

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24621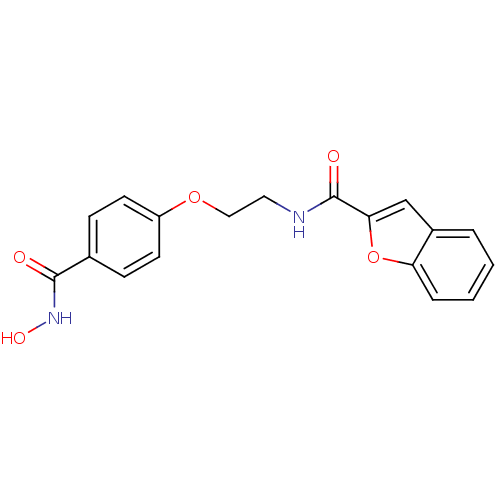 (CG-003 | N-{2-[4-(hydroxycarbamoyl)phenoxy]ethyl}-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24620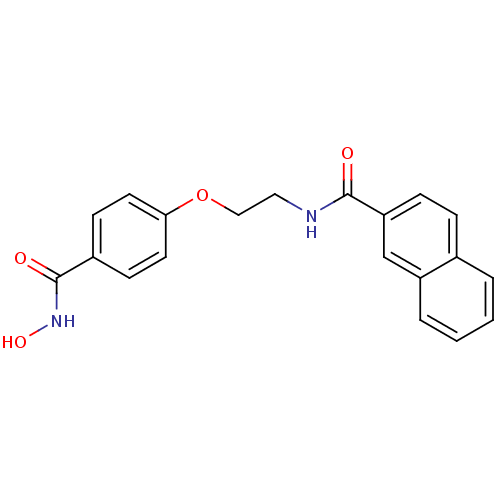 (CG-002 | N-{2-[4-(hydroxycarbamoyl)phenoxy]ethyl}n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | -46.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8.20 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24618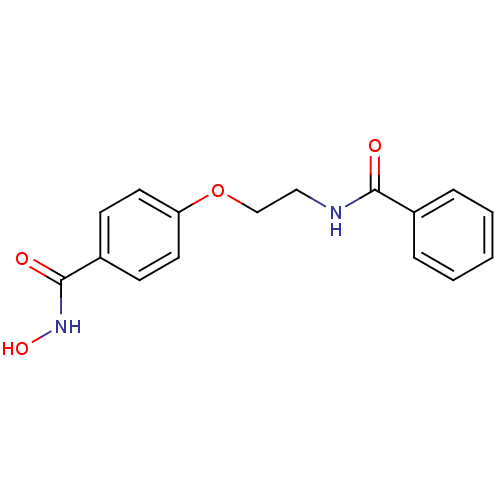 (CG-001 | N-hydroxy-4-[2-(phenylformamido)ethoxy]be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 43 | -41.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 280 | -37.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24623 (4-[2-({3-[(dimethylamino)methyl]-1-benzofuran-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | >-24.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM97672 (US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM97672 (US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM191606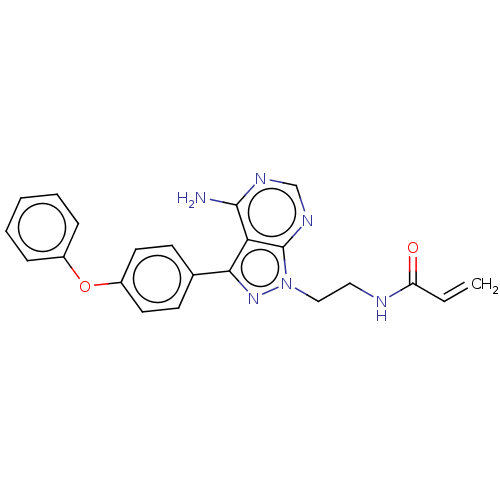 (US9181263, 23 | US9278100, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM191606 (US9181263, 23 | US9278100, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM191605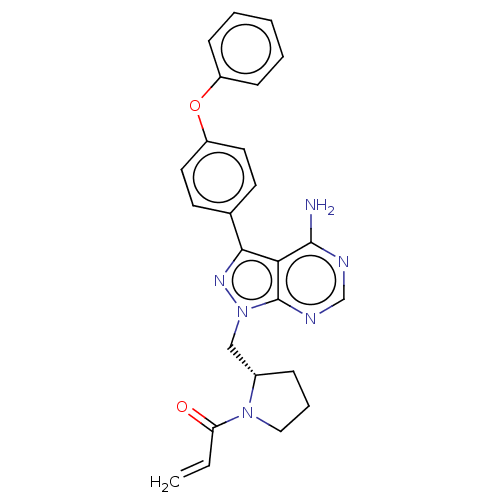 (US9181263, 14 | US9278100, 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM191605 (US9181263, 14 | US9278100, 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fgr (Homo sapiens (Human)) | BDBM97672 (US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fgr (Homo sapiens (Human)) | BDBM97672 (US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM191617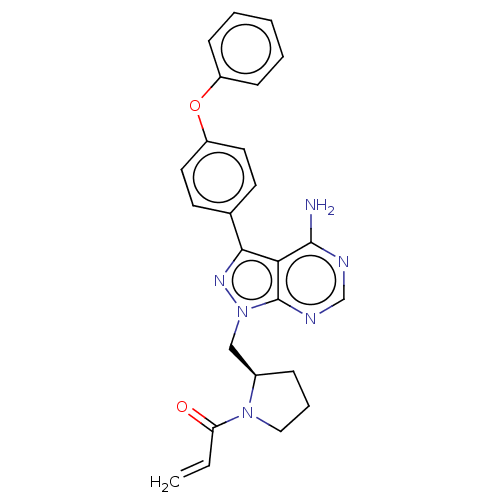 (US9181263, 13 | US9278100, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM191617 (US9181263, 13 | US9278100, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM191622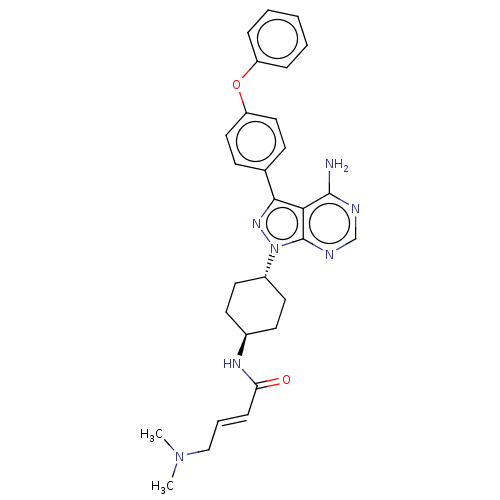 (US9181263, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM220129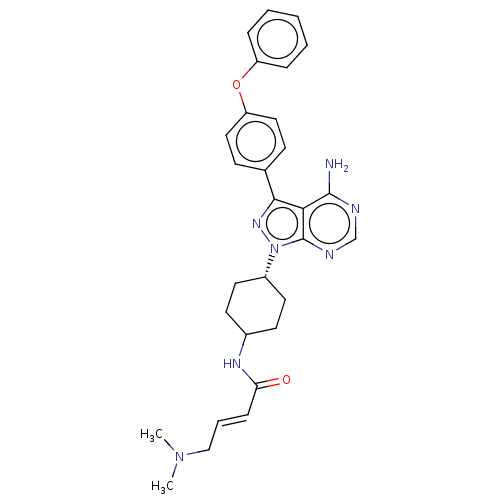 (US9278100, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM97672 (US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytoplasmic tyrosine-protein kinase BMX (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM97672 (US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytoplasmic tyrosine-protein kinase BMX (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM191614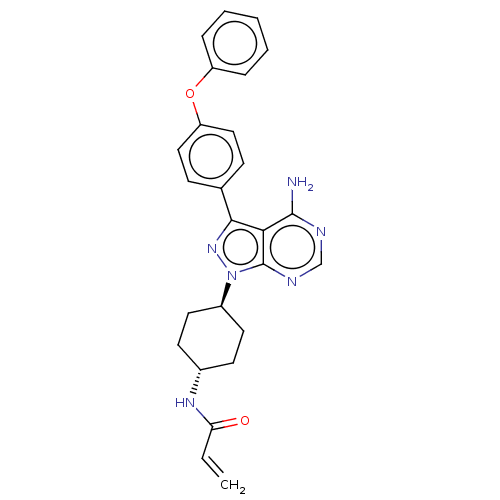 (US9181263, 10 | US9278100, 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM191614 (US9181263, 10 | US9278100, 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM191628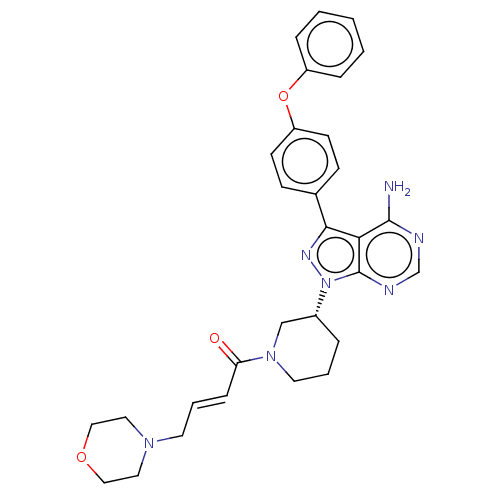 (US9181263, 24 | US9278100, 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM191628 (US9181263, 24 | US9278100, 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM220128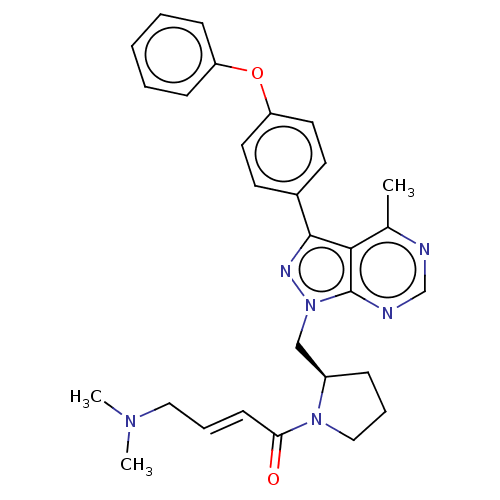 (US9278100, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM191608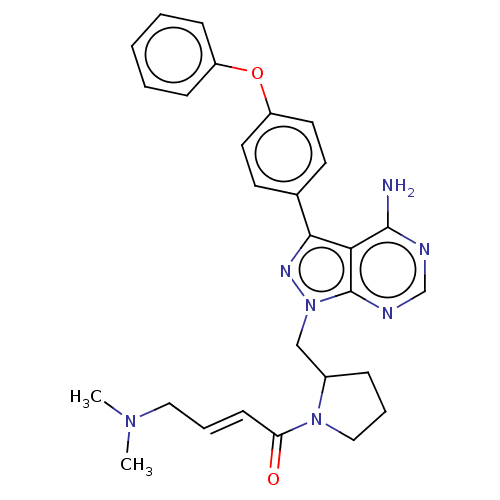 (US9181263, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM97672 (US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM97672 (US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM97672 (US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM97672 (US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM97672 (US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM97672 (US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 308 total ) | Next | Last >> |