 Found 39 hits with Last Name = 'duran' and Initial = 'h'
Found 39 hits with Last Name = 'duran' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A1
(Chick) | BDBM50006730
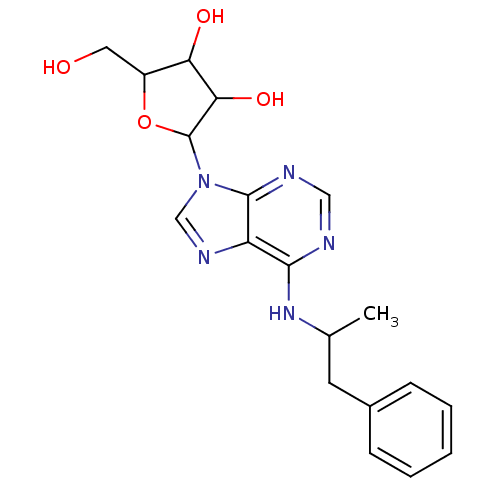
((R)-2-Hydroxymethyl-5-[6-(1-methyl-2-phenyl-ethyla...)Show SMILES CC(Cc1ccccc1)Nc1ncnc2n(cnc12)C1OC(CO)C(O)C1O Show InChI InChI=1S/C19H23N5O4/c1-11(7-12-5-3-2-4-6-12)23-17-14-18(21-9-20-17)24(10-22-14)19-16(27)15(26)13(8-25)28-19/h2-6,9-11,13,15-16,19,25-27H,7-8H2,1H3,(H,20,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Chick) | BDBM50006730

((R)-2-Hydroxymethyl-5-[6-(1-methyl-2-phenyl-ethyla...)Show SMILES CC(Cc1ccccc1)Nc1ncnc2n(cnc12)C1OC(CO)C(O)C1O Show InChI InChI=1S/C19H23N5O4/c1-11(7-12-5-3-2-4-6-12)23-17-14-18(21-9-20-17)24(10-22-14)19-16(27)15(26)13(8-25)28-19/h2-6,9-11,13,15-16,19,25-27H,7-8H2,1H3,(H,20,21,23) | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Chick) | BDBM50009552
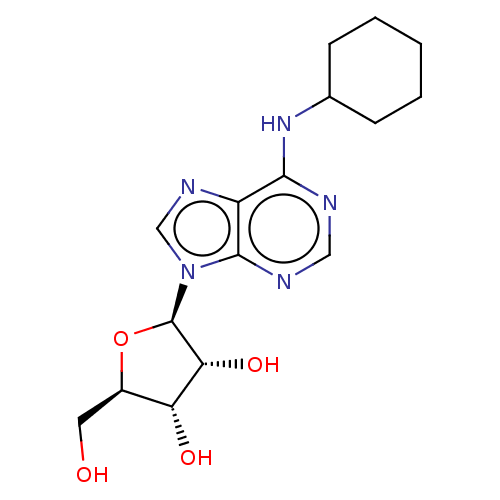
(2-[6-Amino-2-(2-morpholin-4-yl-ethylamino)-purin-9...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCCC3)ncnc12 |r| Show InChI InChI=1S/C16H23N5O4/c22-6-10-12(23)13(24)16(25-10)21-8-19-11-14(17-7-18-15(11)21)20-9-4-2-1-3-5-9/h7-10,12-13,16,22-24H,1-6H2,(H,17,18,20)/t10-,12-,13-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Chick) | BDBM25400

((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)ncnc12 Show InChI InChI=1S/C15H21N5O4/c21-5-9-11(22)12(23)15(24-9)20-7-18-10-13(16-6-17-14(10)20)19-8-3-1-2-4-8/h6-9,11-12,15,21-23H,1-5H2,(H,16,17,19)/t9-,11-,12-,15-/m1/s1 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Chick) | BDBM50034171
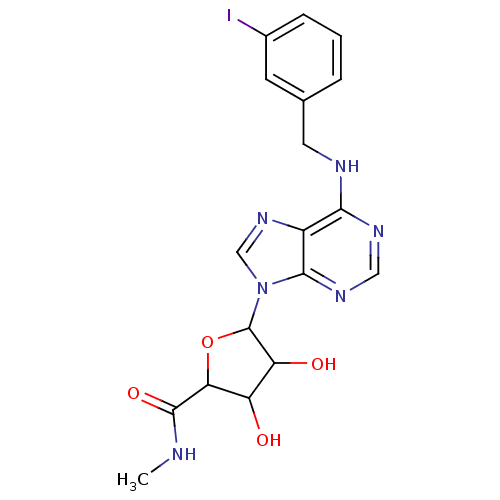
(3,4-Dihydroxy-5-[6-(3-iodo-benzylamino)-purin-9-yl...)Show SMILES CNC(=O)C1OC(C(O)C1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23) | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Chick) | BDBM25400

((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)ncnc12 Show InChI InChI=1S/C15H21N5O4/c21-5-9-11(22)12(23)15(24-9)20-7-18-10-13(16-6-17-14(10)20)19-8-3-1-2-4-8/h6-9,11-12,15,21-23H,1-5H2,(H,16,17,19)/t9-,11-,12-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Chick) | BDBM50008415
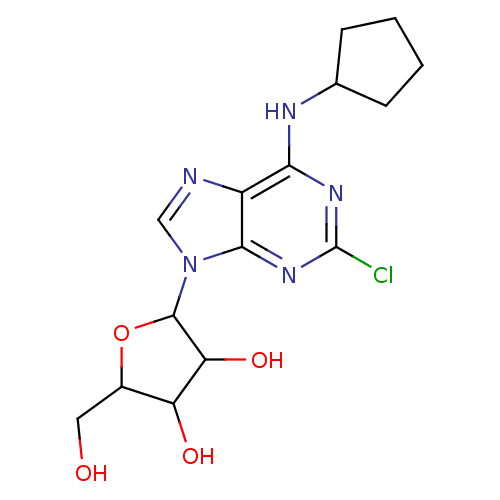
(2-(2-Chloro-6-cyclopentylamino-purin-9-yl)-5-hydro...)Show InChI InChI=1S/C15H20ClN5O4/c16-15-19-12(18-7-3-1-2-4-7)9-13(20-15)21(6-17-9)14-11(24)10(23)8(5-22)25-14/h6-8,10-11,14,22-24H,1-5H2,(H,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Chick) | BDBM85777
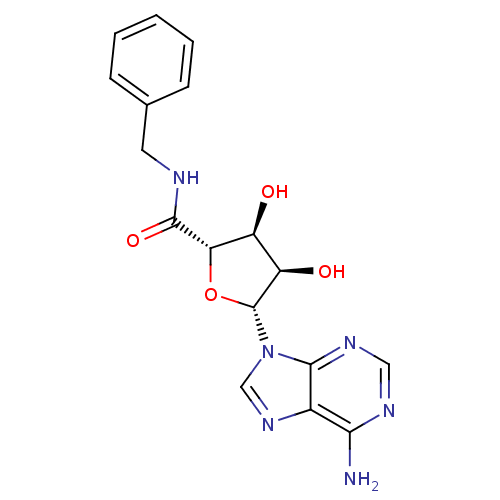
(B-NECA)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCc1ccccc1 Show InChI InChI=1S/C17H18N6O4/c18-14-10-15(21-7-20-14)23(8-22-10)17-12(25)11(24)13(27-17)16(26)19-6-9-4-2-1-3-5-9/h1-5,7-8,11-13,17,24-25H,6H2,(H,19,26)(H2,18,20,21)/t11-,12+,13-,17+/m0/s1 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Chick) | BDBM50008415

(2-(2-Chloro-6-cyclopentylamino-purin-9-yl)-5-hydro...)Show InChI InChI=1S/C15H20ClN5O4/c16-15-19-12(18-7-3-1-2-4-7)9-13(20-15)21(6-17-9)14-11(24)10(23)8(5-22)25-14/h6-8,10-11,14,22-24H,1-5H2,(H,18,19,20) | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Chick) | BDBM85777

(B-NECA)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCc1ccccc1 Show InChI InChI=1S/C17H18N6O4/c18-14-10-15(21-7-20-14)23(8-22-10)17-12(25)11(24)13(27-17)16(26)19-6-9-4-2-1-3-5-9/h1-5,7-8,11-13,17,24-25H,6H2,(H,19,26)(H2,18,20,21)/t11-,12+,13-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Chick) | BDBM82032
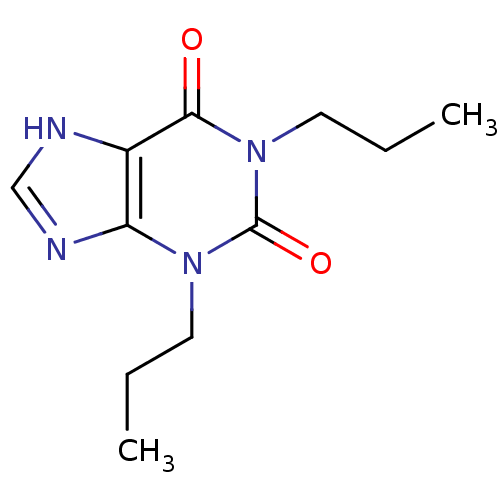
(1,3-Dipropyl-3,7-dihydro-purine-2,6-dione | 1,3-Di...)Show InChI InChI=1S/C11H16N4O2/c1-3-5-14-9-8(12-7-13-9)10(16)15(6-4-2)11(14)17/h7H,3-6H2,1-2H3,(H,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Chick) | BDBM21221

((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 Show InChI InChI=1S/C18H18ClIN6O4/c1-21-16(29)13-11(27)12(28)17(30-13)26-7-23-10-14(24-18(19)25-15(10)26)22-6-8-3-2-4-9(20)5-8/h2-5,7,11-13,17,27-28H,6H2,1H3,(H,21,29)(H,22,24,25)/t11-,12+,13-,17+/m0/s1 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Chick) | BDBM50034171

(3,4-Dihydroxy-5-[6-(3-iodo-benzylamino)-purin-9-yl...)Show SMILES CNC(=O)C1OC(C(O)C1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 16.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Chick) | BDBM50009552

(2-[6-Amino-2-(2-morpholin-4-yl-ethylamino)-purin-9...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCCC3)ncnc12 |r| Show InChI InChI=1S/C16H23N5O4/c22-6-10-12(23)13(24)16(25-10)21-8-19-11-14(17-7-18-15(11)21)20-9-4-2-1-3-5-9/h7-10,12-13,16,22-24H,1-6H2,(H,17,18,20)/t10-,12-,13-,16-/m1/s1 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Chick) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 23.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Chick) | BDBM85036
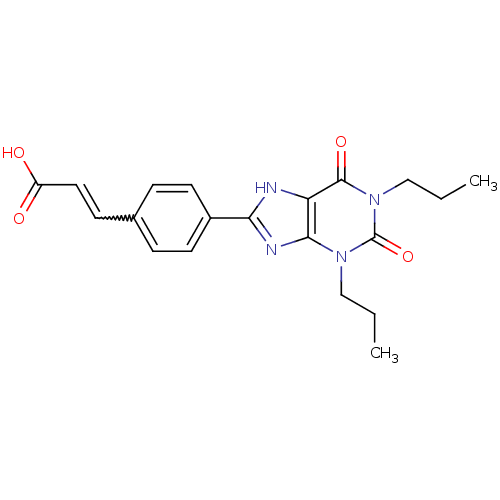
(BW-A1433 | BWA1433 | CAS_129447 | NSC_129447)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(C=CC(O)=O)cc1 |w:21.22| Show InChI InChI=1S/C20H22N4O4/c1-3-11-23-18-16(19(27)24(12-4-2)20(23)28)21-17(22-18)14-8-5-13(6-9-14)7-10-15(25)26/h5-10H,3-4,11-12H2,1-2H3,(H,21,22)(H,25,26)/b10-7+ | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Chick) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 37.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Chick) | BDBM82032

(1,3-Dipropyl-3,7-dihydro-purine-2,6-dione | 1,3-Di...)Show InChI InChI=1S/C11H16N4O2/c1-3-5-14-9-8(12-7-13-9)10(16)15(6-4-2)11(14)17/h7H,3-6H2,1-2H3,(H,12,13) | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 53.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM238298
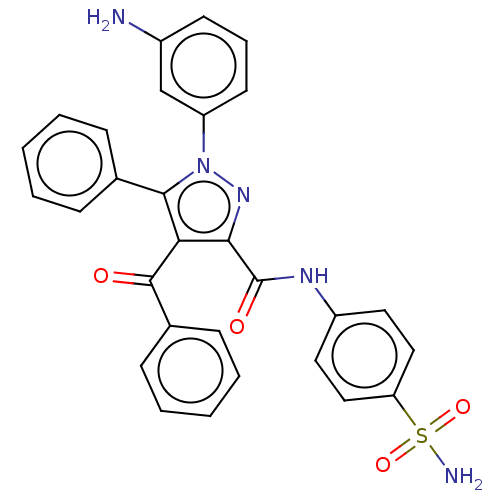
(1-(3-aminophenyl)-4-benzoyl-5-phenyl-N-(4-sulfamoy...)Show SMILES Nc1cccc(c1)-n1nc(C(=O)Nc2ccc(cc2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1 Show InChI InChI=1S/C29H23N5O4S/c30-21-12-7-13-23(18-21)34-27(19-8-3-1-4-9-19)25(28(35)20-10-5-2-6-11-20)26(33-34)29(36)32-22-14-16-24(17-15-22)39(31,37)38/h1-18H,30H2,(H,32,36)(H2,31,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 55 | -41.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Dumlupinar University
| Assay Description
CA activity was assayed in inhibition studies by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion o... |
J Enzyme Inhib Med Chem 28: 328-36 (2013)
Article DOI: 10.3109/14756366.2011.651465
BindingDB Entry DOI: 10.7270/Q2V986ZJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Chick) | BDBM85037
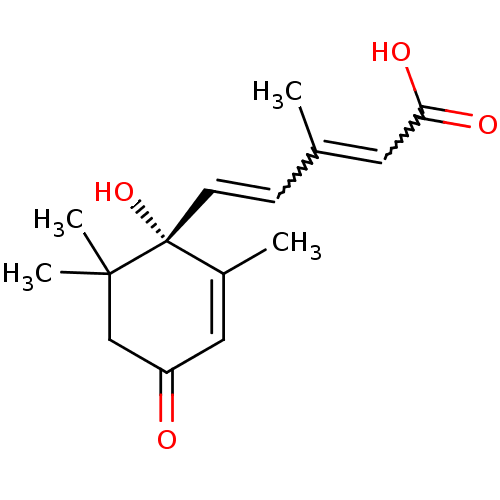
(ABA | CAS_14375-45-2)Show SMILES CC(C=C[C@@]1(O)C(C)=CC(=O)CC1(C)C)=CC(O)=O |r,w:2.1,15.16,c:7| Show InChI InChI=1S/C15H20O4/c1-10(7-13(17)18)5-6-15(19)11(2)8-12(16)9-14(15,3)4/h5-8,19H,9H2,1-4H3,(H,17,18)/t15-/m1/s1 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 56.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM238299
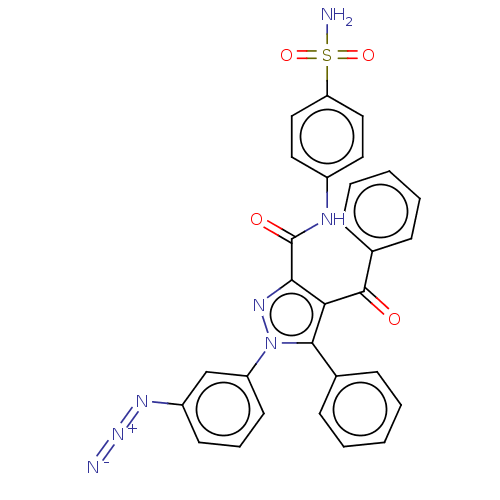
(1-(3-azidophenyl)-4-benzoyl-5-phenyl-N-(4-sulfamoy...)Show SMILES NS(=O)(=O)c1ccc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(c2)N=[N+]=[N-])cc1 Show InChI InChI=1S/C29H21N7O4S/c30-35-33-22-12-7-13-23(18-22)36-27(19-8-3-1-4-9-19)25(28(37)20-10-5-2-6-11-20)26(34-36)29(38)32-21-14-16-24(17-15-21)41(31,39)40/h1-18H,(H,32,38)(H2,31,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 64 | -41.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Dumlupinar University
| Assay Description
CA activity was assayed in inhibition studies by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion o... |
J Enzyme Inhib Med Chem 28: 328-36 (2013)
Article DOI: 10.3109/14756366.2011.651465
BindingDB Entry DOI: 10.7270/Q2V986ZJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Chick) | BDBM21221

((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 Show InChI InChI=1S/C18H18ClIN6O4/c1-21-16(29)13-11(27)12(28)17(30-13)26-7-23-10-14(24-18(19)25-15(10)26)22-6-8-3-2-4-9(20)5-8/h2-5,7,11-13,17,27-28H,6H2,1H3,(H,21,29)(H,22,24,25)/t11-,12+,13-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 74.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM238298

(1-(3-aminophenyl)-4-benzoyl-5-phenyl-N-(4-sulfamoy...)Show SMILES Nc1cccc(c1)-n1nc(C(=O)Nc2ccc(cc2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1 Show InChI InChI=1S/C29H23N5O4S/c30-21-12-7-13-23(18-21)34-27(19-8-3-1-4-9-19)25(28(35)20-10-5-2-6-11-20)26(33-34)29(36)32-22-14-16-24(17-15-22)39(31,37)38/h1-18H,30H2,(H,32,36)(H2,31,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 108 | -39.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Dumlupinar University
| Assay Description
CA activity was assayed in inhibition studies by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion o... |
J Enzyme Inhib Med Chem 28: 328-36 (2013)
Article DOI: 10.3109/14756366.2011.651465
BindingDB Entry DOI: 10.7270/Q2V986ZJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM238299

(1-(3-azidophenyl)-4-benzoyl-5-phenyl-N-(4-sulfamoy...)Show SMILES NS(=O)(=O)c1ccc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(c2)N=[N+]=[N-])cc1 Show InChI InChI=1S/C29H21N7O4S/c30-35-33-22-12-7-13-23(18-22)36-27(19-8-3-1-4-9-19)25(28(37)20-10-5-2-6-11-20)26(34-36)29(38)32-21-14-16-24(17-15-21)41(31,39)40/h1-18H,(H,32,38)(H2,31,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 129 | -39.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Dumlupinar University
| Assay Description
CA activity was assayed in inhibition studies by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion o... |
J Enzyme Inhib Med Chem 28: 328-36 (2013)
Article DOI: 10.3109/14756366.2011.651465
BindingDB Entry DOI: 10.7270/Q2V986ZJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM238300

(4-benzoyl-1-(3-((2-hydroxy-4-oxopent-2-en-3-yl) di...)Show SMILES [#6]-[#6](=O)-[#6](=[#7]\[#7]-c1cccc(c1)-n1nc(-[#6](=O)-[#7]-c2ccc(cc2)S([#7])(=O)=O)c(-[#6](=O)-c2ccccc2)c1-c1ccccc1)\[#6](-[#6])=O Show InChI InChI=1S/C34H28N6O6S/c1-21(41)30(22(2)42)38-37-26-14-9-15-27(20-26)40-32(23-10-5-3-6-11-23)29(33(43)24-12-7-4-8-13-24)31(39-40)34(44)36-25-16-18-28(19-17-25)47(35,45)46/h3-20,37H,1-2H3,(H,36,44)(H2,35,45,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 160 | -38.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Dumlupinar University
| Assay Description
CA activity was assayed in inhibition studies by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion o... |
J Enzyme Inhib Med Chem 28: 328-36 (2013)
Article DOI: 10.3109/14756366.2011.651465
BindingDB Entry DOI: 10.7270/Q2V986ZJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM238302

(4-benzoyl-1-(3-((3-hydroxy-1-oxo-1-phenylbut-2-en-...)Show SMILES CC(=O)C(=N/Nc1cccc(c1)-n1nc(C(=O)Nc2ccc(cc2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1)\C(=O)c1ccccc1 Show InChI InChI=1S/C39H30N6O6S/c1-25(46)34(38(48)28-16-9-4-10-17-28)43-42-30-18-11-19-31(24-30)45-36(26-12-5-2-6-13-26)33(37(47)27-14-7-3-8-15-27)35(44-45)39(49)41-29-20-22-32(23-21-29)52(40,50)51/h2-24,42H,1H3,(H,41,49)(H2,40,50,51)/b43-34+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 166 | -38.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Dumlupinar University
| Assay Description
CA activity was assayed in inhibition studies by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion o... |
J Enzyme Inhib Med Chem 28: 328-36 (2013)
Article DOI: 10.3109/14756366.2011.651465
BindingDB Entry DOI: 10.7270/Q2V986ZJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Chick) | BDBM85036

(BW-A1433 | BWA1433 | CAS_129447 | NSC_129447)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(C=CC(O)=O)cc1 |w:21.22| Show InChI InChI=1S/C20H22N4O4/c1-3-11-23-18-16(19(27)24(12-4-2)20(23)28)21-17(22-18)14-8-5-13(6-9-14)7-10-15(25)26/h5-10H,3-4,11-12H2,1-2H3,(H,21,22)(H,25,26)/b10-7+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM238301
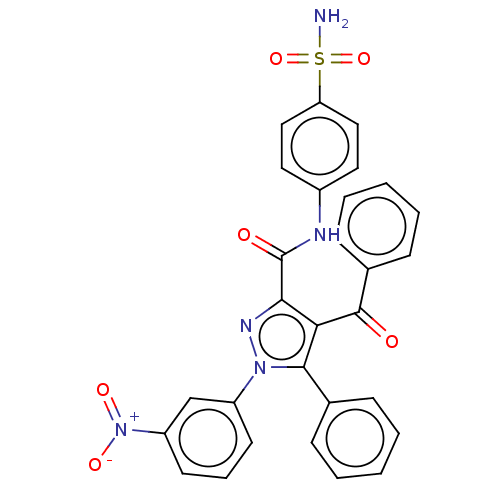
(4-benzoyl-1-(3-nitrophenyl)-5-phenyl-N-(4-sulfamoy...)Show SMILES NS(=O)(=O)c1ccc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(c2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C29H21N5O6S/c30-41(39,40)24-16-14-21(15-17-24)31-29(36)26-25(28(35)20-10-5-2-6-11-20)27(19-8-3-1-4-9-19)33(32-26)22-12-7-13-23(18-22)34(37)38/h1-18H,(H,31,36)(H2,30,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 215 | -38.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Dumlupinar University
| Assay Description
CA activity was assayed in inhibition studies by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion o... |
J Enzyme Inhib Med Chem 28: 328-36 (2013)
Article DOI: 10.3109/14756366.2011.651465
BindingDB Entry DOI: 10.7270/Q2V986ZJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM238301

(4-benzoyl-1-(3-nitrophenyl)-5-phenyl-N-(4-sulfamoy...)Show SMILES NS(=O)(=O)c1ccc(NC(=O)c2nn(c(c2C(=O)c2ccccc2)-c2ccccc2)-c2cccc(c2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C29H21N5O6S/c30-41(39,40)24-16-14-21(15-17-24)31-29(36)26-25(28(35)20-10-5-2-6-11-20)27(19-8-3-1-4-9-19)33(32-26)22-12-7-13-23(18-22)34(37)38/h1-18H,(H,31,36)(H2,30,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 337 | -36.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Dumlupinar University
| Assay Description
CA activity was assayed in inhibition studies by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion o... |
J Enzyme Inhib Med Chem 28: 328-36 (2013)
Article DOI: 10.3109/14756366.2011.651465
BindingDB Entry DOI: 10.7270/Q2V986ZJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM238302

(4-benzoyl-1-(3-((3-hydroxy-1-oxo-1-phenylbut-2-en-...)Show SMILES CC(=O)C(=N/Nc1cccc(c1)-n1nc(C(=O)Nc2ccc(cc2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1)\C(=O)c1ccccc1 Show InChI InChI=1S/C39H30N6O6S/c1-25(46)34(38(48)28-16-9-4-10-17-28)43-42-30-18-11-19-31(24-30)45-36(26-12-5-2-6-13-26)33(37(47)27-14-7-3-8-15-27)35(44-45)39(49)41-29-20-22-32(23-21-29)52(40,50)51/h2-24,42H,1H3,(H,41,49)(H2,40,50,51)/b43-34+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 521 | -35.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Dumlupinar University
| Assay Description
CA activity was assayed in inhibition studies by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion o... |
J Enzyme Inhib Med Chem 28: 328-36 (2013)
Article DOI: 10.3109/14756366.2011.651465
BindingDB Entry DOI: 10.7270/Q2V986ZJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM238300

(4-benzoyl-1-(3-((2-hydroxy-4-oxopent-2-en-3-yl) di...)Show SMILES [#6]-[#6](=O)-[#6](=[#7]\[#7]-c1cccc(c1)-n1nc(-[#6](=O)-[#7]-c2ccc(cc2)S([#7])(=O)=O)c(-[#6](=O)-c2ccccc2)c1-c1ccccc1)\[#6](-[#6])=O Show InChI InChI=1S/C34H28N6O6S/c1-21(41)30(22(2)42)38-37-26-14-9-15-27(20-26)40-32(23-10-5-3-6-11-23)29(33(43)24-12-7-4-8-13-24)31(39-40)34(44)36-25-16-18-28(19-17-25)47(35,45)46/h3-20,37H,1-2H3,(H,36,44)(H2,35,45,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 595 | -35.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Dumlupinar University
| Assay Description
CA activity was assayed in inhibition studies by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion o... |
J Enzyme Inhib Med Chem 28: 328-36 (2013)
Article DOI: 10.3109/14756366.2011.651465
BindingDB Entry DOI: 10.7270/Q2V986ZJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Chick) | BDBM85775
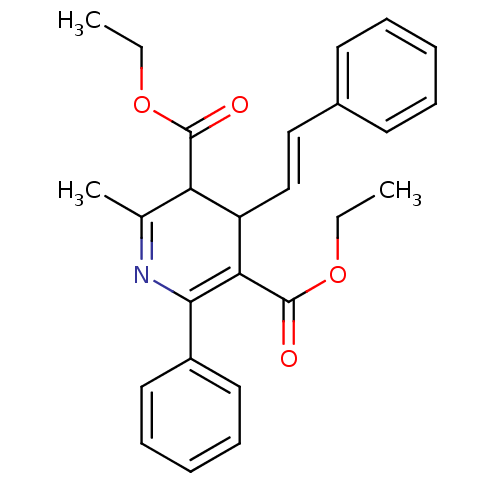
(CHEMBL315984 | MRS1097)Show SMILES CCOC(=O)C1C(\C=C\c2ccccc2)C(C(=O)OCC)=C(N=C1C)c1ccccc1 |c:21,23| Show InChI InChI=1S/C26H27NO4/c1-4-30-25(28)22-18(3)27-24(20-14-10-7-11-15-20)23(26(29)31-5-2)21(22)17-16-19-12-8-6-9-13-19/h6-17,21-22H,4-5H2,1-3H3/b17-16+ | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Chick) | BDBM85776
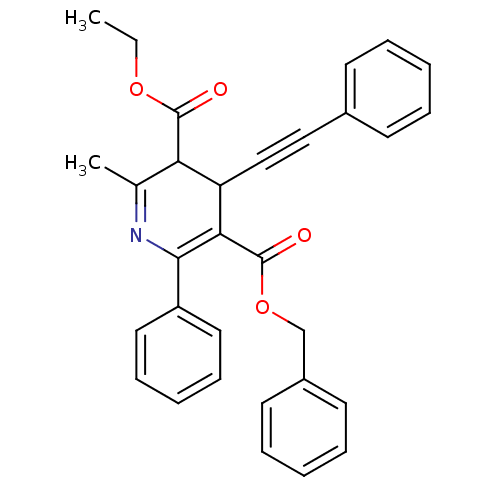
(CAS_393594 | CHEMBL89852 | MRS1191 | NSC_393594)Show SMILES CCOC(=O)C1C(C#Cc2ccccc2)C(C(=O)OCc2ccccc2)=C(N=C1C)c1ccccc1 |c:27,29| Show InChI InChI=1S/C31H27NO4/c1-3-35-30(33)27-22(2)32-29(25-17-11-6-12-18-25)28(26(27)20-19-23-13-7-4-8-14-23)31(34)36-21-24-15-9-5-10-16-24/h4-18,26-27H,3,21H2,1-2H3 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
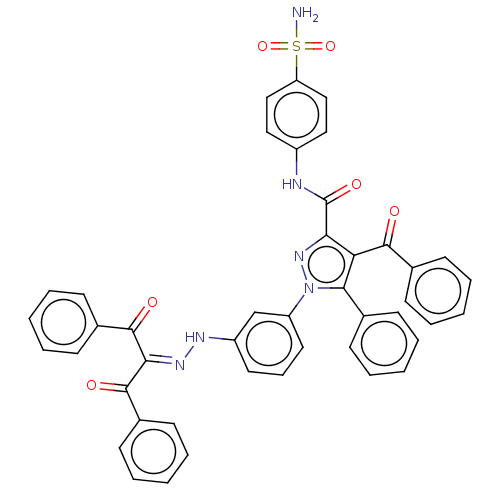
(Homo sapiens (Human)) | BDBM238304
(4-benzoyl-1-(3-(2-(1,3-dioxo-1,3-diphenylpropan-2-...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#7]-[#6](=O)-c2nn(c(c2-[#6](=O)-c2ccccc2)-c2ccccc2)-c2cccc(-[#7]\[#7]=[#6](\[#6](=O)-c3ccccc3)-[#6](=O)-c3ccccc3)c2)cc1 Show InChI InChI=1S/C44H32N6O6S/c45-57(55,56)36-26-24-33(25-27-36)46-44(54)38-37(41(51)30-16-7-2-8-17-30)40(29-14-5-1-6-15-29)50(49-38)35-23-13-22-34(28-35)47-48-39(42(52)31-18-9-3-10-19-31)43(53)32-20-11-4-12-21-32/h1-28,47H,(H,46,54)(H2,45,55,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.95E+3 | -32.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Dumlupinar University
| Assay Description
CA activity was assayed in inhibition studies by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion o... |
J Enzyme Inhib Med Chem 28: 328-36 (2013)
Article DOI: 10.3109/14756366.2011.651465
BindingDB Entry DOI: 10.7270/Q2V986ZJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
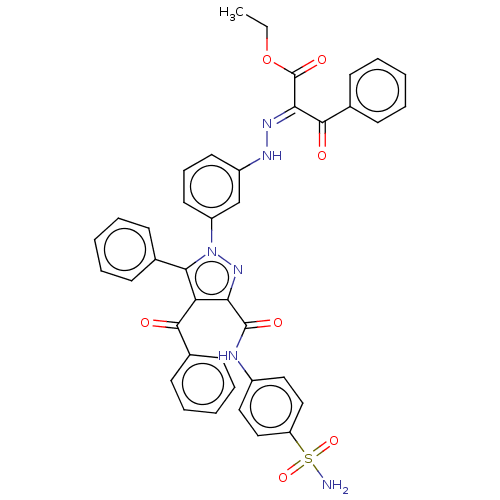
(Homo sapiens (Human)) | BDBM238303
(Ethyl-2-(2-(3-(4-benzoyl-5-phenyl-3-(4-sulfamoylph...)Show SMILES CCOC(=O)C(=N\Nc1cccc(c1)-n1nc(C(=O)Nc2ccc(cc2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1)\C(=O)c1ccccc1 Show InChI InChI=1S/C40H32N6O7S/c1-2-53-40(50)35(38(48)28-17-10-5-11-18-28)44-43-30-19-12-20-31(25-30)46-36(26-13-6-3-7-14-26)33(37(47)27-15-8-4-9-16-27)34(45-46)39(49)42-29-21-23-32(24-22-29)54(41,51)52/h3-25,43H,2H2,1H3,(H,42,49)(H2,41,51,52)/b44-35+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90E+3 | -31.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Dumlupinar University
| Assay Description
CA activity was assayed in inhibition studies by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion o... |
J Enzyme Inhib Med Chem 28: 328-36 (2013)
Article DOI: 10.3109/14756366.2011.651465
BindingDB Entry DOI: 10.7270/Q2V986ZJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
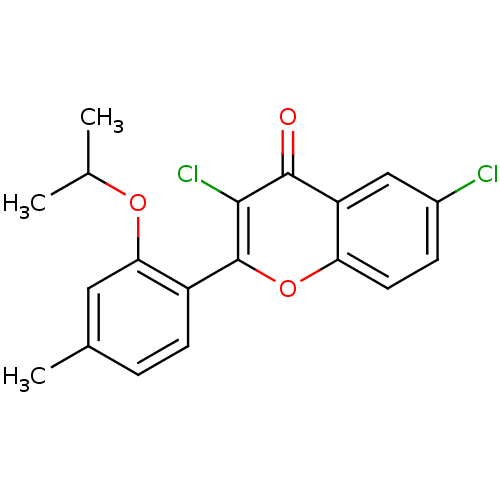
(Chick) | BDBM50053924
(3,6-Dichloro-2-(2-isopropoxy-4-methyl-phenyl)-chro...)Show InChI InChI=1S/C19H16Cl2O3/c1-10(2)23-16-8-11(3)4-6-13(16)19-17(21)18(22)14-9-12(20)5-7-15(14)24-19/h4-10H,1-3H3 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Chick) | BDBM85775

(CHEMBL315984 | MRS1097)Show SMILES CCOC(=O)C1C(\C=C\c2ccccc2)C(C(=O)OCC)=C(N=C1C)c1ccccc1 |c:21,23| Show InChI InChI=1S/C26H27NO4/c1-4-30-25(28)22-18(3)27-24(20-14-10-7-11-15-20)23(26(29)31-5-2)21(22)17-16-19-12-8-6-9-13-19/h6-17,21-22H,4-5H2,1-3H3/b17-16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 81-6 (2001)
Article DOI: 10.1007/s002100000340
BindingDB Entry DOI: 10.7270/Q25Q4TNC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM238304

(4-benzoyl-1-(3-(2-(1,3-dioxo-1,3-diphenylpropan-2-...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#7]-[#6](=O)-c2nn(c(c2-[#6](=O)-c2ccccc2)-c2ccccc2)-c2cccc(-[#7]\[#7]=[#6](\[#6](=O)-c3ccccc3)-[#6](=O)-c3ccccc3)c2)cc1 Show InChI InChI=1S/C44H32N6O6S/c45-57(55,56)36-26-24-33(25-27-36)46-44(54)38-37(41(51)30-16-7-2-8-17-30)40(29-14-5-1-6-15-29)50(49-38)35-23-13-22-34(28-35)47-48-39(42(52)31-18-9-3-10-19-31)43(53)32-20-11-4-12-21-32/h1-28,47H,(H,46,54)(H2,45,55,56) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10E+3 | -30.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Dumlupinar University
| Assay Description
CA activity was assayed in inhibition studies by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion o... |
J Enzyme Inhib Med Chem 28: 328-36 (2013)
Article DOI: 10.3109/14756366.2011.651465
BindingDB Entry DOI: 10.7270/Q2V986ZJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM238303

(Ethyl-2-(2-(3-(4-benzoyl-5-phenyl-3-(4-sulfamoylph...)Show SMILES CCOC(=O)C(=N\Nc1cccc(c1)-n1nc(C(=O)Nc2ccc(cc2)S(N)(=O)=O)c(C(=O)c2ccccc2)c1-c1ccccc1)\C(=O)c1ccccc1 Show InChI InChI=1S/C40H32N6O7S/c1-2-53-40(50)35(38(48)28-17-10-5-11-18-28)44-43-30-19-12-20-31(25-30)46-36(26-13-6-3-7-14-26)33(37(47)27-15-8-4-9-16-27)34(45-46)39(49)42-29-21-23-32(24-22-29)54(41,51)52/h3-25,43H,2H2,1H3,(H,42,49)(H2,41,51,52)/b44-35+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.24E+3 | -29.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Dumlupinar University
| Assay Description
CA activity was assayed in inhibition studies by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion o... |
J Enzyme Inhib Med Chem 28: 328-36 (2013)
Article DOI: 10.3109/14756366.2011.651465
BindingDB Entry DOI: 10.7270/Q2V986ZJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data