 Found 261 hits with Last Name = 'parry' and Initial = 'dm'
Found 261 hits with Last Name = 'parry' and Initial = 'dm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437403

(CHEMBL2408771)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-2-[#6]-[#6]-[#6]-[#7]-[#6]-2-[#6]-1 Show InChI InChI=1S/C26H32N6O3/c1-17(2)11-13-31-22-23(28-25(31)30-14-19-10-7-12-27-20(19)15-30)29(3)26(35)32(24(22)34)16-21(33)18-8-5-4-6-9-18/h4-6,8-9,11,19-20,27H,7,10,12-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427749
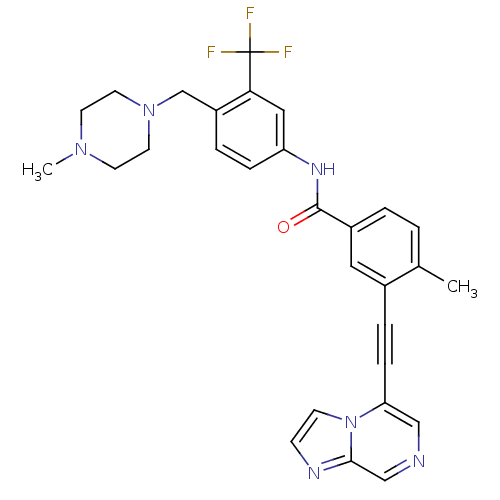
(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437403

(CHEMBL2408771)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-2-[#6]-[#6]-[#6]-[#7]-[#6]-2-[#6]-1 Show InChI InChI=1S/C26H32N6O3/c1-17(2)11-13-31-22-23(28-25(31)30-14-19-10-7-12-27-20(19)15-30)29(3)26(35)32(24(22)34)16-21(33)18-8-5-4-6-9-18/h4-6,8-9,11,19-20,27H,7,10,12-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427748
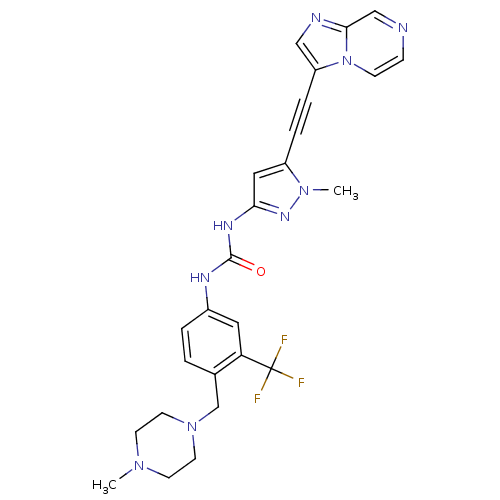
(CHEMBL2324925)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cnccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-31-24-16-30-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427749

(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type Abl1 kinase (unknown origin) |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50228407

(8-((R)-3-amino-piperidin-1-yl)-3-methyl-7-(3-methy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-1 Show InChI InChI=1S/C24H30N6O3/c1-16(2)11-13-29-20-21(26-23(29)28-12-7-10-18(25)14-28)27(3)24(33)30(22(20)32)15-19(31)17-8-5-4-6-9-17/h4-6,8-9,11,18H,7,10,12-15,25H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377170
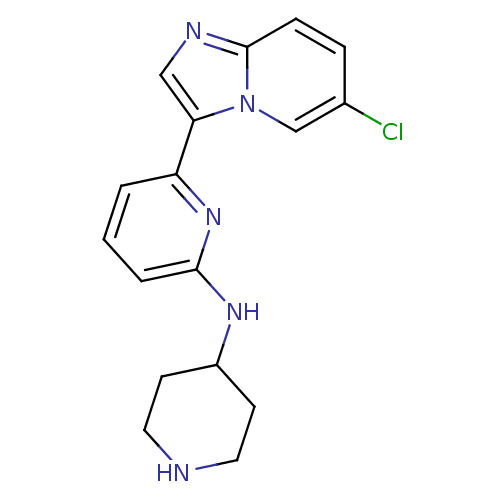
(CHEMBL256570 | US11254667, Compound I-2 | US115422...)Show InChI InChI=1S/C17H18ClN5/c18-12-4-5-17-20-10-15(23(17)11-12)14-2-1-3-16(22-14)21-13-6-8-19-9-7-13/h1-5,10-11,13,19H,6-9H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437404
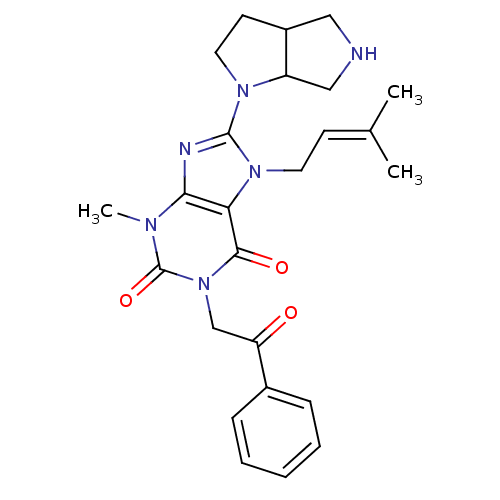
(CHEMBL2408655)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-2-[#6]-[#7]-[#6]-[#6]-1-2 Show InChI InChI=1S/C25H30N6O3/c1-16(2)9-11-30-21-22(27-24(30)29-12-10-18-13-26-14-19(18)29)28(3)25(34)31(23(21)33)15-20(32)17-7-5-4-6-8-17/h4-9,18-19,26H,10-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377180
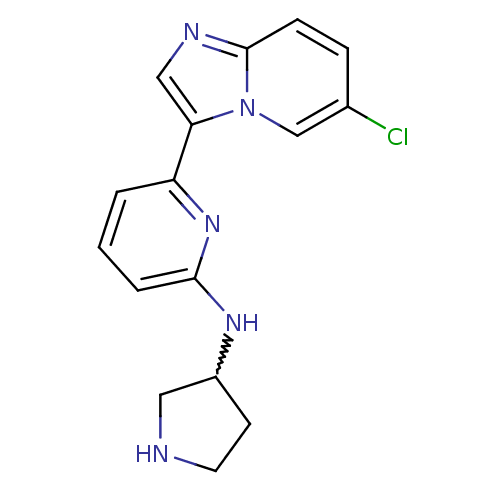
(CHEMBL401633)Show SMILES Clc1ccc2ncc(-c3cccc(NC4CCNC4)n3)n2c1 |w:14.13| Show InChI InChI=1S/C16H16ClN5/c17-11-4-5-16-19-9-14(22(16)10-11)13-2-1-3-15(21-13)20-12-6-7-18-8-12/h1-5,9-10,12,18H,6-8H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427747
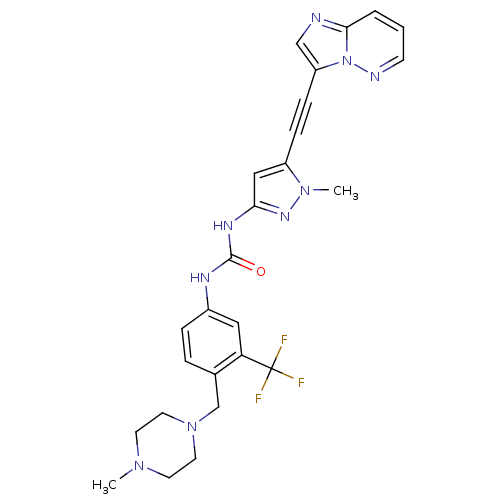
(CHEMBL2324926)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cccnn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-10-12-37(13-11-35)17-18-5-6-19(14-22(18)26(27,28)29)32-25(39)33-23-15-20(36(2)34-23)7-8-21-16-30-24-4-3-9-31-38(21)24/h3-6,9,14-16H,10-13,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type Abl1 kinase (unknown origin) |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228407

(8-((R)-3-amino-piperidin-1-yl)-3-methyl-7-(3-methy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-1 Show InChI InChI=1S/C24H30N6O3/c1-16(2)11-13-29-20-21(26-23(29)28-12-7-10-18(25)14-28)27(3)24(33)30(22(20)32)15-19(31)17-8-5-4-6-9-17/h4-6,8-9,11,18H,7,10,12-15,25H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427749

(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427749

(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427748

(CHEMBL2324925)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cnccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-31-24-16-30-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type Abl1 kinase (unknown origin) |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228407

(8-((R)-3-amino-piperidin-1-yl)-3-methyl-7-(3-methy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-1 Show InChI InChI=1S/C24H30N6O3/c1-16(2)11-13-29-20-21(26-23(29)28-12-7-10-18(25)14-28)27(3)24(33)30(22(20)32)15-19(31)17-8-5-4-6-9-17/h4-6,8-9,11,18H,7,10,12-15,25H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437404

(CHEMBL2408655)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-2-[#6]-[#7]-[#6]-[#6]-1-2 Show InChI InChI=1S/C25H30N6O3/c1-16(2)9-11-30-21-22(27-24(30)29-12-10-18-13-26-14-19(18)29)28(3)25(34)31(23(21)33)15-20(32)17-7-5-4-6-8-17/h4-9,18-19,26H,10-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427750
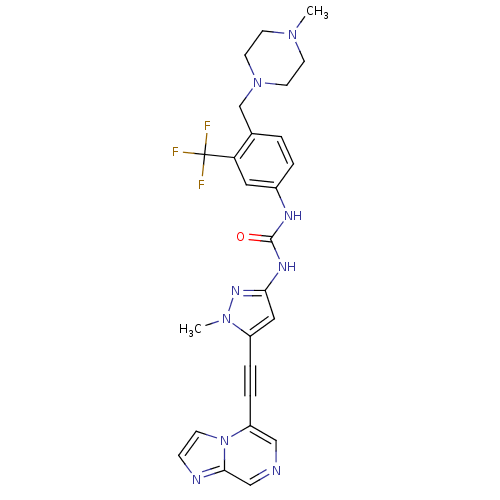
(CHEMBL2324923)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cncc5nccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-30-16-24-31-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type Abl1 kinase (unknown origin) |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50437403

(CHEMBL2408771)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-2-[#6]-[#6]-[#6]-[#7]-[#6]-2-[#6]-1 Show InChI InChI=1S/C26H32N6O3/c1-17(2)11-13-31-22-23(28-25(31)30-14-19-10-7-12-27-20(19)15-30)29(3)26(35)32(24(22)34)16-21(33)18-8-5-4-6-9-18/h4-6,8-9,11,19-20,27H,7,10,12-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427749

(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427750

(CHEMBL2324923)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cncc5nccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-30-16-24-31-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377165
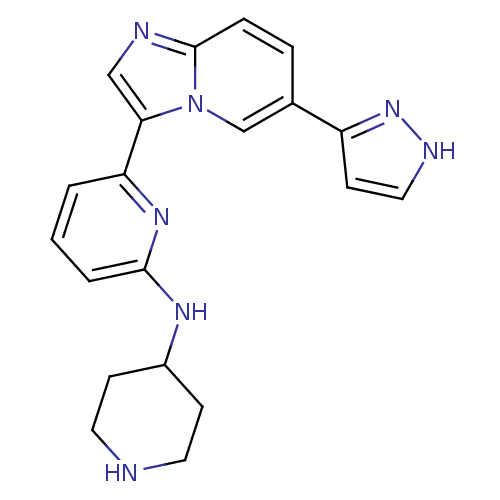
(CHEMBL255867)Show SMILES C1CC(CCN1)Nc1cccc(n1)-c1cnc2ccc(cn12)-c1cc[nH]n1 Show InChI InChI=1S/C20H21N7/c1-2-17(25-19(3-1)24-15-6-9-21-10-7-15)18-12-22-20-5-4-14(13-27(18)20)16-8-11-23-26-16/h1-5,8,11-13,15,21H,6-7,9-10H2,(H,23,26)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427748

(CHEMBL2324925)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cnccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-31-24-16-30-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377175
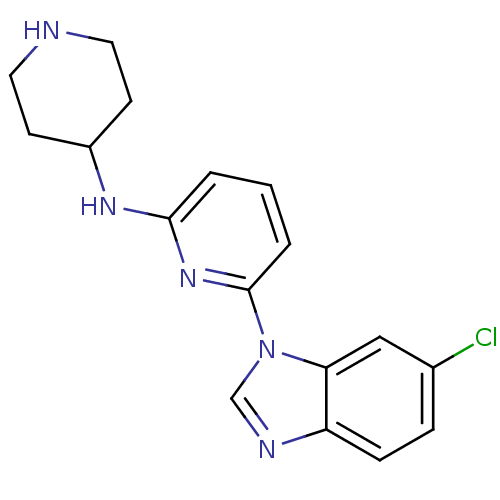
(CHEMBL436653)Show InChI InChI=1S/C17H18ClN5/c18-12-4-5-14-15(10-12)23(11-20-14)17-3-1-2-16(22-17)21-13-6-8-19-9-7-13/h1-5,10-11,13,19H,6-9H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50271563
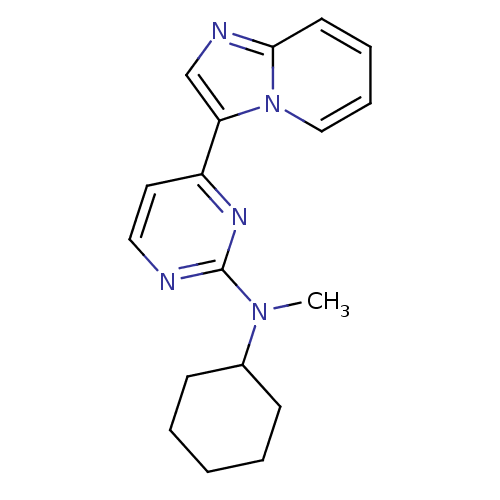
(CHEMBL482708 | N-cyclohexyl-4-(H-imidazo[1,2-a]pyr...)Show InChI InChI=1S/C18H21N5/c1-22(14-7-3-2-4-8-14)18-19-11-10-15(21-18)16-13-20-17-9-5-6-12-23(16)17/h5-6,9-14H,2-4,7-8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 (unknown origin) |
Bioorg Med Chem Lett 18: 3291-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.039
BindingDB Entry DOI: 10.7270/Q2NZ87FX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377168

(CHEMBL255873)Show InChI InChI=1S/C18H18N6/c19-10-13-4-5-18-21-11-16(24(18)12-13)15-2-1-3-17(23-15)22-14-6-8-20-9-7-14/h1-5,11-12,14,20H,6-9H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377163

(CHEMBL403358)Show InChI InChI=1S/C18H20N6O/c19-18(25)12-4-5-17-21-10-15(24(17)11-12)14-2-1-3-16(23-14)22-13-6-8-20-9-7-13/h1-5,10-11,13,20H,6-9H2,(H2,19,25)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377181
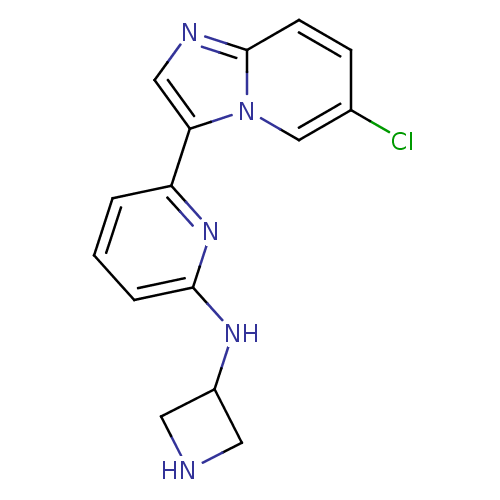
(CHEMBL256963 | US11254667, Compound I-15 | US11542...)Show InChI InChI=1S/C15H14ClN5/c16-10-4-5-15-18-8-13(21(15)9-10)12-2-1-3-14(20-12)19-11-6-17-7-11/h1-5,8-9,11,17H,6-7H2,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377169

(CHEMBL402629)Show InChI InChI=1S/C18H21N5O/c1-24-14-5-6-18-20-11-16(23(18)12-14)15-3-2-4-17(22-15)21-13-7-9-19-10-8-13/h2-6,11-13,19H,7-10H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427747

(CHEMBL2324926)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cccnn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-10-12-37(13-11-35)17-18-5-6-19(14-22(18)26(27,28)29)32-25(39)33-23-15-20(36(2)34-23)7-8-21-16-30-24-4-3-9-31-38(21)24/h3-6,9,14-16H,10-13,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437395
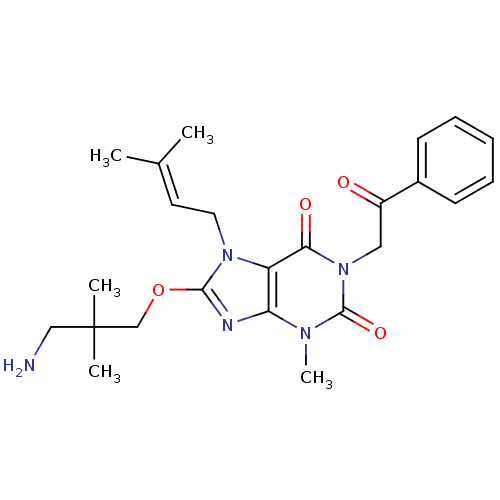
(CHEMBL2408638)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]C([#6])([#6])[#6]-[#7])nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 Show InChI InChI=1S/C24H31N5O4/c1-16(2)11-12-28-19-20(26-22(28)33-15-24(3,4)14-25)27(5)23(32)29(21(19)31)13-18(30)17-9-7-6-8-10-17/h6-11H,12-15,25H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377186

(CHEMBL403433)Show InChI InChI=1S/C16H17N5O/c1-22-12-5-6-16-18-9-14(21(16)10-12)13-3-2-4-15(20-13)19-11-7-17-8-11/h2-6,9-11,17H,7-8H2,1H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377166
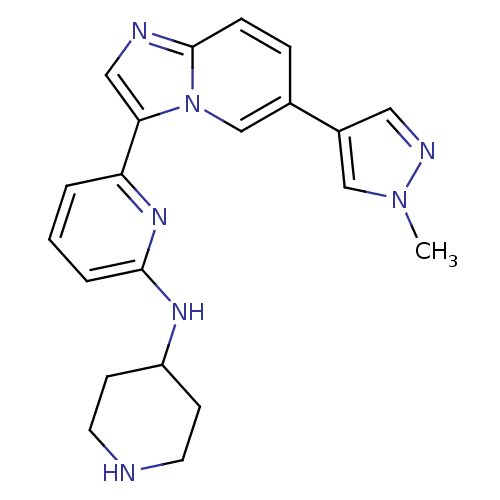
(CHEMBL255446)Show SMILES Cn1cc(cn1)-c1ccc2ncc(-c3cccc(NC4CCNCC4)n3)n2c1 Show InChI InChI=1S/C21H23N7/c1-27-13-16(11-24-27)15-5-6-21-23-12-19(28(21)14-15)18-3-2-4-20(26-18)25-17-7-9-22-10-8-17/h2-6,11-14,17,22H,7-10H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427748

(CHEMBL2324925)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cnccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-31-24-16-30-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377167
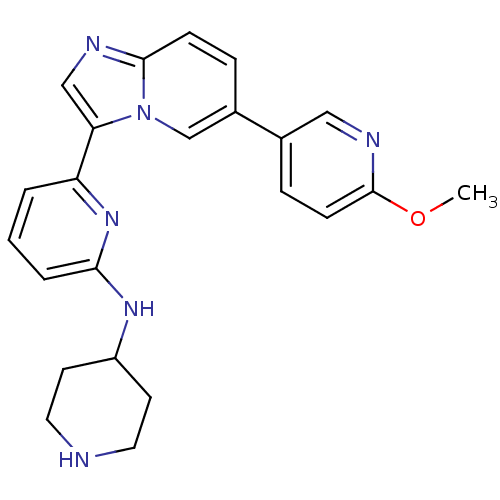
(CHEMBL402361)Show SMILES COc1ccc(cn1)-c1ccc2ncc(-c3cccc(NC4CCNCC4)n3)n2c1 Show InChI InChI=1S/C23H24N6O/c1-30-23-8-6-16(13-26-23)17-5-7-22-25-14-20(29(22)15-17)19-3-2-4-21(28-19)27-18-9-11-24-12-10-18/h2-8,13-15,18,24H,9-12H2,1H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427742
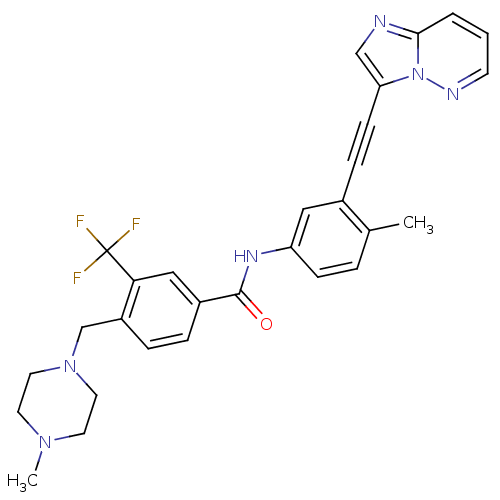
(CHEMBL2324930)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(c2)C#Cc2cnc3cccnn23)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-9-24(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)35-28(39)22-6-7-23(26(17-22)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50437404

(CHEMBL2408655)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-2-[#6]-[#7]-[#6]-[#6]-1-2 Show InChI InChI=1S/C25H30N6O3/c1-16(2)9-11-30-21-22(27-24(30)29-12-10-18-13-26-14-19(18)29)28(3)25(34)31(23(21)33)15-20(32)17-7-5-4-6-8-17/h4-9,18-19,26H,10-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377179
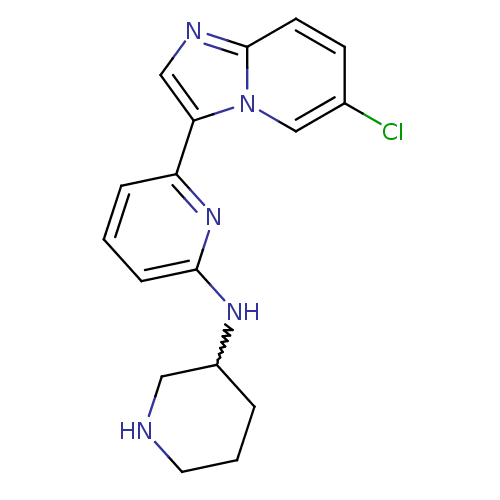
(CHEMBL256961)Show SMILES Clc1ccc2ncc(-c3cccc(NC4CCCNC4)n3)n2c1 |w:14.13| Show InChI InChI=1S/C17H18ClN5/c18-12-6-7-17-20-10-15(23(17)11-12)14-4-1-5-16(22-14)21-13-3-2-8-19-9-13/h1,4-7,10-11,13,19H,2-3,8-9H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437395

(CHEMBL2408638)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]C([#6])([#6])[#6]-[#7])nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 Show InChI InChI=1S/C24H31N5O4/c1-16(2)11-12-28-19-20(26-22(28)33-15-24(3,4)14-25)27(5)23(32)29(21(19)31)13-18(30)17-9-7-6-8-10-17/h6-11H,12-15,25H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427748

(CHEMBL2324925)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cnccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-31-24-16-30-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377172
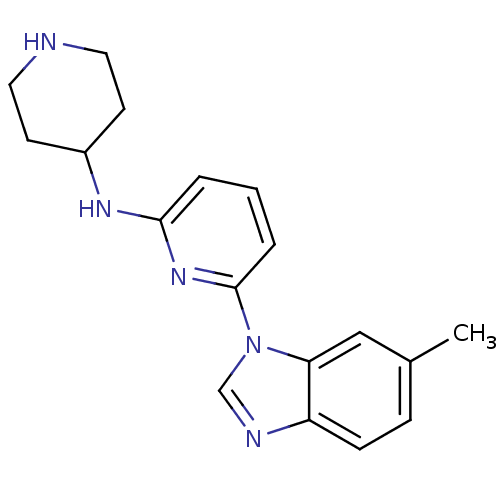
(CHEMBL404473)Show InChI InChI=1S/C18H21N5/c1-13-5-6-15-16(11-13)23(12-20-15)18-4-2-3-17(22-18)21-14-7-9-19-10-8-14/h2-6,11-12,14,19H,7-10H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50138394
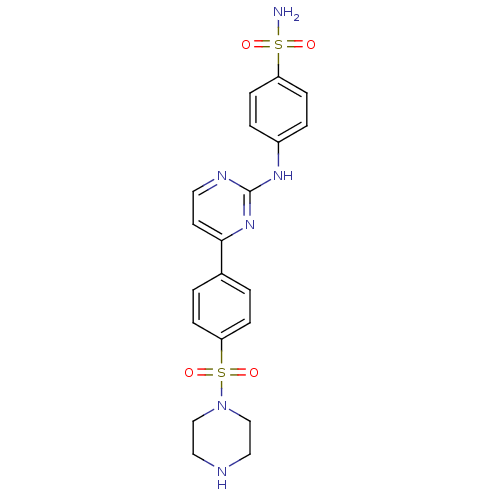
(4-{4-[4-(Piperazine-1-sulfonyl)-phenyl]-pyrimidin-...)Show SMILES NS(=O)(=O)c1ccc(Nc2nccc(n2)-c2ccc(cc2)S(=O)(=O)N2CCNCC2)cc1 Show InChI InChI=1S/C20H22N6O4S2/c21-31(27,28)17-7-3-16(4-8-17)24-20-23-10-9-19(25-20)15-1-5-18(6-2-15)32(29,30)26-13-11-22-12-14-26/h1-10,22H,11-14H2,(H2,21,27,28)(H,23,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against IKK2 |
Bioorg Med Chem Lett 14: 409-12 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ036V |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50437404

(CHEMBL2408655)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-2-[#6]-[#7]-[#6]-[#6]-1-2 Show InChI InChI=1S/C25H30N6O3/c1-16(2)9-11-30-21-22(27-24(30)29-12-10-18-13-26-14-19(18)29)28(3)25(34)31(23(21)33)15-20(32)17-7-5-4-6-8-17/h4-9,18-19,26H,10-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427750

(CHEMBL2324923)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cncc5nccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-30-16-24-31-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427747

(CHEMBL2324926)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cccnn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-10-12-37(13-11-35)17-18-5-6-19(14-22(18)26(27,28)29)32-25(39)33-23-15-20(36(2)34-23)7-8-21-16-30-24-4-3-9-31-38(21)24/h3-6,9,14-16H,10-13,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427750

(CHEMBL2324923)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cncc5nccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-30-16-24-31-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427745
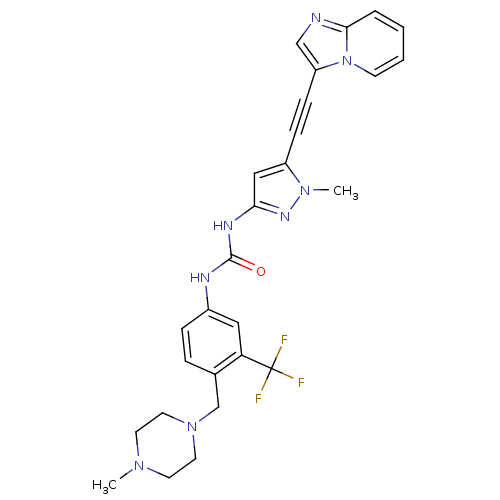
(CHEMBL2324927)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5ccccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C27H27F3N8O/c1-35-11-13-37(14-12-35)18-19-6-7-20(15-23(19)27(28,29)30)32-26(39)33-24-16-21(36(2)34-24)8-9-22-17-31-25-5-3-4-10-38(22)25/h3-7,10,15-17H,11-14,18H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427741
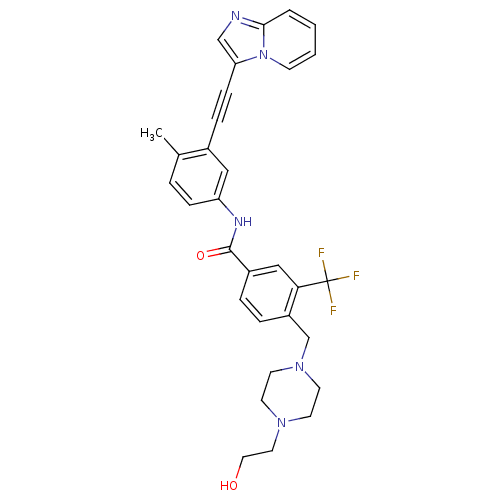
(CHEMBL2324931)Show SMILES Cc1ccc(NC(=O)c2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)cc1C#Cc1cnc2ccccn12 Show InChI InChI=1S/C31H30F3N5O2/c1-22-5-9-26(18-23(22)8-10-27-20-35-29-4-2-3-11-39(27)29)36-30(41)24-6-7-25(28(19-24)31(32,33)34)21-38-14-12-37(13-15-38)16-17-40/h2-7,9,11,18-20,40H,12-17,21H2,1H3,(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427744
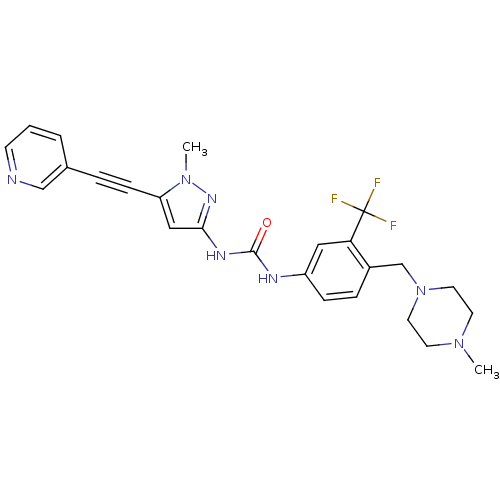
(CHEMBL2324928)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cccnc4)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C25H26F3N7O/c1-33-10-12-35(13-11-33)17-19-6-7-20(14-22(19)25(26,27)28)30-24(36)31-23-15-21(34(2)32-23)8-5-18-4-3-9-29-16-18/h3-4,6-7,9,14-16H,10-13,17H2,1-2H3,(H2,30,31,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427750

(CHEMBL2324923)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cncc5nccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-30-16-24-31-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427742

(CHEMBL2324930)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(c2)C#Cc2cnc3cccnn23)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-9-24(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)35-28(39)22-6-7-23(26(17-22)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data