

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasoactive intestinal polypeptide receptor 1 (Homo sapiens (Human)) | BDBM50435130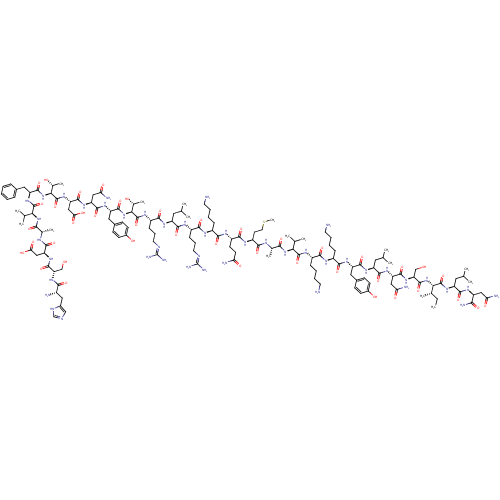 (CHEMBL1893324) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human VPAC1 receptor by radioligand displacement assay | Eur J Med Chem 63: 85-94 (2013) Article DOI: 10.1016/j.ejmech.2013.01.044 BindingDB Entry DOI: 10.7270/Q2JH3NKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50015490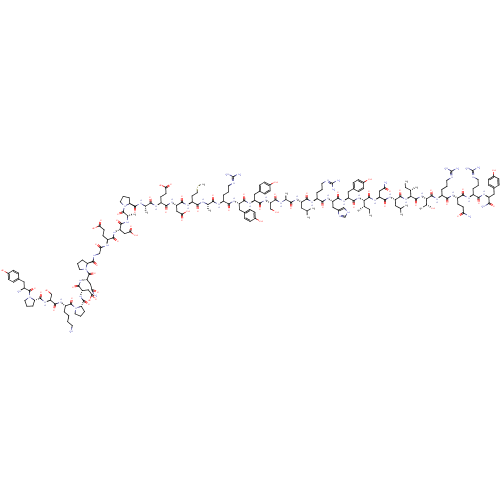 (CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human neuropeptide Y receptor type 2 by radioligand displacement assay | Eur J Med Chem 63: 85-94 (2013) Article DOI: 10.1016/j.ejmech.2013.01.044 BindingDB Entry DOI: 10.7270/Q2JH3NKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50015490 (CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human neuropeptide Y receptor type 1 by radioligand displacement assay | Eur J Med Chem 63: 85-94 (2013) Article DOI: 10.1016/j.ejmech.2013.01.044 BindingDB Entry DOI: 10.7270/Q2JH3NKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50063266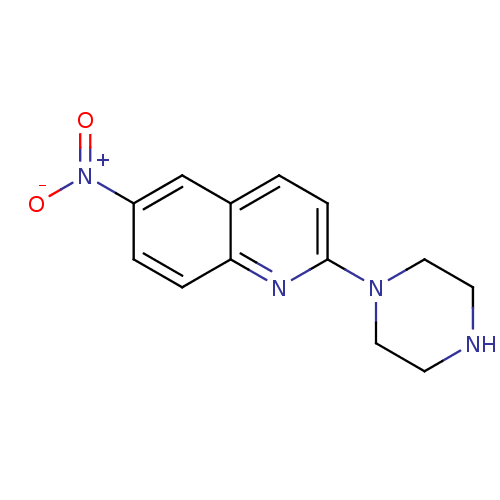 (6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Binding affinity towards Serotonin transporter was determined in rat forebrain membrane | J Med Chem 42: 1556-75 (1999) Article DOI: 10.1021/jm981112s BindingDB Entry DOI: 10.7270/Q2D50NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50335566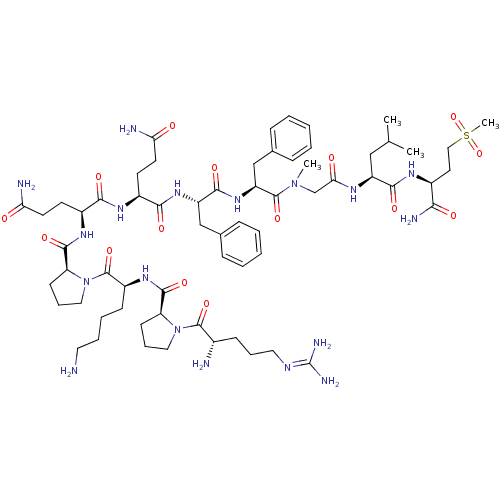 (CHEMBL1651026 | Substance P [Sar9,Met(O2)11]) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human NK1 receptor by radioligand displacement assay | Eur J Med Chem 63: 85-94 (2013) Article DOI: 10.1016/j.ejmech.2013.01.044 BindingDB Entry DOI: 10.7270/Q2JH3NKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50007568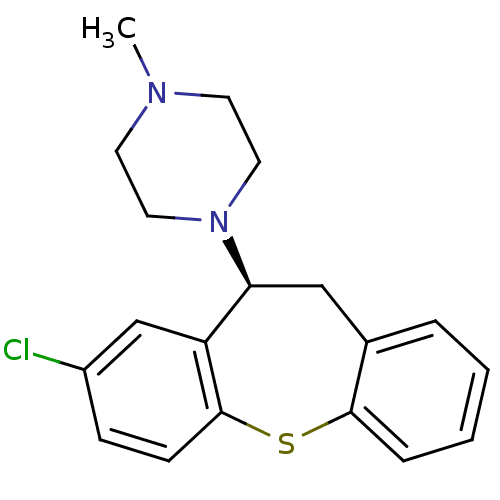 (1-((S)-8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Half-maximal inhibition of [3H]ketanserin binding to 5-hydroxytryptamine 2A receptor in rat cerebral cortex homogenate | J Med Chem 45: 344-59 (2002) BindingDB Entry DOI: 10.7270/Q2TX3G26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)-RAT) | BDBM50164588 (1-[1-(2-Chloro-phenyl)-cyclopropyl]-6-fluoro-2-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ G. D'Annunzio Curated by ChEMBL | Assay Description Inhibitory constant for Dopamine receptor D1-like | J Med Chem 48: 2646-54 (2005) Article DOI: 10.1021/jm040889k BindingDB Entry DOI: 10.7270/Q2QR4XW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50541723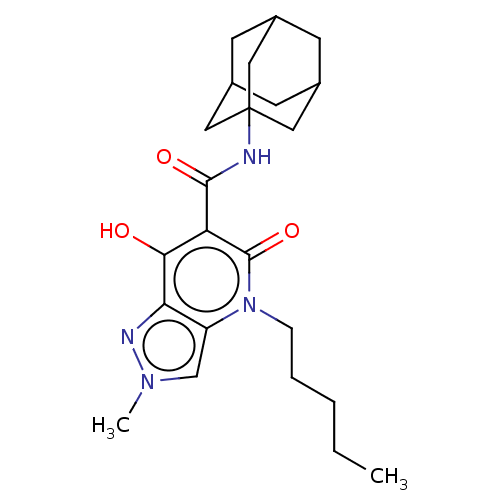 (CHEMBL4649122) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from recombinant human CB2R expressed in HEK293 cell membranes | J Med Chem 63: 7369-7391 (2020) Article DOI: 10.1021/acs.jmedchem.0c00595 BindingDB Entry DOI: 10.7270/Q2N01B3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM22872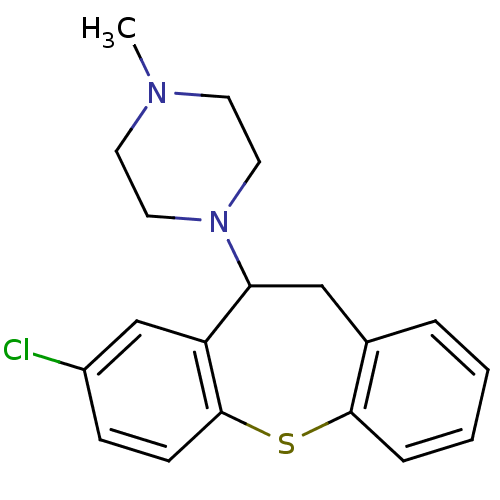 (1-(3-chloro-5,6-dihydrobenzo[b][1]benzothiepin-5-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Half-maximal inhibition of [3H]ketanserin binding to 5-hydroxytryptamine 2A receptor in rat cerebral cortex homogenate | J Med Chem 45: 344-59 (2002) BindingDB Entry DOI: 10.7270/Q2TX3G26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50063263 (6-(4-Methyl-piperazin-1-yl)-7,8,9,10-tetrahydro-ph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]granisetron binding to 5-hydroxytryptamine 3 receptor (5-HT 3 receptor) of rat cortex and hippocampus tissue | J Med Chem 42: 1556-75 (1999) Article DOI: 10.1021/jm981112s BindingDB Entry DOI: 10.7270/Q2D50NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130880 (CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human NTS1 receptor by radioligand displacement assay | Eur J Med Chem 63: 85-94 (2013) Article DOI: 10.1016/j.ejmech.2013.01.044 BindingDB Entry DOI: 10.7270/Q2JH3NKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM35254 (2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Half-maximal inhibition of [3H]7-OH-DPAT binding to Histamine H1 receptor in rat tissue homogenate | J Med Chem 45: 344-59 (2002) BindingDB Entry DOI: 10.7270/Q2TX3G26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50540490 (CHEMBL4645580) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human recombinant muscarinic receptor M1 expressed in CHO-K1 cell membranes incubated for 2 hrs by scintillation countin... | J Med Chem 63: 5763-5782 (2020) Article DOI: 10.1021/acs.jmedchem.9b02100 BindingDB Entry DOI: 10.7270/Q20R9SX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM82423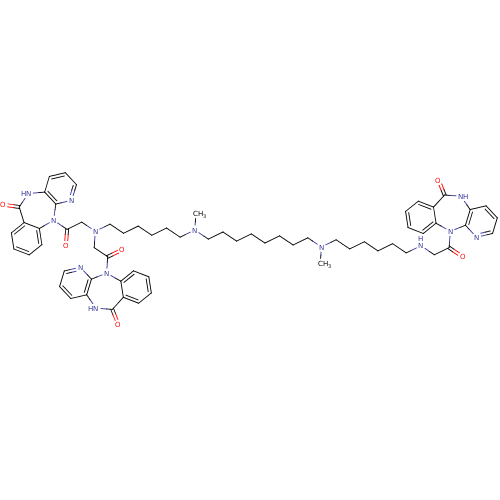 (CAS_132947 | NSC_132947 | TRIPITRAMINE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]N-methylscopolamine chloride from human cloned muscarinic M2 receptor expressed in CHOK1 cells | Bioorg Med Chem 16: 7311-20 (2008) Article DOI: 10.1016/j.bmc.2008.06.025 BindingDB Entry DOI: 10.7270/Q24F1S07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50004767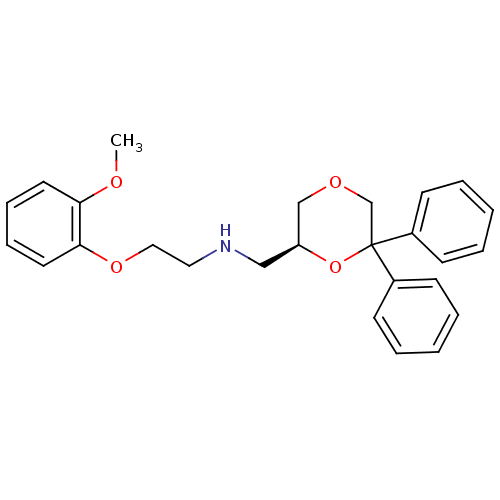 (CHEMBL2312536) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.309 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from recombinant human 5HT1A receptor expressed in human HeLa cell membranes after 30 mins by liquid scintillation coun... | Eur J Med Chem 125: 233-244 (2017) Article DOI: 10.1016/j.ejmech.2016.09.026 BindingDB Entry DOI: 10.7270/Q21C20BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50257034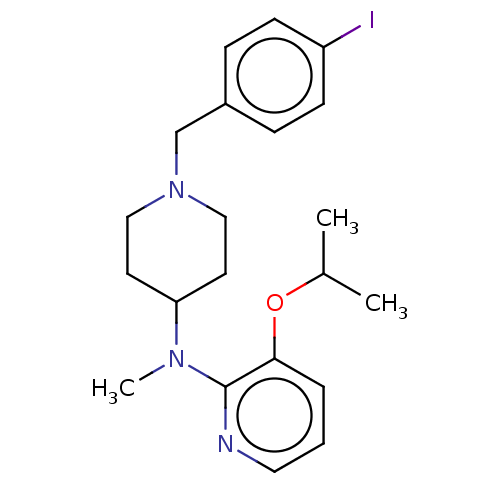 (CHEMBL4096543) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50007567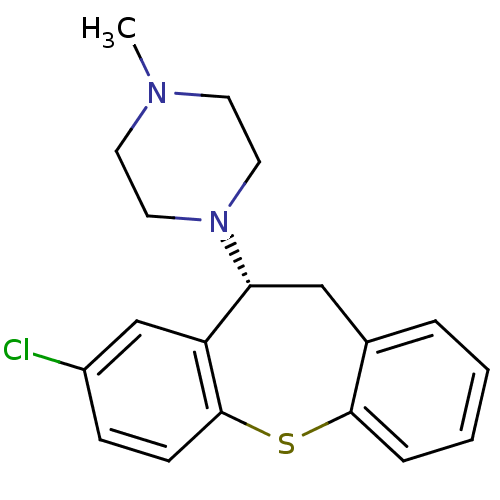 (1-(8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-10-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Half-maximal inhibition of [3H]ketanserin binding to 5-hydroxytryptamine 2A receptor in rat cerebral cortex homogenate | J Med Chem 45: 344-59 (2002) BindingDB Entry DOI: 10.7270/Q2TX3G26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50257034 (CHEMBL4096543) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scuola di Scienze del Farmaco e dei Prodotti della Salute , UniversitÓ di Camerino , Via S. Agostino 1 , 62032 Camerino , Italy. Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D4 receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method | J Med Chem 61: 3712-3725 (2018) Article DOI: 10.1021/acs.jmedchem.8b00265 BindingDB Entry DOI: 10.7270/Q20K2C2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human recombinant 5-HT1A receptor expressed in human HeLa cells incubated for 30 mins by radioligand competition ... | Eur J Med Chem 168: 461-473 (2019) Article DOI: 10.1016/j.ejmech.2019.02.056 BindingDB Entry DOI: 10.7270/Q2WM1HT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50054088 ((2R,3R)-2-(Methyl-propyl-amino)-3-phenyl-indan-5-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D2 in rat striatal homogenate by [3H]-spiperone displacement. | J Med Chem 39: 4238-46 (1996) Article DOI: 10.1021/jm960318v BindingDB Entry DOI: 10.7270/Q2WQ04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50054088 ((2R,3R)-2-(Methyl-propyl-amino)-3-phenyl-indan-5-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D2 in rat striatal homogenate by [3H]-spiperone displacement. | J Med Chem 39: 4238-46 (1996) Article DOI: 10.1021/jm960318v BindingDB Entry DOI: 10.7270/Q2WQ04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50026917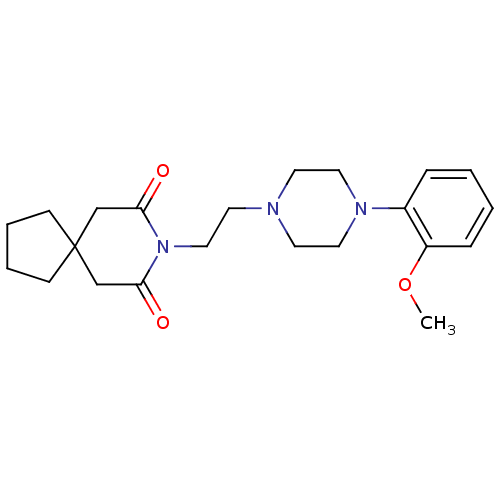 (8-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-8-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human recombinant 5-HT1A receptor expressed in human HeLa cells incubated for 30 mins by radioligand competition ... | Eur J Med Chem 168: 461-473 (2019) Article DOI: 10.1016/j.ejmech.2019.02.056 BindingDB Entry DOI: 10.7270/Q2WM1HT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50026917 (8-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-8-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from recombinant human 5HT1A receptor expressed in human HeLa cell membranes after 30 mins by liquid scintillation coun... | Eur J Med Chem 125: 233-244 (2017) Article DOI: 10.1016/j.ejmech.2016.09.026 BindingDB Entry DOI: 10.7270/Q21C20BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50004768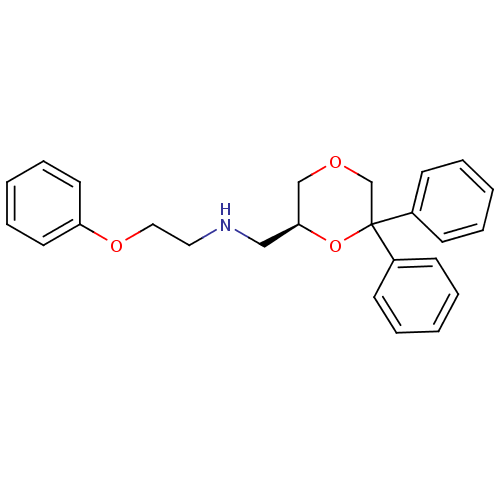 (CHEMBL2312538) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from recombinant human 5HT1A receptor expressed in human HeLa cell membranes after 30 mins by liquid scintillation coun... | Eur J Med Chem 125: 233-244 (2017) Article DOI: 10.1016/j.ejmech.2016.09.026 BindingDB Entry DOI: 10.7270/Q21C20BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50401953 (CHEMBL2207643) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50076415 (2-[2-(4-Methyl-piperazin-1-yl)-4-phenyl-quinolin-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]granisetron binding to 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 42: 1556-75 (1999) Article DOI: 10.1021/jm981112s BindingDB Entry DOI: 10.7270/Q2D50NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50007568 (1-((S)-8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Half-maximal inhibition of [3H]-7-OH-DPAT binding to Dopamine receptor D3 in rat tissue homogenate | J Med Chem 45: 344-59 (2002) BindingDB Entry DOI: 10.7270/Q2TX3G26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007568 (1-((S)-8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Half-maximal inhibition of [3H]spiperone binding to Dopamine receptor D2 in rat striatal homogenate | J Med Chem 45: 344-59 (2002) BindingDB Entry DOI: 10.7270/Q2TX3G26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50589760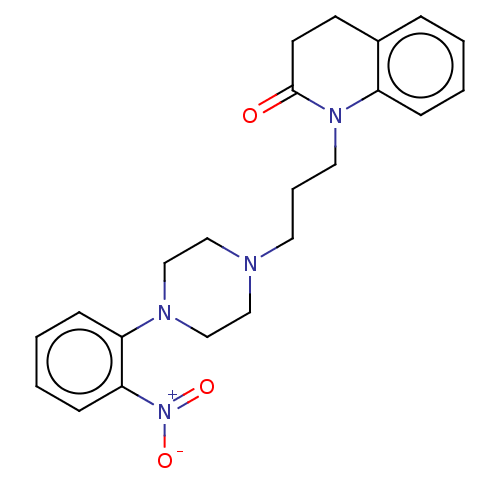 (CHEMBL5198170) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00840 BindingDB Entry DOI: 10.7270/Q2ST7TTC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM85093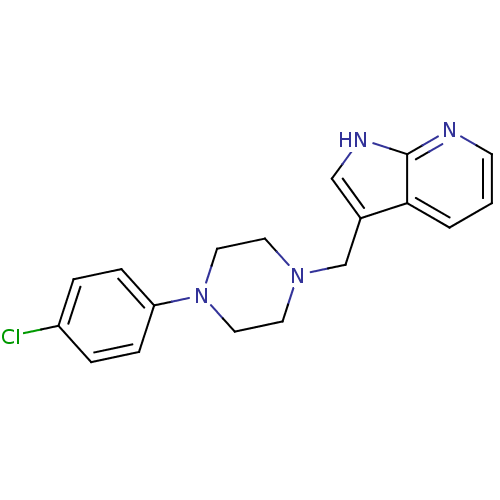 (CAS_3853 | CHEMBL267014 | CHEMBL555670 | L 745,870...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50054084 ((2R,3R)-2-(Allyl-methyl-amino)-3-phenyl-indan-5-ol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D2 in rat striatal homogenate by [3H]-spiperone displacement. | J Med Chem 39: 4238-46 (1996) Article DOI: 10.1021/jm960318v BindingDB Entry DOI: 10.7270/Q2WQ04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50054084 ((2R,3R)-2-(Allyl-methyl-amino)-3-phenyl-indan-5-ol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D2 in rat striatal homogenate by [3H]-spiperone displacement. | J Med Chem 39: 4238-46 (1996) Article DOI: 10.1021/jm960318v BindingDB Entry DOI: 10.7270/Q2WQ04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galanin receptor type 1 (Homo sapiens (Human)) | BDBM50378616 (GALANIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human GAL1 receptor by radioligand displacement assay | Eur J Med Chem 63: 85-94 (2013) Article DOI: 10.1016/j.ejmech.2013.01.044 BindingDB Entry DOI: 10.7270/Q2JH3NKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50333104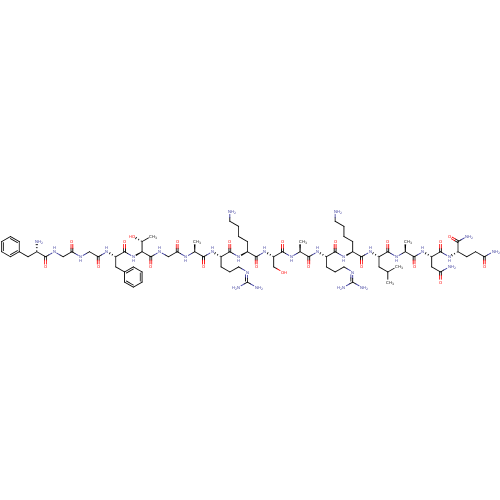 (CHEMBL389521 | H-FGGFTGARKSARKLANQ-NH2 | N/OFQ-NH2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human NOP receptor by radioligand displacement assay | Eur J Med Chem 63: 85-94 (2013) Article DOI: 10.1016/j.ejmech.2013.01.044 BindingDB Entry DOI: 10.7270/Q2JH3NKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50608788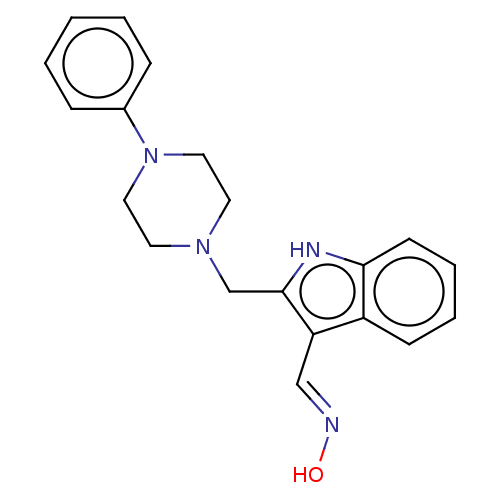 (CHEMBL5285358) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Sus scrofa) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]SCH-23390 from dopamine D1-like receptor in porcine striata homogenate | J Med Chem 49: 6848-57 (2006) Article DOI: 10.1021/jm0604979 BindingDB Entry DOI: 10.7270/Q25T3M92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM22872 (1-(3-chloro-5,6-dihydrobenzo[b][1]benzothiepin-5-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Half-maximal inhibition of [3H]spiperone binding to Dopamine receptor D2 in rat striatal homogenate | J Med Chem 45: 344-59 (2002) BindingDB Entry DOI: 10.7270/Q2TX3G26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50589757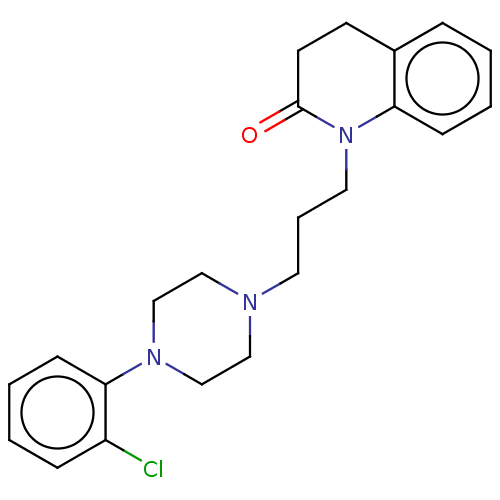 (CHEMBL5172201) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00840 BindingDB Entry DOI: 10.7270/Q2ST7TTC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50094505 (2-((4-phenylpiperazin-1-yl)methyl)-1H-indole-5-car...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50540458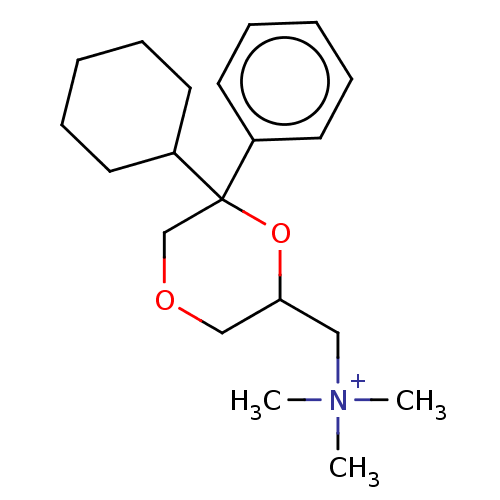 (CHEMBL4634230) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.525 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human recombinant muscarinic receptor M1 expressed in CHO-K1 cell membranes incubated for 2 hrs by scintillation countin... | J Med Chem 63: 5763-5782 (2020) Article DOI: 10.1021/acs.jmedchem.9b02100 BindingDB Entry DOI: 10.7270/Q20R9SX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50004766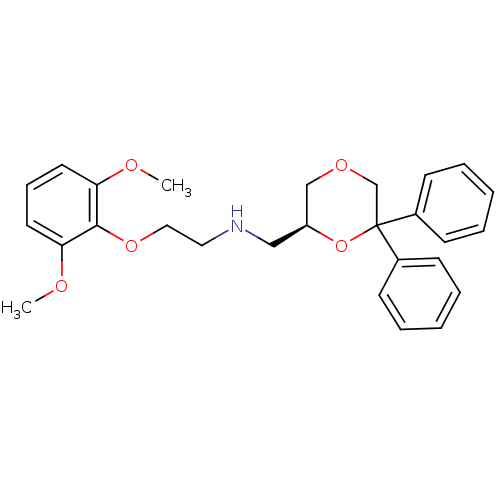 (CHEMBL2312226) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from recombinant human 5HT1A receptor expressed in human HeLa cell membranes after 30 mins by liquid scintillation coun... | Eur J Med Chem 125: 233-244 (2017) Article DOI: 10.1016/j.ejmech.2016.09.026 BindingDB Entry DOI: 10.7270/Q21C20BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50259021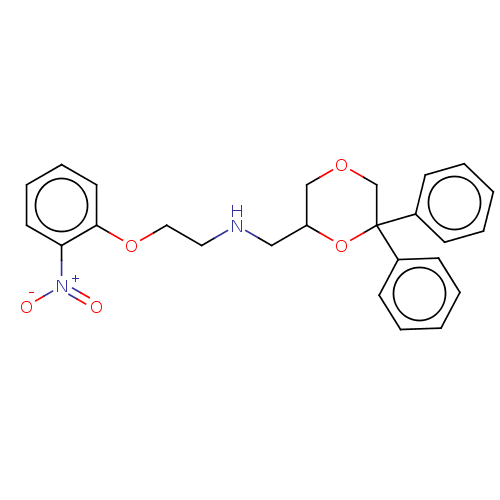 (CHEMBL4067083) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from recombinant human 5HT1A receptor expressed in human HeLa cell membranes after 30 mins by liquid scintillation coun... | Eur J Med Chem 125: 233-244 (2017) Article DOI: 10.1016/j.ejmech.2016.09.026 BindingDB Entry DOI: 10.7270/Q21C20BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50004769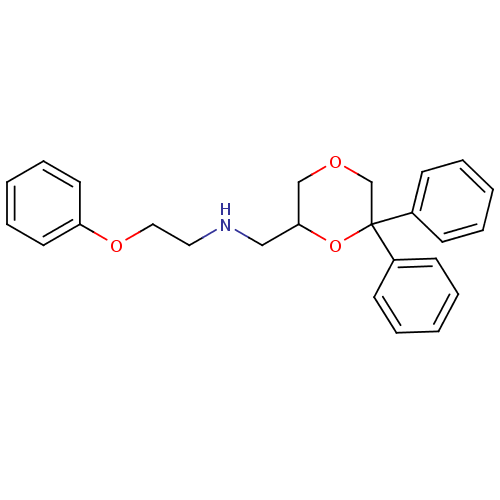 (CHEMBL2312537) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.589 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human recombinant 5-HT1A receptor expressed in human HeLa cells incubated for 30 mins by radioligand competition ... | Eur J Med Chem 168: 461-473 (2019) Article DOI: 10.1016/j.ejmech.2019.02.056 BindingDB Entry DOI: 10.7270/Q2WM1HT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50004769 (CHEMBL2312537) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.589 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from recombinant human 5HT1A receptor expressed in human HeLa cell membranes after 30 mins by liquid scintillation coun... | Eur J Med Chem 125: 233-244 (2017) Article DOI: 10.1016/j.ejmech.2016.09.026 BindingDB Entry DOI: 10.7270/Q21C20BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50054089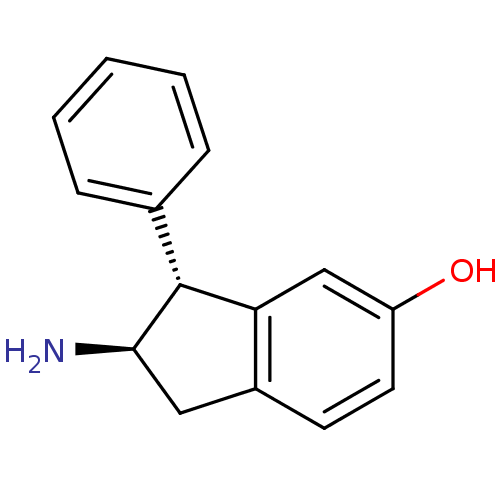 ((2R,3R)-2-Amino-3-phenyl-indan-5-ol | CHEMBL135224) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D2 in rat striatal homogenate by [3H]-spiperone displacement. | J Med Chem 39: 4238-46 (1996) Article DOI: 10.1021/jm960318v BindingDB Entry DOI: 10.7270/Q2WQ04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50054089 ((2R,3R)-2-Amino-3-phenyl-indan-5-ol | CHEMBL135224) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D2 in rat striatal homogenate by [3H]-spiperone displacement. | J Med Chem 39: 4238-46 (1996) Article DOI: 10.1021/jm960318v BindingDB Entry DOI: 10.7270/Q2WQ04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50076401 (CHEMBL41511 | [2-(4-Methyl-piperazin-1-yl)-4-pheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]granisetron binding to 5-hydroxytryptamine 3 receptor (5-HT 3 receptor) of rat cortex and hippocampus tissue | J Med Chem 42: 1556-75 (1999) Article DOI: 10.1021/jm981112s BindingDB Entry DOI: 10.7270/Q2D50NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50076416 (2-[4-(2-Fluoro-phenyl)-2-(4-methyl-piperazin-1-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]granisetron binding to 5-hydroxytryptamine 3 receptor (5-HT 3 receptor) of rat cortex and hippocampus tissue | J Med Chem 42: 1556-75 (1999) Article DOI: 10.1021/jm981112s BindingDB Entry DOI: 10.7270/Q2D50NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50412783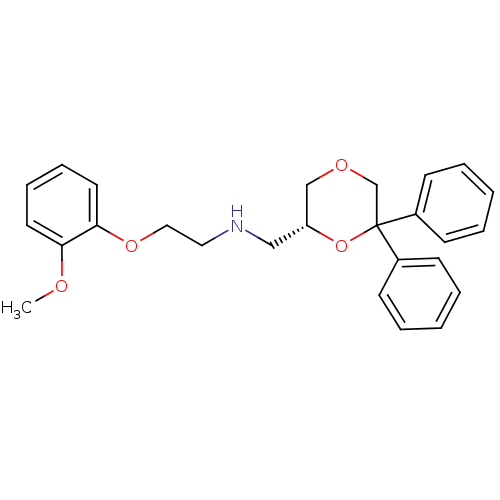 (CHEMBL493486) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from recombinant human 5HT1A receptor expressed in human HeLa cell membranes after 30 mins by liquid scintillation coun... | Eur J Med Chem 125: 233-244 (2017) Article DOI: 10.1016/j.ejmech.2016.09.026 BindingDB Entry DOI: 10.7270/Q21C20BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50589757 (CHEMBL5172201) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00840 BindingDB Entry DOI: 10.7270/Q2ST7TTC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2158 total ) | Next | Last >> |