Bioorg Med Chem Lett 4: 1365-1368 (1994)
Article DOI: 10.1016/S0960-894X(01)80363-2
BindingDB Entry DOI: 10.7270/Q26M381K |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403332
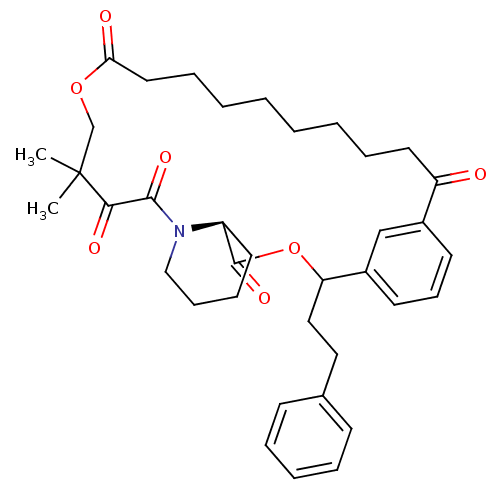
(CHEMBL323633)Show SMILES CC1(C)COC(=O)CCCCCCCCC(=O)c2cccc(c2)C(CCc2ccccc2)OC(=O)[C@@H]2CCCCN2C(=O)C1=O Show InChI InChI=1S/C37H47NO7/c1-37(2)26-44-33(40)21-11-6-4-3-5-10-20-31(39)28-17-14-18-29(25-28)32(23-22-27-15-8-7-9-16-27)45-36(43)30-19-12-13-24-38(30)35(42)34(37)41/h7-9,14-18,25,30,32H,3-6,10-13,19-24,26H2,1-2H3/t30-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12) |
Bioorg Med Chem Lett 4: 325-328 (1994)
Article DOI: 10.1016/S0960-894X(01)80137-2
BindingDB Entry DOI: 10.7270/Q21Z45M7 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50044879

((4aR,10bR)-8-Chloro-4,10b-dimethyl-1,4,4a,5,6,10b-...)Show InChI InChI=1S/C15H18ClNO/c1-15-8-7-14(18)17(2)13(15)6-3-10-9-11(16)4-5-12(10)15/h4-5,9,13H,3,6-8H2,1-2H3/t13-,15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Apparent inhibition constant towards human Steroid 5-alpha-reductase type I |
Bioorg Med Chem Lett 4: 2327-2330 (1994)
Article DOI: 10.1016/0960-894X(94)85034-8
BindingDB Entry DOI: 10.7270/Q25Q4X8P |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407307
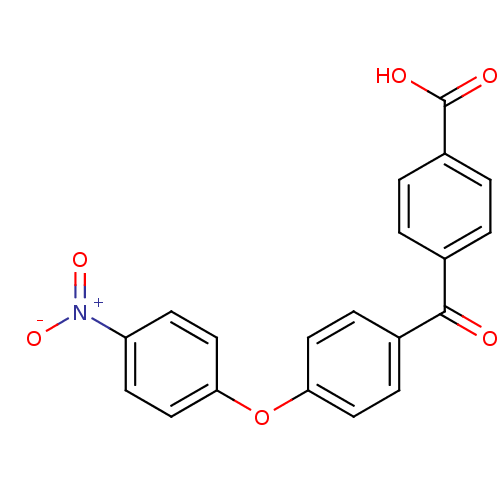
(CHEMBL285651)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(Oc2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C20H13NO6/c22-19(13-1-3-15(4-2-13)20(23)24)14-5-9-17(10-6-14)27-18-11-7-16(8-12-18)21(25)26/h1-12H,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50366682
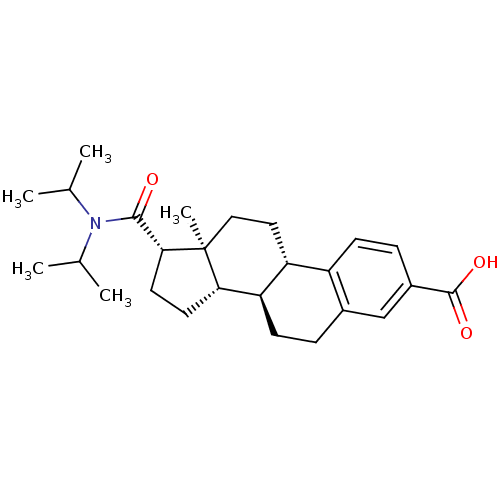
(CHEMBL1627395)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CC[C@H]2[C@@H]3CCc4cc(ccc4[C@H]3CC[C@]12C)C(O)=O Show InChI InChI=1S/C26H37NO3/c1-15(2)27(16(3)4)24(28)23-11-10-22-21-9-6-17-14-18(25(29)30)7-8-19(17)20(21)12-13-26(22,23)5/h7-8,14-16,20-23H,6,9-13H2,1-5H3,(H,29,30)/t20-,21-,22+,23-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against human prostatic Steroid 5-alpha-reductase |
Bioorg Med Chem Lett 1: 27-32 (1991)
Article DOI: 10.1016/S0960-894X(01)81084-2
BindingDB Entry DOI: 10.7270/Q29P31J1 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50043604

((8S,9S,10R,13S,14S,17S)-17-(tert-butylcarbamoyl)-1...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC=C4C=C(CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O |c:15,t:13| Show InChI InChI=1S/C25H37NO3/c1-23(2,3)26-21(27)20-9-8-18-17-7-6-16-14-15(22(28)29)10-12-24(16,4)19(17)11-13-25(18,20)5/h6,14,17-20H,7-13H2,1-5H3,(H,26,27)(H,28,29)/t17-,18-,19-,20+,24-,25-/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Steroid 5-alpha-reductase was determined in rat ventral prostates expressed as apparent inhibition constant; Ran... |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407316
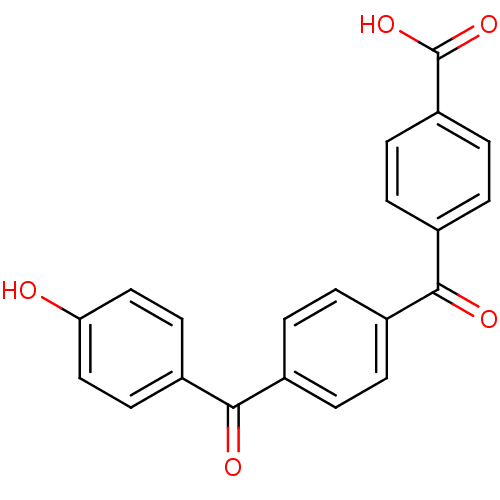
(CHEMBL286372)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(=O)c1ccc(O)cc1 Show InChI InChI=1S/C21H14O5/c22-18-11-9-16(10-12-18)20(24)14-3-1-13(2-4-14)19(23)15-5-7-17(8-6-15)21(25)26/h1-12,22H,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403335
(CHEMBL325145)Show SMILES CC1(C)CCC(=O)CCCCC(=O)OCC(C)(C)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]1CCc1ccccc1 Show InChI InChI=1S/C32H45NO7/c1-31(2)20-19-24(34)14-8-9-16-27(35)39-22-32(3,4)28(36)29(37)33-21-11-10-15-25(33)30(38)40-26(31)18-17-23-12-6-5-7-13-23/h5-7,12-13,25-26H,8-11,14-22H2,1-4H3/t25-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12) |
Bioorg Med Chem Lett 4: 325-328 (1994)
Article DOI: 10.1016/S0960-894X(01)80137-2
BindingDB Entry DOI: 10.7270/Q21Z45M7 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50452770

(CHEMBL2311127)Show SMILES [H][C@@]12CC[C@H](C(=O)N(C(C)C)C(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])C(F)=C(CC[C@]12C)C(O)=O |c:29| Show InChI InChI=1S/C27H42FNO3/c1-15(2)29(16(3)4)24(30)22-10-9-19-17-7-8-21-23(28)18(25(31)32)11-13-26(21,5)20(17)12-14-27(19,22)6/h15-17,19-22H,7-14H2,1-6H3,(H,31,32)/t17-,19-,20-,21-,22+,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50057479
(7-Bromo-9,10-dihydro-phenanthrene-2-carboxylic aci...)Show InChI InChI=1S/C15H11BrO2/c16-12-4-6-14-10(8-12)2-1-9-7-11(15(17)18)3-5-13(9)14/h3-8H,1-2H2,(H,17,18) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50391269

(CHEMBL48467)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC[C@H]4C=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:17| Show InChI InChI=1S/C27H43NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h15-17,19-23H,7-14H2,1-6H3,(H,30,31)/t19-,20?,21?,22?,23+,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against 5-alpha reductase was determined in human prostatic tissue expressed as apparent inhibition constant |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data