 Found 302 hits with Last Name = 'girard' and Initial = 'gr'
Found 302 hits with Last Name = 'girard' and Initial = 'gr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244020
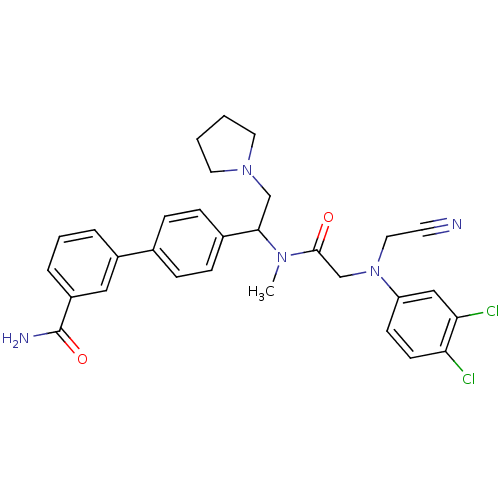
(4'-[1-({2-[Cyanomethyl-(3,4-dichloro-phenyl)-amino...)Show SMILES CN(C(CN1CCCC1)c1ccc(cc1)-c1cccc(c1)C(N)=O)C(=O)CN(CC#N)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C30H31Cl2N5O2/c1-35(29(38)20-37(16-13-33)25-11-12-26(31)27(32)18-25)28(19-36-14-2-3-15-36)22-9-7-21(8-10-22)23-5-4-6-24(17-23)30(34)39/h4-12,17-18,28H,2-3,14-16,19-20H2,1H3,(H2,34,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377220
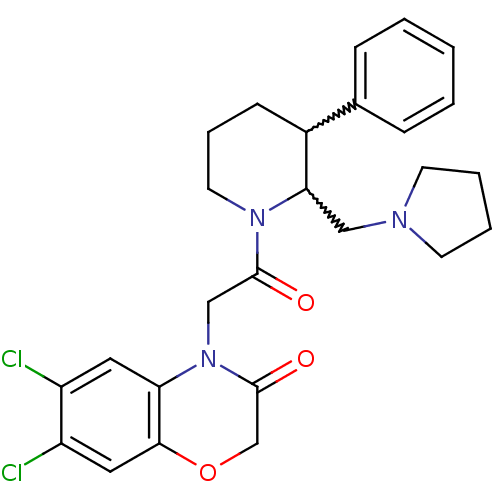
(CHEMBL255509)Show SMILES Clc1cc2OCC(=O)N(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2cc1Cl |w:17.18,16.25| Show InChI InChI=1S/C26H29Cl2N3O3/c27-20-13-22-24(14-21(20)28)34-17-26(33)31(22)16-25(32)30-12-6-9-19(18-7-2-1-3-8-18)23(30)15-29-10-4-5-11-29/h1-3,7-8,13-14,19,23H,4-6,9-12,15-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377218
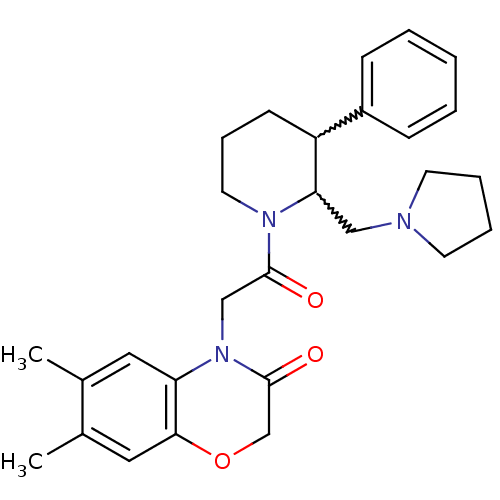
(CHEMBL257171)Show SMILES Cc1cc2OCC(=O)N(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2cc1C |w:17.18,16.25| Show InChI InChI=1S/C28H35N3O3/c1-20-15-24-26(16-21(20)2)34-19-28(33)31(24)18-27(32)30-14-8-11-23(22-9-4-3-5-10-22)25(30)17-29-12-6-7-13-29/h3-5,9-10,15-16,23,25H,6-8,11-14,17-19H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377217
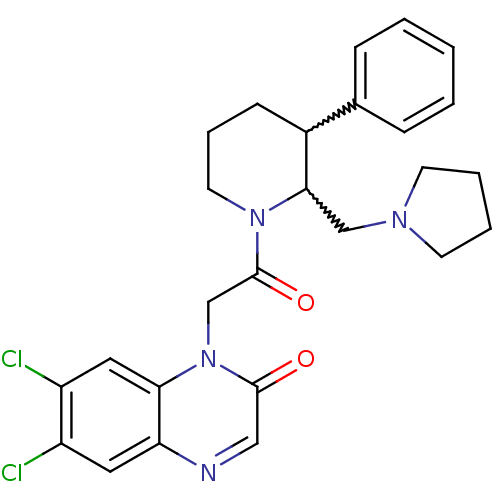
(CHEMBL256989)Show SMILES Clc1cc2ncc(=O)n(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2cc1Cl |w:17.18,16.25| Show InChI InChI=1S/C26H28Cl2N4O2/c27-20-13-22-23(14-21(20)28)32(25(33)15-29-22)17-26(34)31-12-6-9-19(18-7-2-1-3-8-18)24(31)16-30-10-4-5-11-30/h1-3,7-8,13-15,19,24H,4-6,9-12,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377215
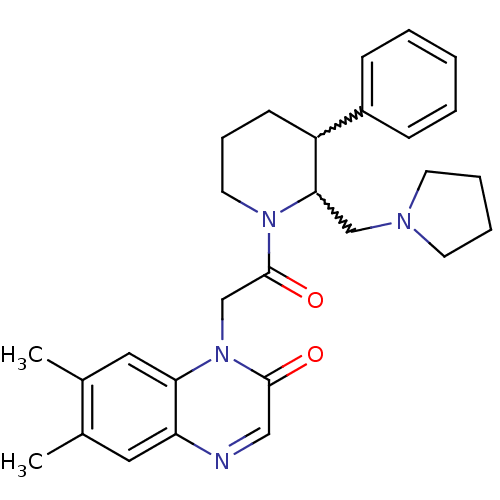
(CHEMBL257415)Show SMILES Cc1cc2ncc(=O)n(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2cc1C |w:17.18,16.25| Show InChI InChI=1S/C28H34N4O2/c1-20-15-24-25(16-21(20)2)32(27(33)17-29-24)19-28(34)31-14-8-11-23(22-9-4-3-5-10-22)26(31)18-30-12-6-7-13-30/h3-5,9-10,15-17,23,26H,6-8,11-14,18-19H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377227

(CHEMBL255462)Show SMILES Clc1ccc2sc(=O)n(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2c1 |w:16.25,17.18| Show InChI InChI=1S/C25H28ClN3O2S/c26-19-10-11-23-21(15-19)29(25(31)32-23)17-24(30)28-14-6-9-20(18-7-2-1-3-8-18)22(28)16-27-12-4-5-13-27/h1-3,7-8,10-11,15,20,22H,4-6,9,12-14,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377224
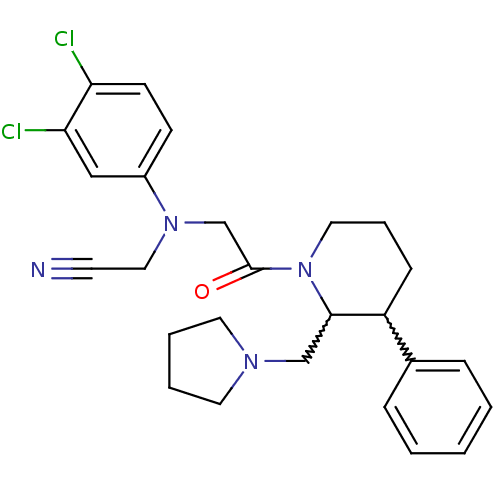
(CHEMBL257767)Show SMILES Clc1ccc(cc1Cl)N(CC#N)CC(=O)N1CCCC(C1CN1CCCC1)c1ccccc1 |w:19.29,20.22| Show InChI InChI=1S/C26H30Cl2N4O/c27-23-11-10-21(17-24(23)28)31(16-12-29)19-26(33)32-15-6-9-22(20-7-2-1-3-8-20)25(32)18-30-13-4-5-14-30/h1-3,7-8,10-11,17,22,25H,4-6,9,13-16,18-19H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377219
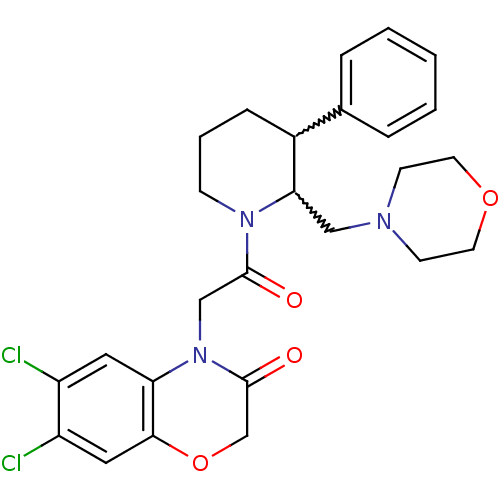
(CHEMBL402520)Show SMILES Clc1cc2OCC(=O)N(CC(=O)N3CCCC(C3CN3CCOCC3)c3ccccc3)c2cc1Cl |w:16.26,17.18| Show InChI InChI=1S/C26H29Cl2N3O4/c27-20-13-22-24(14-21(20)28)35-17-26(33)31(22)16-25(32)30-8-4-7-19(18-5-2-1-3-6-18)23(30)15-29-9-11-34-12-10-29/h1-3,5-6,13-14,19,23H,4,7-12,15-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244022
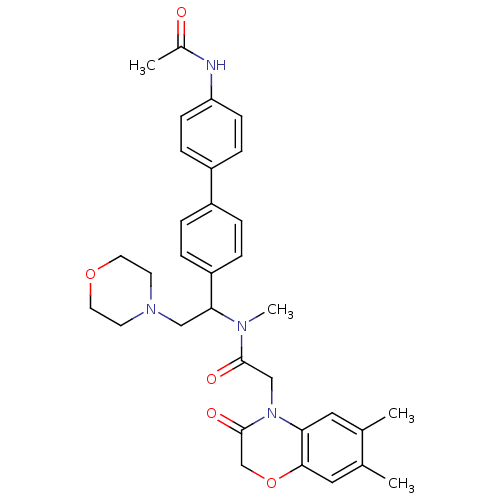
(CHEMBL449192 | N-[1-(4'-Acetylamino-biphenyl-4-yl)...)Show SMILES CN(C(CN1CCOCC1)c1ccc(cc1)-c1ccc(NC(C)=O)cc1)C(=O)CN1C(=O)COc2cc(C)c(C)cc12 Show InChI InChI=1S/C33H38N4O5/c1-22-17-29-31(18-23(22)2)42-21-33(40)37(29)20-32(39)35(4)30(19-36-13-15-41-16-14-36)27-7-5-25(6-8-27)26-9-11-28(12-10-26)34-24(3)38/h5-12,17-18,30H,13-16,19-21H2,1-4H3,(H,34,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM13014
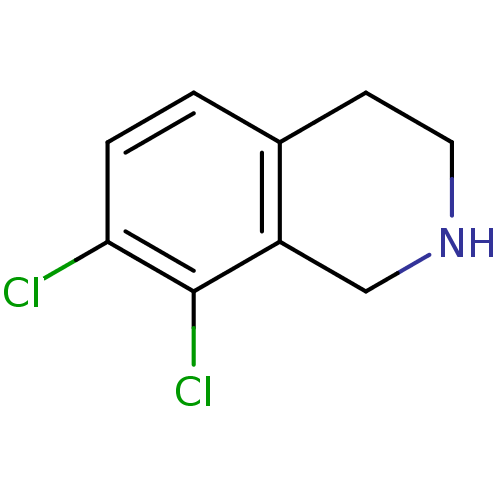
(7,8-Dichloro-1,2,3,4-tetrahydro-isoquinoline; hydr...)Show InChI InChI=1S/C9H9Cl2N/c10-8-2-1-6-3-4-12-5-7(6)9(8)11/h1-2,12H,3-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Dissociation constant(Ki) of compound was determined to measure PNMT-inhibitory potency |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377216
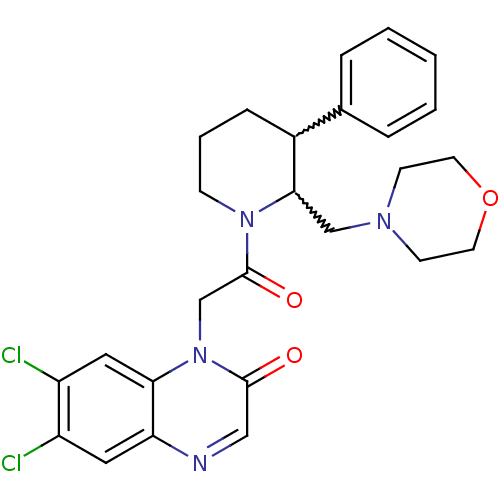
(CHEMBL256988)Show SMILES Clc1cc2ncc(=O)n(CC(=O)N3CCCC(C3CN3CCOCC3)c3ccccc3)c2cc1Cl |w:17.18,16.26| Show InChI InChI=1S/C26H28Cl2N4O3/c27-20-13-22-23(14-21(20)28)32(25(33)15-29-22)17-26(34)31-8-4-7-19(18-5-2-1-3-6-18)24(31)16-30-9-11-35-12-10-30/h1-3,5-6,13-15,19,24H,4,7-12,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244019
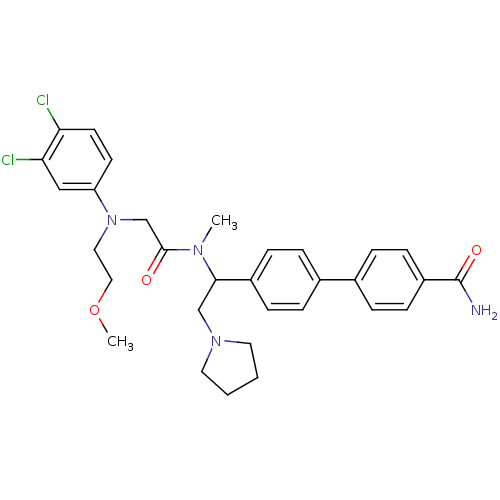
(4'-[1-({2-[(3,4-Dichloro-phenyl)-(2-methoxy-ethyl)...)Show SMILES COCCN(CC(=O)N(C)C(CN1CCCC1)c1ccc(cc1)-c1ccc(cc1)C(N)=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C31H36Cl2N4O3/c1-35(30(38)21-37(17-18-40-2)26-13-14-27(32)28(33)19-26)29(20-36-15-3-4-16-36)24-9-5-22(6-10-24)23-7-11-25(12-8-23)31(34)39/h5-14,19,29H,3-4,15-18,20-21H2,1-2H3,(H2,34,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM13014

(7,8-Dichloro-1,2,3,4-tetrahydro-isoquinoline; hydr...)Show InChI InChI=1S/C9H9Cl2N/c10-8-2-1-6-3-4-12-5-7(6)9(8)11/h1-2,12H,3-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for phenylethanolamine N-methyl-transferase was determined. |
J Med Chem 32: 1566-71 (1989)
BindingDB Entry DOI: 10.7270/Q2QZ2BJV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244021
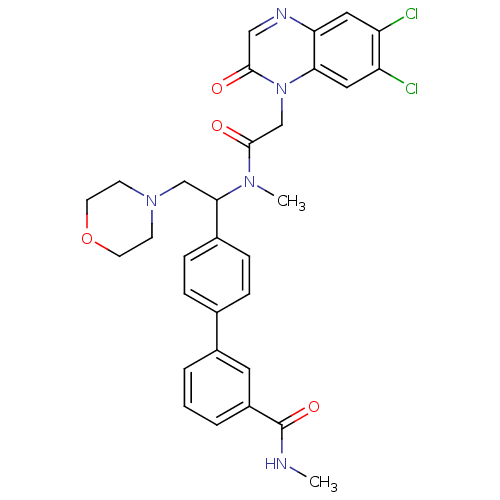
(4'-(1-{[2-(6,7-Dichloro-2-oxo-2H-quinoxalin-1-yl)-...)Show SMILES CNC(=O)c1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)Cn1c2cc(Cl)c(Cl)cc2ncc1=O Show InChI InChI=1S/C31H31Cl2N5O4/c1-34-31(41)23-5-3-4-22(14-23)20-6-8-21(9-7-20)28(18-37-10-12-42-13-11-37)36(2)30(40)19-38-27-16-25(33)24(32)15-26(27)35-17-29(38)39/h3-9,14-17,28H,10-13,18-19H2,1-2H3,(H,34,41) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244065
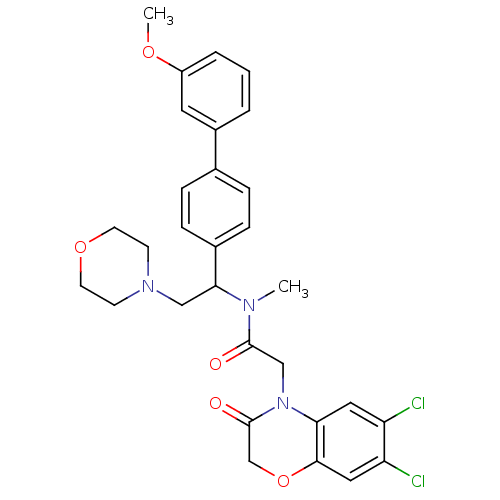
(2-(6,7-Dichloro-3-oxo-2,3-dihydro-benzo[1,4]oxazin...)Show SMILES COc1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 Show InChI InChI=1S/C30H31Cl2N3O5/c1-33(29(36)18-35-26-15-24(31)25(32)16-28(26)40-19-30(35)37)27(17-34-10-12-39-13-11-34)21-8-6-20(7-9-21)22-4-3-5-23(14-22)38-2/h3-9,14-16,27H,10-13,17-19H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377229
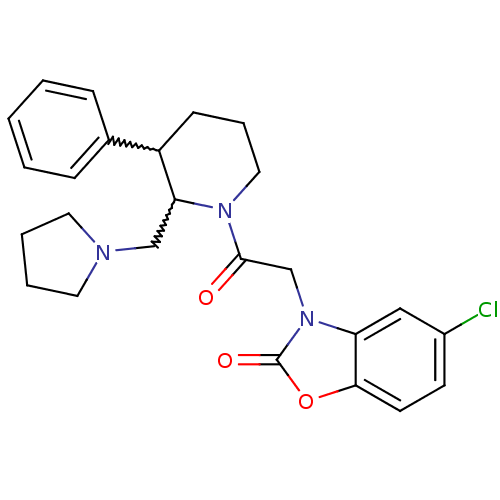
(CHEMBL257150)Show SMILES Clc1ccc2oc(=O)n(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2c1 |w:16.25,17.18| Show InChI InChI=1S/C25H28ClN3O3/c26-19-10-11-23-21(15-19)29(25(31)32-23)17-24(30)28-14-6-9-20(18-7-2-1-3-8-18)22(28)16-27-12-4-5-13-27/h1-3,7-8,10-11,15,20,22H,4-6,9,12-14,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377214
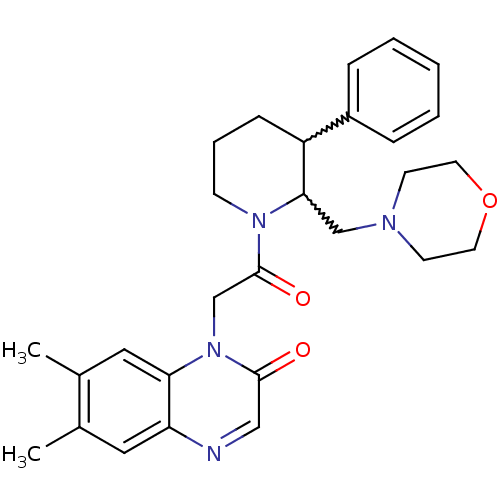
(CHEMBL256721)Show SMILES Cc1cc2ncc(=O)n(CC(=O)N3CCCC(C3CN3CCOCC3)c3ccccc3)c2cc1C |w:17.18,16.26| Show InChI InChI=1S/C28H34N4O3/c1-20-15-24-25(16-21(20)2)32(27(33)17-29-24)19-28(34)31-10-6-9-23(22-7-4-3-5-8-22)26(31)18-30-11-13-35-14-12-30/h3-5,7-8,15-17,23,26H,6,9-14,18-19H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50239135
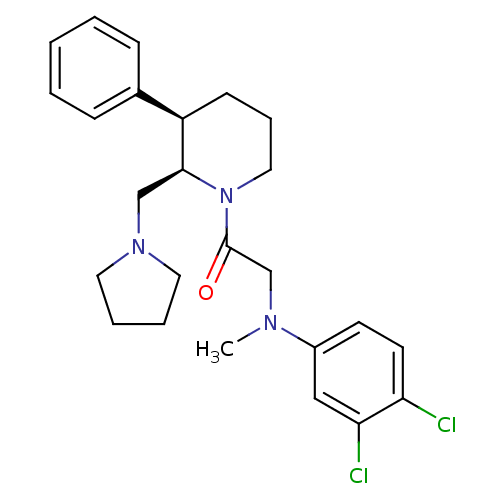
(2-((3,4-dichlorophenyl)(methyl)amino)-1-((2R,3R)-3...)Show SMILES CN(CC(=O)N1CCC[C@@H]([C@@H]1CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C25H31Cl2N3O/c1-28(20-11-12-22(26)23(27)16-20)18-25(31)30-15-7-10-21(19-8-3-2-4-9-19)24(30)17-29-13-5-6-14-29/h2-4,8-9,11-12,16,21,24H,5-7,10,13-15,17-18H2,1H3/t21-,24+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377226

(CHEMBL255460)Show SMILES Clc1ccc2sc(=O)n(CC(=O)N3CCCC(C3CN3CCOCC3)c3ccccc3)c2c1 |w:16.26,17.18| Show InChI InChI=1S/C25H28ClN3O3S/c26-19-8-9-23-21(15-19)29(25(31)33-23)17-24(30)28-10-4-7-20(18-5-2-1-3-6-18)22(28)16-27-11-13-32-14-12-27/h1-3,5-6,8-9,15,20,22H,4,7,10-14,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377223
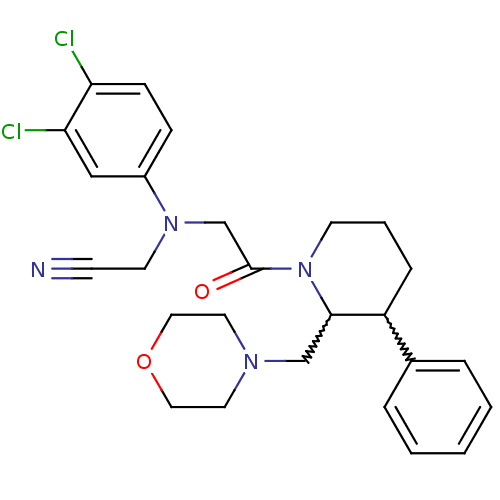
(CHEMBL258251)Show SMILES Clc1ccc(cc1Cl)N(CC#N)CC(=O)N1CCCC(C1CN1CCOCC1)c1ccccc1 |w:19.30,20.22| Show InChI InChI=1S/C26H30Cl2N4O2/c27-23-9-8-21(17-24(23)28)31(12-10-29)19-26(33)32-11-4-7-22(20-5-2-1-3-6-20)25(32)18-30-13-15-34-16-14-30/h1-3,5-6,8-9,17,22,25H,4,7,11-16,18-19H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244018
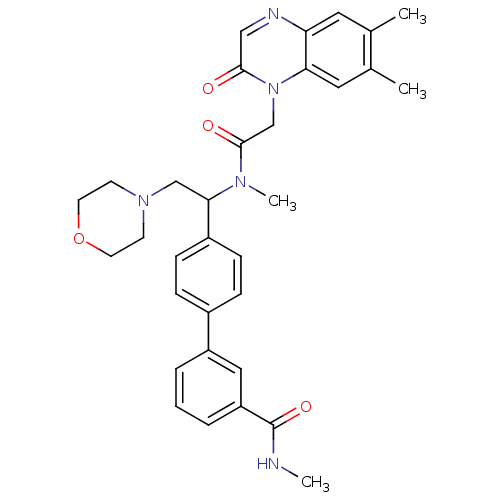
(4'-(1-{[2-(6,7-Dimethyl-2-oxo-2H-quinoxalin-1-yl)-...)Show SMILES CNC(=O)c1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)Cn1c2cc(C)c(C)cc2ncc1=O Show InChI InChI=1S/C33H37N5O4/c1-22-16-28-29(17-23(22)2)38(31(39)19-35-28)21-32(40)36(4)30(20-37-12-14-42-15-13-37)25-10-8-24(9-11-25)26-6-5-7-27(18-26)33(41)34-3/h5-11,16-19,30H,12-15,20-21H2,1-4H3,(H,34,41) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377222
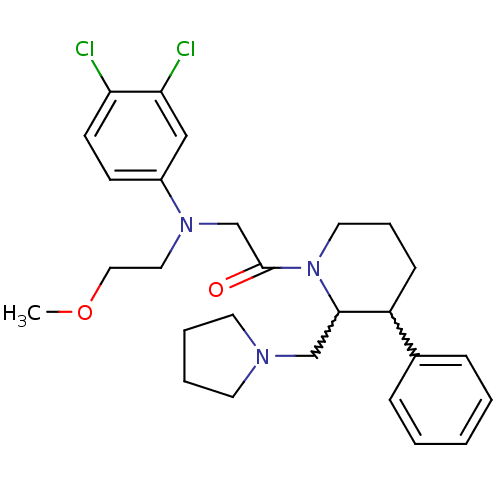
(CHEMBL256937)Show SMILES COCCN(CC(=O)N1CCCC(C1CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 |w:12.21,13.14| Show InChI InChI=1S/C27H35Cl2N3O2/c1-34-17-16-31(22-11-12-24(28)25(29)18-22)20-27(33)32-15-7-10-23(21-8-3-2-4-9-21)26(32)19-30-13-5-6-14-30/h2-4,8-9,11-12,18,23,26H,5-7,10,13-17,19-20H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Bos taurus (bovine)) | BDBM50018237
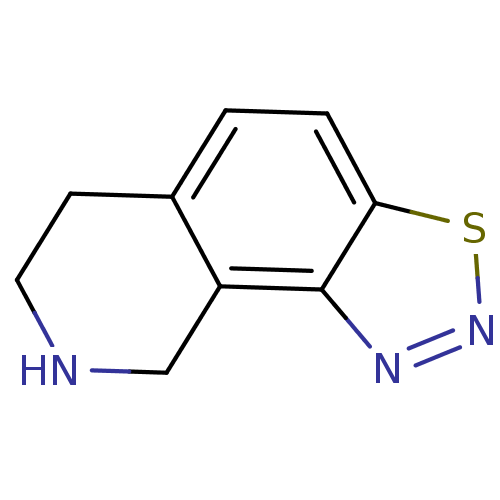
(6,7,8,9-Tetrahydro-3-thia-1,2,8-triaza-cyclopenta[...)Show InChI InChI=1S/C9H9N3S/c1-2-8-9(11-12-13-8)7-5-10-4-3-6(1)7/h1-2,10H,3-5H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for phenylethanolamine N-methyl-transferase was determined. |
J Med Chem 32: 1566-71 (1989)
BindingDB Entry DOI: 10.7270/Q2QZ2BJV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243919
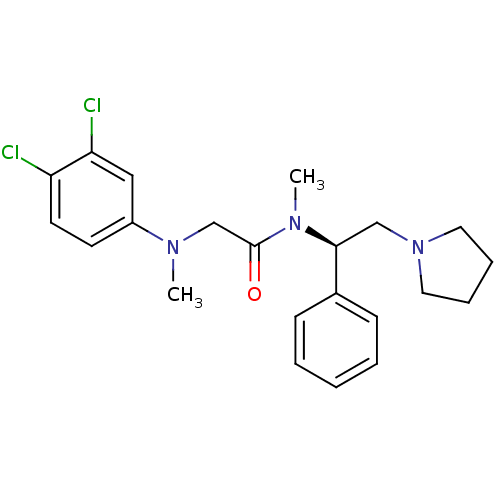
((R)-2-((3,4-dichlorophenyl)(methyl)amino)-N-methyl...)Show SMILES CN(CC(=O)N(C)[C@@H](CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C22H27Cl2N3O/c1-25(18-10-11-19(23)20(24)14-18)16-22(28)26(2)21(15-27-12-6-7-13-27)17-8-4-3-5-9-17/h3-5,8-11,14,21H,6-7,12-13,15-16H2,1-2H3/t21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243970
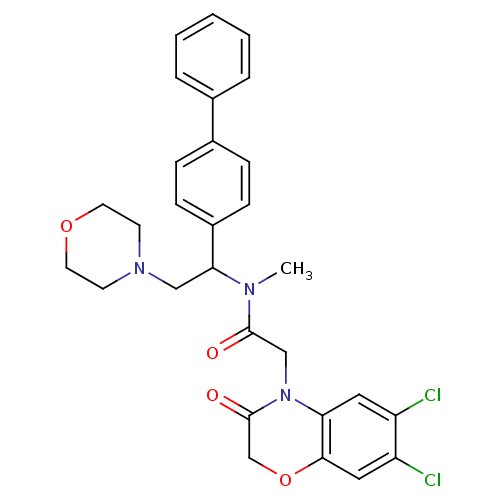
((+/-)N-(1-Biphenyl-4-yl-2-morpholin-4-yl-ethyl)-2-...)Show SMILES CN(C(CN1CCOCC1)c1ccc(cc1)-c1ccccc1)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 Show InChI InChI=1S/C29H29Cl2N3O4/c1-32(28(35)18-34-25-15-23(30)24(31)16-27(25)38-19-29(34)36)26(17-33-11-13-37-14-12-33)22-9-7-21(8-10-22)20-5-3-2-4-6-20/h2-10,15-16,26H,11-14,17-19H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243868
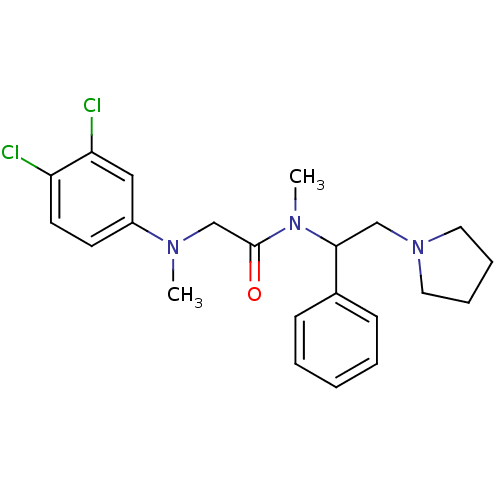
((+/-)-2-((3,4-dichlorophenyl)(methyl)amino)-N-meth...)Show SMILES CN(CC(=O)N(C)C(CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H27Cl2N3O/c1-25(18-10-11-19(23)20(24)14-18)16-22(28)26(2)21(15-27-12-6-7-13-27)17-8-4-3-5-9-17/h3-5,8-11,14,21H,6-7,12-13,15-16H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50240153
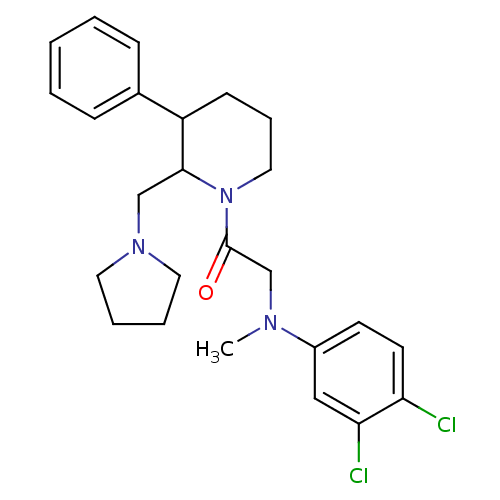
((+/-)-2-((3,4-dichlorophenyl)(methyl)amino)-1-(3-p...)Show SMILES CN(CC(=O)N1CCCC(C1CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C25H31Cl2N3O/c1-28(20-11-12-22(26)23(27)16-20)18-25(31)30-15-7-10-21(19-8-3-2-4-9-19)24(30)17-29-13-5-6-14-29/h2-4,8-9,11-12,16,21,24H,5-7,10,13-15,17-18H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243971
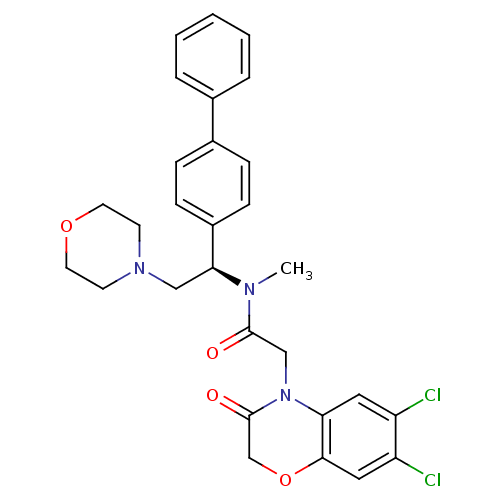
(CHEMBL453075 | N-((R)-1-Biphenyl-4-yl-2-morpholin-...)Show SMILES CN([C@@H](CN1CCOCC1)c1ccc(cc1)-c1ccccc1)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 |r| Show InChI InChI=1S/C29H29Cl2N3O4/c1-32(28(35)18-34-25-15-23(30)24(31)16-27(25)38-19-29(34)36)26(17-33-11-13-37-14-12-33)22-9-7-21(8-10-22)20-5-3-2-4-6-20/h2-10,15-16,26H,11-14,17-19H2,1H3/t26-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50240153

((+/-)-2-((3,4-dichlorophenyl)(methyl)amino)-1-(3-p...)Show SMILES CN(CC(=O)N1CCCC(C1CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C25H31Cl2N3O/c1-28(20-11-12-22(26)23(27)16-20)18-25(31)30-15-7-10-21(19-8-3-2-4-9-19)24(30)17-29-13-5-6-14-29/h2-4,8-9,11-12,16,21,24H,5-7,10,13-15,17-18H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243921
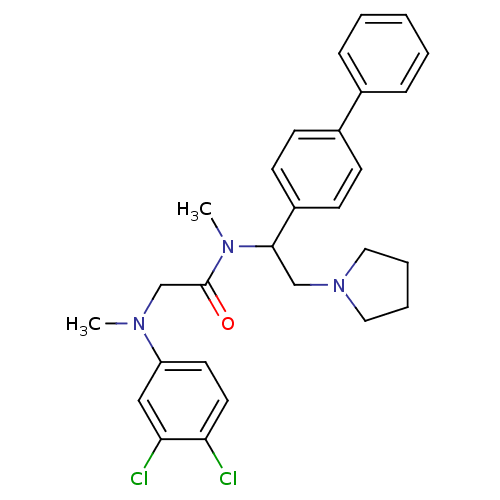
(CHEMBL488642 | N-(1-Biphenyl-4-yl-2-pyrrolidin-1-y...)Show SMILES CN(CC(=O)N(C)C(CN1CCCC1)c1ccc(cc1)-c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C28H31Cl2N3O/c1-31(24-14-15-25(29)26(30)18-24)20-28(34)32(2)27(19-33-16-6-7-17-33)23-12-10-22(11-13-23)21-8-4-3-5-9-21/h3-5,8-15,18,27H,6-7,16-17,19-20H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029108

(6,7,8-Trichloro-1,2,3,4-tetrahydro-isoquinoline | ...)Show InChI InChI=1S/C9H8Cl3N/c10-7-3-5-1-2-13-4-6(5)8(11)9(7)12/h3,13H,1-2,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029102

(7-Chloro-1,2,3,4-tetrahydro-isoquinoline | CHEMBL1...)Show InChI InChI=1S/C9H10ClN/c10-9-2-1-7-3-4-11-6-8(7)5-9/h1-2,5,11H,3-4,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Dissociation constant(Ki) of compound was determined to measure PNMT-inhibitory potency |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029107

(8-Chloro-1,2,3,4-tetrahydro-isoquinoline | CHEMBL1...)Show InChI InChI=1S/C9H10ClN/c10-9-3-1-2-7-4-5-11-6-8(7)9/h1-3,11H,4-6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377228
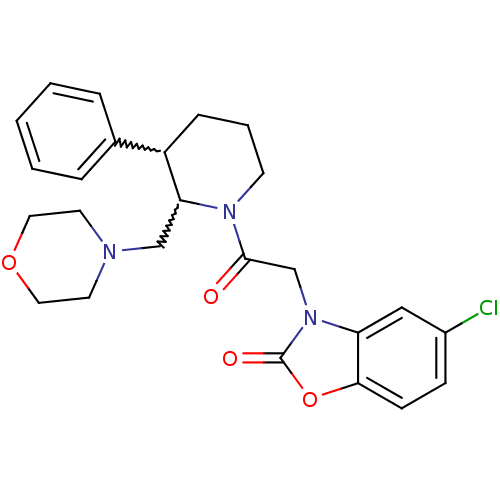
(CHEMBL404289)Show SMILES Clc1ccc2oc(=O)n(CC(=O)N3CCCC(C3CN3CCOCC3)c3ccccc3)c2c1 |w:16.26,17.18| Show InChI InChI=1S/C25H28ClN3O4/c26-19-8-9-23-21(15-19)29(25(31)33-23)17-24(30)28-10-4-7-20(18-5-2-1-3-6-18)22(28)16-27-11-13-32-14-12-27/h1-3,5-6,8-9,15,20,22H,4,7,10-14,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029101
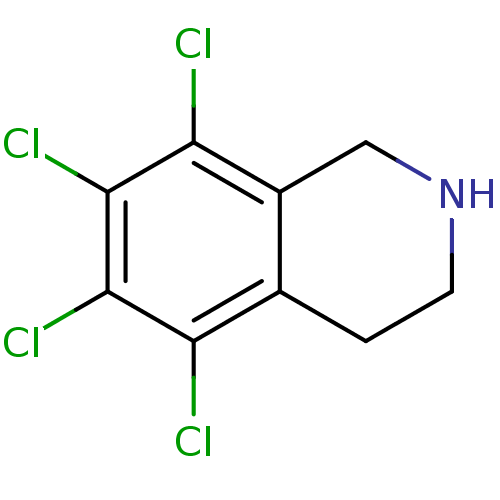
(5,6,7,8-Tetrachloro-1,2,3,4-tetrahydro-isoquinolin...)Show InChI InChI=1S/C9H7Cl4N/c10-6-4-1-2-14-3-5(4)7(11)9(13)8(6)12/h14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Dissociation constant(Ki) of compound was determined to measure Phenylethanolamine N-methyltransferase inhibitory potency |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029099

(5,7,8-Trichloro-1,2,3,4-tetrahydro-isoquinoline | ...)Show InChI InChI=1S/C9H8Cl3N/c10-7-3-8(11)9(12)6-4-13-2-1-5(6)7/h3,13H,1-2,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Dissociation constant(Ki) of compound was determined to measure PNMT-inhibitory potency |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377225
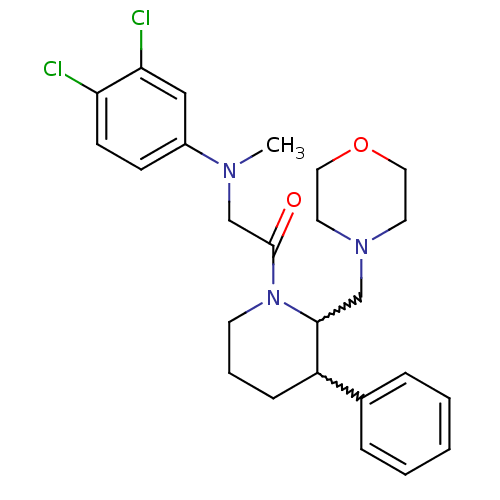
(CHEMBL402805)Show SMILES CN(CC(=O)N1CCCC(C1CN1CCOCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 |w:9.19,10.11| Show InChI InChI=1S/C25H31Cl2N3O2/c1-28(20-9-10-22(26)23(27)16-20)18-25(31)30-11-5-8-21(19-6-3-2-4-7-19)24(30)17-29-12-14-32-15-13-29/h2-4,6-7,9-10,16,21,24H,5,8,11-15,17-18H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377221
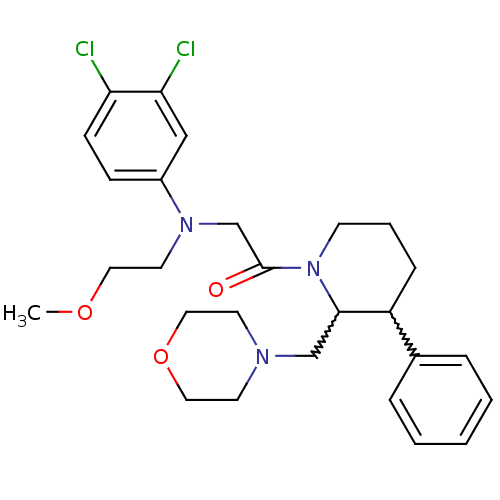
(CHEMBL402813)Show SMILES COCCN(CC(=O)N1CCCC(C1CN1CCOCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 |w:12.22,13.14| Show InChI InChI=1S/C27H35Cl2N3O3/c1-34-15-14-31(22-9-10-24(28)25(29)18-22)20-27(33)32-11-5-8-23(21-6-3-2-4-7-21)26(32)19-30-12-16-35-17-13-30/h2-4,6-7,9-10,18,23,26H,5,8,11-17,19-20H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243969
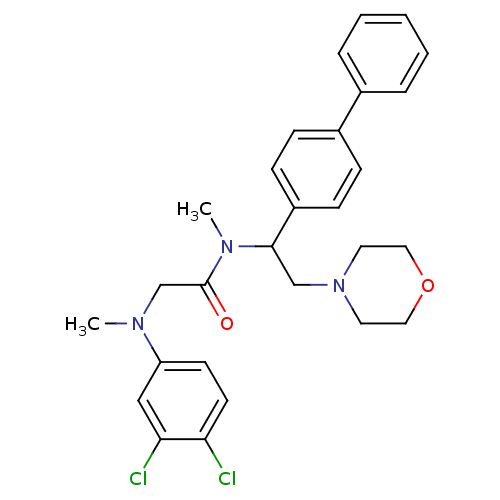
(CHEMBL452298 | N-(1-Biphenyl-4-yl-2-morpholin-4-yl...)Show SMILES CN(CC(=O)N(C)C(CN1CCOCC1)c1ccc(cc1)-c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C28H31Cl2N3O2/c1-31(24-12-13-25(29)26(30)18-24)20-28(34)32(2)27(19-33-14-16-35-17-15-33)23-10-8-22(9-11-23)21-6-4-3-5-7-21/h3-13,18,27H,14-17,19-20H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244017
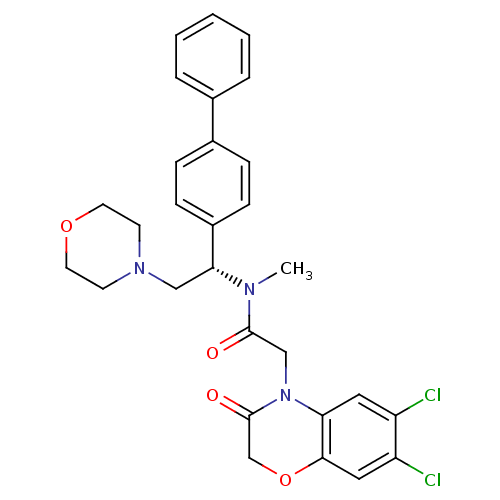
(CHEMBL452808 | N-((S)-1-Biphenyl-4-yl-2-morpholin-...)Show SMILES CN([C@H](CN1CCOCC1)c1ccc(cc1)-c1ccccc1)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 |r| Show InChI InChI=1S/C29H29Cl2N3O4/c1-32(28(35)18-34-25-15-23(30)24(31)16-27(25)38-19-29(34)36)26(17-33-11-13-37-14-12-33)22-9-7-21(8-10-22)20-5-3-2-4-6-20/h2-10,15-16,26H,11-14,17-19H2,1H3/t26-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50241424
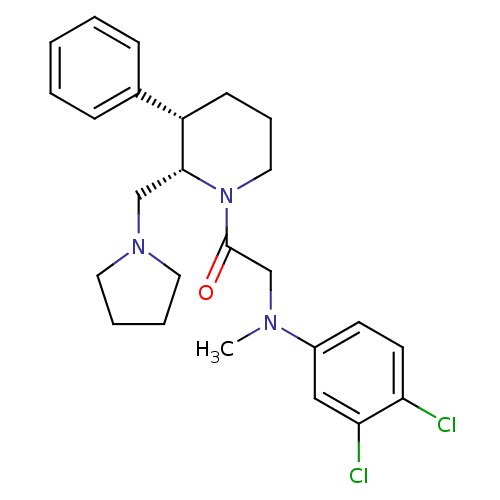
(2-((3,4-dichlorophenyl)(methyl)amino)-1-((2S,3S)-3...)Show SMILES CN(CC(=O)N1CCC[C@H]([C@H]1CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C25H31Cl2N3O/c1-28(20-11-12-22(26)23(27)16-20)18-25(31)30-15-7-10-21(19-8-3-2-4-9-19)24(30)17-29-13-5-6-14-29/h2-4,8-9,11-12,16,21,24H,5-7,10,13-15,17-18H2,1H3/t21-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243920
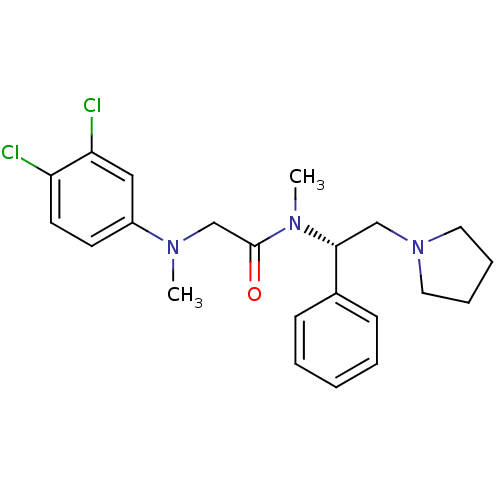
((S)-2-((3,4-dichlorophenyl)(methyl)amino)-N-methyl...)Show SMILES CN(CC(=O)N(C)[C@H](CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C22H27Cl2N3O/c1-25(18-10-11-19(23)20(24)14-18)16-22(28)26(2)21(15-27-12-6-7-13-27)17-8-4-3-5-9-17/h3-5,8-11,14,21H,6-7,12-13,15-16H2,1-2H3/t21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50007344
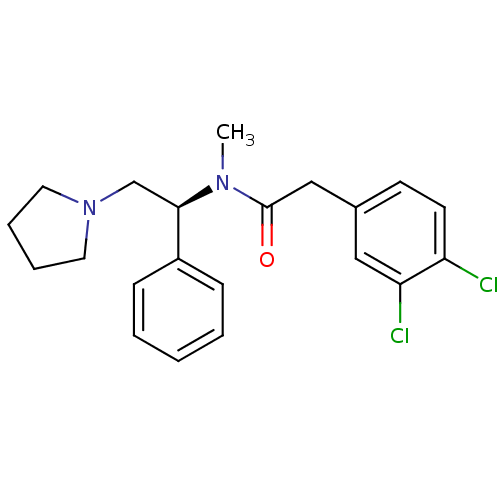
((S)-2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C21H24Cl2N2O/c1-24(21(26)14-16-9-10-18(22)19(23)13-16)20(15-25-11-5-6-12-25)17-7-3-2-4-8-17/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3/t20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50011972

(CHEMBL385189 | Sar-Arg-Val-Tyr-Ile-His-Pro-Thi-OH)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1cccs1)C(O)=O Show InChI InChI=1S/C47H69N13O10S/c1-6-27(4)39(44(67)56-34(21-29-23-51-25-53-29)45(68)60-18-8-12-36(60)42(65)57-35(46(69)70)22-31-10-9-19-71-31)59-41(64)33(20-28-13-15-30(61)16-14-28)55-43(66)38(26(2)3)58-40(63)32(54-37(62)24-50-5)11-7-17-52-47(48)49/h9-10,13-16,19,23,25-27,32-36,38-39,50,61H,6-8,11-12,17-18,20-22,24H2,1-5H3,(H,51,53)(H,54,62)(H,55,66)(H,56,67)(H,57,65)(H,58,63)(H,59,64)(H,69,70)(H4,48,49,52)/t27-,32-,33-,34-,35-,36-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of [125I]-AII specific binding towards angiotensin II receptor in rat mesenteric membranes. |
J Med Chem 34: 1514-7 (1991)
BindingDB Entry DOI: 10.7270/Q2TD9WBV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230790

(CHEMBL292892)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1[N+]([O-])=O)C(O)=O Show InChI InChI=1S/C23H23N3O6S/c1-2-3-6-21-24-13-18(10-17(23(29)30)11-19-5-4-9-33-19)25(21)14-16-8-7-15(22(27)28)12-20(16)26(31)32/h4-5,7-10,12-13H,2-3,6,11,14H2,1H3,(H,27,28)(H,29,30)/b17-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282363
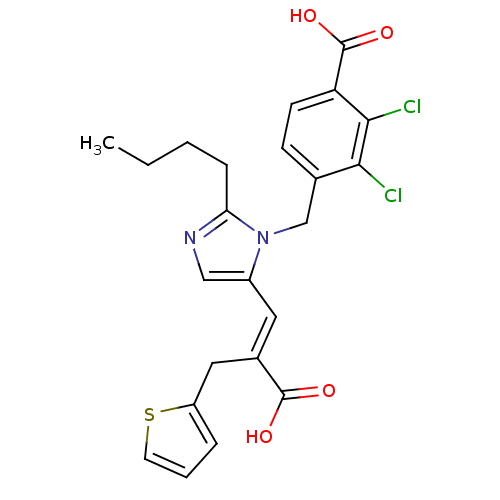
(4-[2-Butyl-5-((E)-2-carboxy-3-thiophen-2-yl-propen...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(Cl)c1Cl Show InChI InChI=1S/C23H22Cl2N2O4S/c1-2-3-6-19-26-12-16(10-15(22(28)29)11-17-5-4-9-32-17)27(19)13-14-7-8-18(23(30)31)21(25)20(14)24/h4-5,7-10,12H,2-3,6,11,13H2,1H3,(H,28,29)(H,30,31)/b15-10+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50 |
Bioorg Med Chem Lett 4: 23-28 (1994)
Article DOI: 10.1016/S0960-894X(01)81116-1
BindingDB Entry DOI: 10.7270/Q29G5MRF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230779

(CHEMBL294686)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)c2nn[nH]n2)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N6O2S/c1-2-3-6-21-24-14-19(29(21)15-16-7-9-17(10-8-16)23(30)31)12-18(22-25-27-28-26-22)13-20-5-4-11-32-20/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,30,31)(H,25,26,27,28)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230811
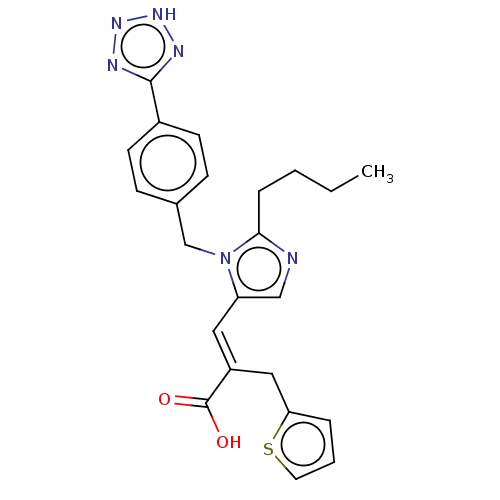
(CHEMBL293091)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)-c1nn[nH]n1 Show InChI InChI=1S/C23H24N6O2S/c1-2-3-6-21-24-14-19(12-18(23(30)31)13-20-5-4-11-32-20)29(21)15-16-7-9-17(10-8-16)22-25-27-28-26-22/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,30,31)(H,25,26,27,28)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230778
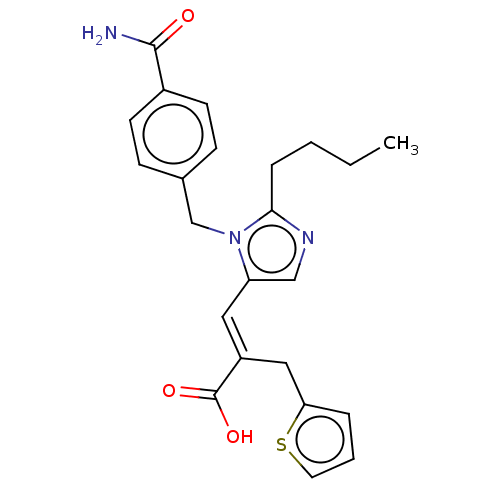
(CHEMBL56211)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(N)=O Show InChI InChI=1S/C23H25N3O3S/c1-2-3-6-21-25-14-19(12-18(23(28)29)13-20-5-4-11-30-20)26(21)15-16-7-9-17(10-8-16)22(24)27/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H2,24,27)(H,28,29)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230771
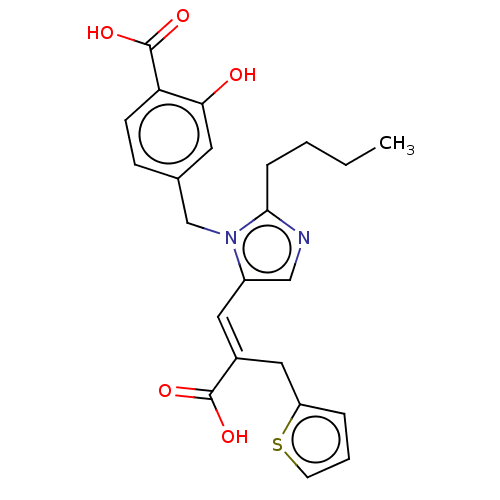
(CHEMBL55510)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(O)c1 Show InChI InChI=1S/C23H24N2O5S/c1-2-3-6-21-24-13-17(11-16(22(27)28)12-18-5-4-9-31-18)25(21)14-15-7-8-19(23(29)30)20(26)10-15/h4-5,7-11,13,26H,2-3,6,12,14H2,1H3,(H,27,28)(H,29,30)/b16-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data