

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246899 ((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University College of Medicine Curated by ChEMBL | Assay Description Inhibition of PSMA (unknown origin) | Bioorg Med Chem Lett 28: 572-576 (2018) Article DOI: 10.1016/j.bmcl.2018.01.047 BindingDB Entry DOI: 10.7270/Q2RN3BGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50366620 (RESINIFERATOXIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0426 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50247744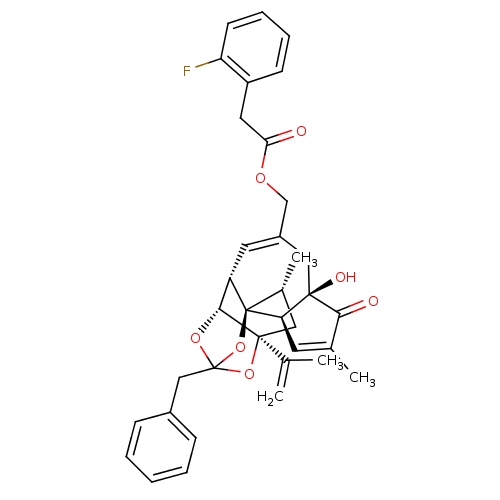 (CHEMBL504725 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50454862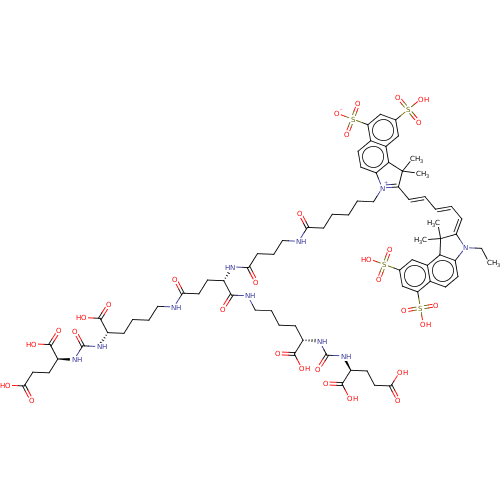 (CHEMBL4211875) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University College of Medicine Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell lysates incubated for 2 hrs in presence of N-acetylaspartylglutamate by Amplex red glutamic acid/glutamate oxi... | Bioorg Med Chem Lett 28: 572-576 (2018) Article DOI: 10.1016/j.bmcl.2018.01.047 BindingDB Entry DOI: 10.7270/Q2RN3BGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50247741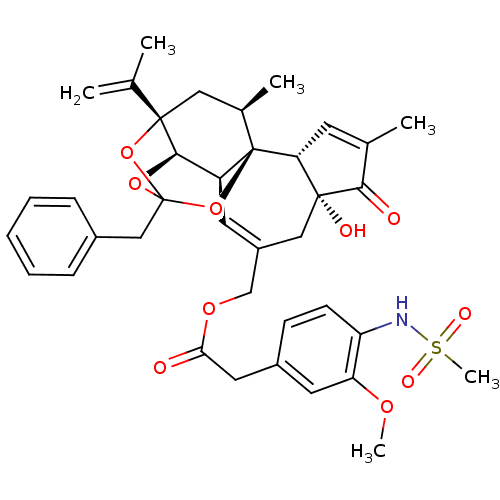 (CHEMBL503101 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.228 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180197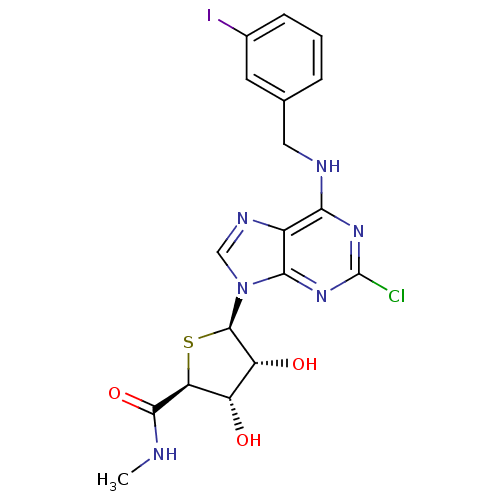 ((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Binding affinity to human adenosine A3 receptor | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50454861 (CHEMBL4206989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University College of Medicine Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell lysates incubated for 2 hrs in presence of N-acetylaspartylglutamate by Amplex red glutamic acid/glutamate oxi... | Bioorg Med Chem Lett 28: 572-576 (2018) Article DOI: 10.1016/j.bmcl.2018.01.047 BindingDB Entry DOI: 10.7270/Q2RN3BGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50247749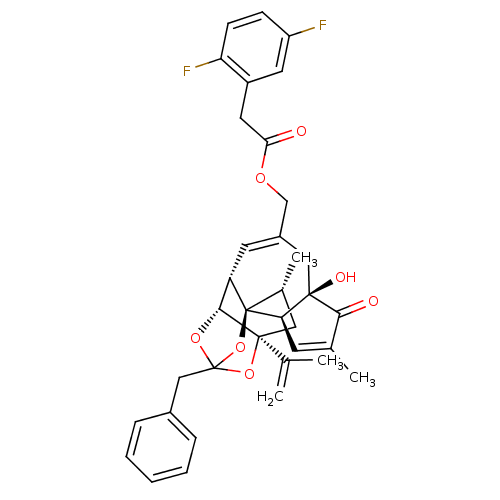 (CHEMBL510583 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50454860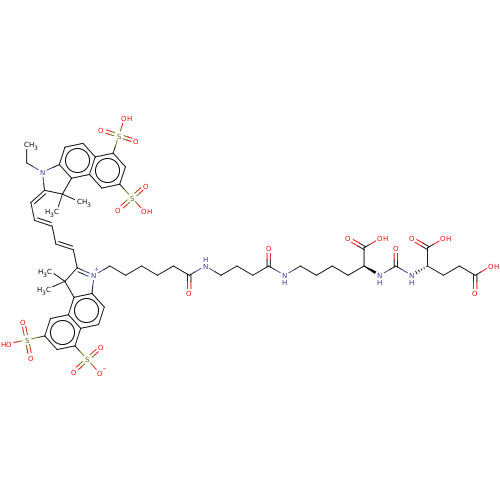 (CHEMBL4202635) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University College of Medicine Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell lysates incubated for 2 hrs in presence of N-acetylaspartylglutamate by Amplex red glutamic acid/glutamate oxi... | Bioorg Med Chem Lett 28: 572-576 (2018) Article DOI: 10.1016/j.bmcl.2018.01.047 BindingDB Entry DOI: 10.7270/Q2RN3BGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50172117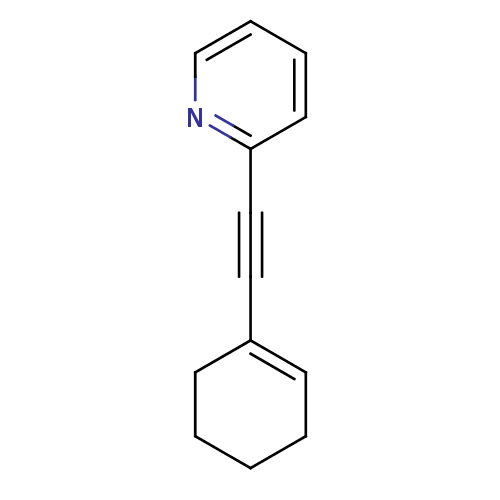 (2-Cyclohex-1-enylethynyl-pyridine | CHEMBL196643) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from mGlu5 receptor of rat cortical membrane | Bioorg Med Chem Lett 15: 4589-93 (2005) Article DOI: 10.1016/j.bmcl.2005.06.099 BindingDB Entry DOI: 10.7270/Q2DB81C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50172136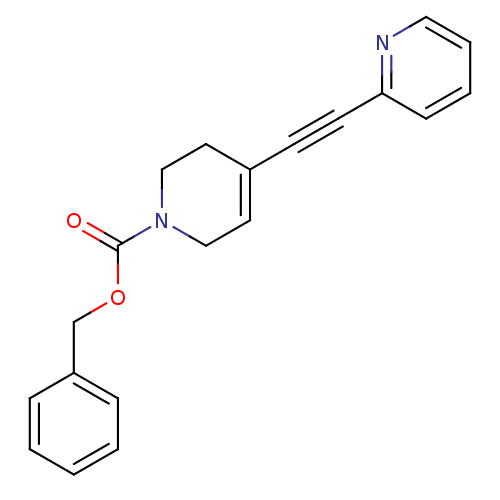 (4-Pyridin-2-ylethynyl-3,6-dihydro-2H-pyridine-1-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from mGlu5 receptor of rat cortical membrane | Bioorg Med Chem Lett 15: 4589-93 (2005) Article DOI: 10.1016/j.bmcl.2005.06.099 BindingDB Entry DOI: 10.7270/Q2DB81C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50247742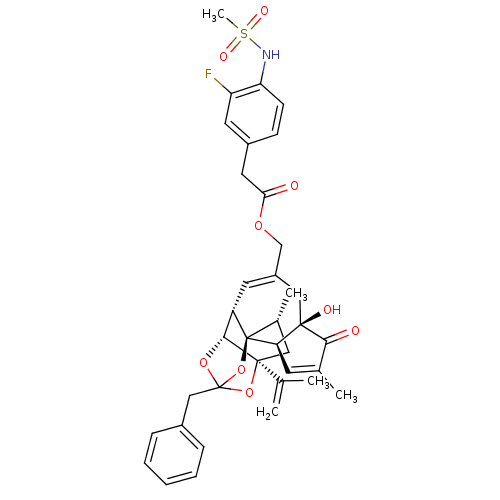 (CHEMBL509154 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50247748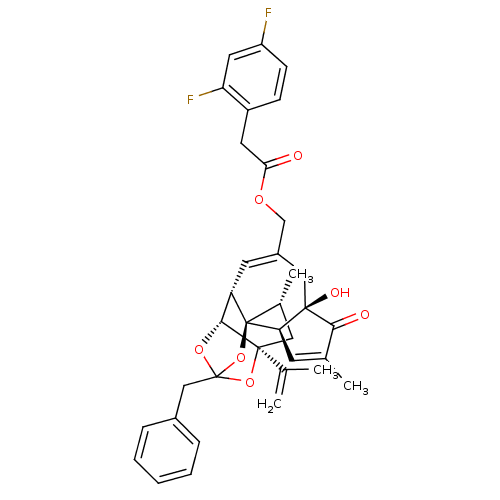 (CHEMBL510228 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50247751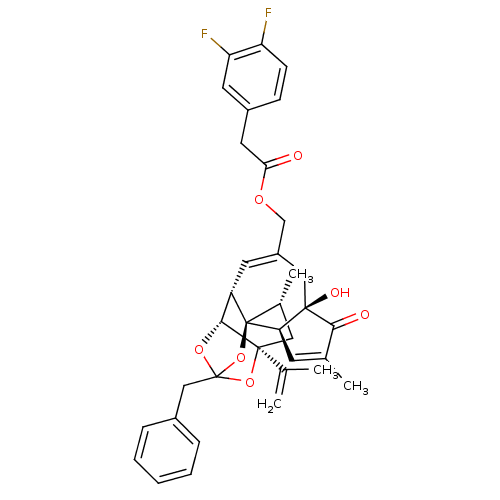 (CHEMBL449201 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50149792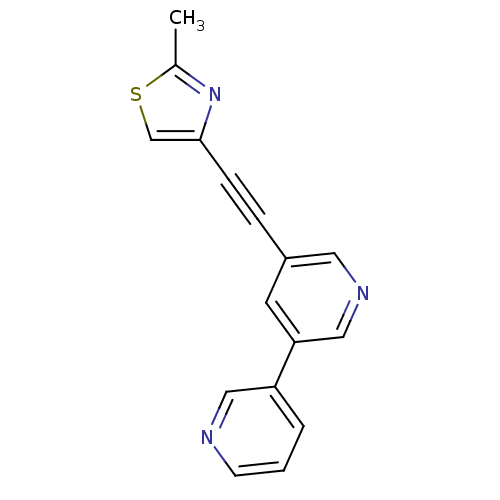 (5-(2-Methyl-thiazol-4-ylethynyl)-[3,3'']bipyridiny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement by compound of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from rat cortical membranes | Bioorg Med Chem Lett 14: 3993-6 (2004) Article DOI: 10.1016/j.bmcl.2004.05.037 BindingDB Entry DOI: 10.7270/Q2T43SKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50293031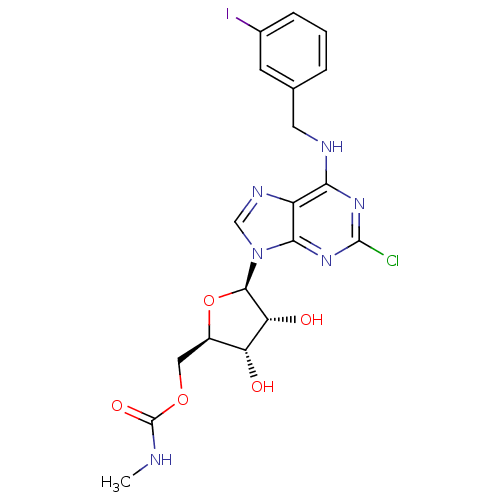 (2-chloro-N6-(3-iodobenzyl)-5'-N-methylcarbamoylade...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Binding affinity to human adenosine A3 receptor | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50172123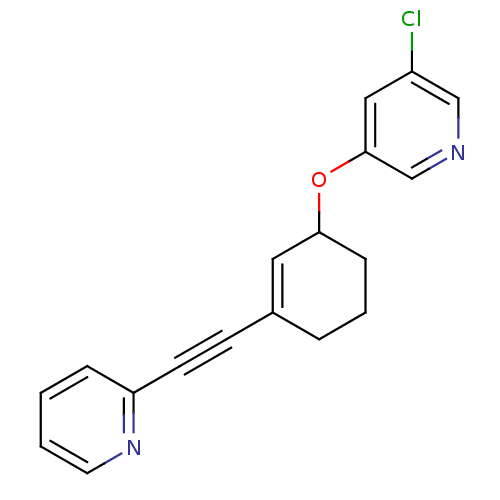 (3-Chloro-5-(3-pyridin-2-ylethynyl-cyclohex-2-enylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from mGlu5 receptor of rat cortical membrane | Bioorg Med Chem Lett 15: 4589-93 (2005) Article DOI: 10.1016/j.bmcl.2005.06.099 BindingDB Entry DOI: 10.7270/Q2DB81C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras guanyl-releasing protein 3 (Homo sapiens (Human)) | BDBM50244866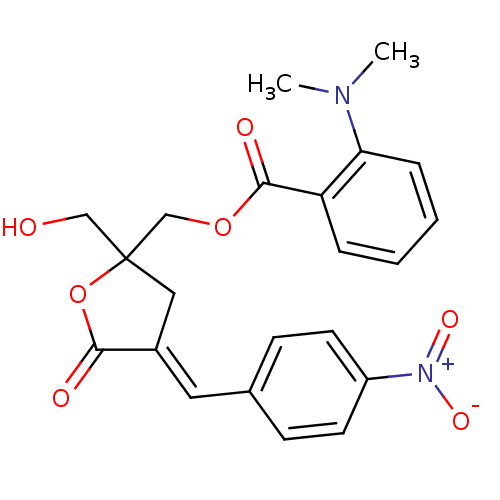 (CHEMBL511820 | rac-(E)-{2-(Hydroxymethyl)-4-[(4-ni...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Binding affinity to RasGRP3 (unknown origin) | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50154997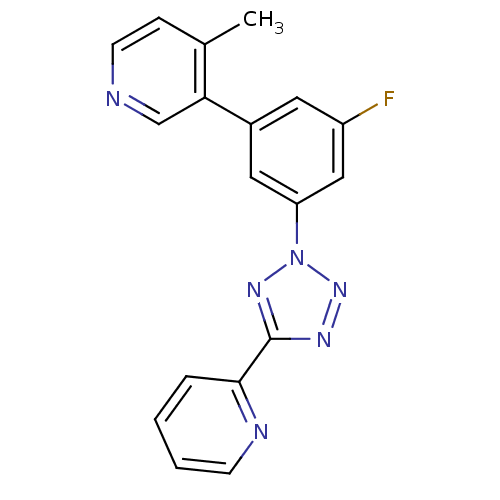 (3-[3-Fluoro-5-(5-pyridin-2-yl-tetrazol-2-yl)-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Metabotropic glutamate receptor was determined by displacing [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from rat cortica... | Bioorg Med Chem Lett 14: 5481-4 (2004) Article DOI: 10.1016/j.bmcl.2004.09.018 BindingDB Entry DOI: 10.7270/Q25M657V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50172130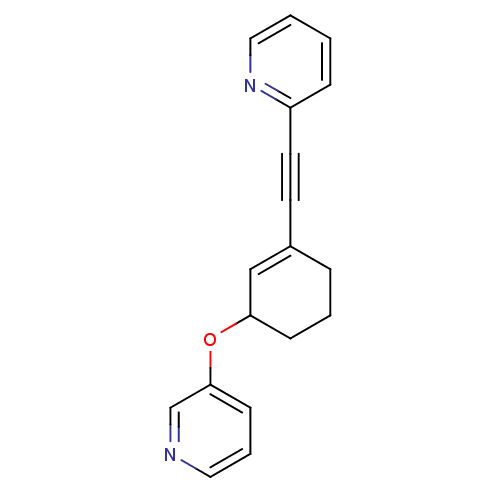 (2-{[3-(pyridin-3-yloxy)cyclohex-1-en-1-yl]ethynyl}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from mGlu5 receptor of rat cortical membrane | Bioorg Med Chem Lett 15: 4589-93 (2005) Article DOI: 10.1016/j.bmcl.2005.06.099 BindingDB Entry DOI: 10.7270/Q2DB81C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50154997 (3-[3-Fluoro-5-(5-pyridin-2-yl-tetrazol-2-yl)-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Metabotropic glutamate receptor was determined by displacing [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from rat cortica... | Bioorg Med Chem Lett 14: 5477-80 (2004) Article DOI: 10.1016/j.bmcl.2004.09.011 BindingDB Entry DOI: 10.7270/Q29C6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50149801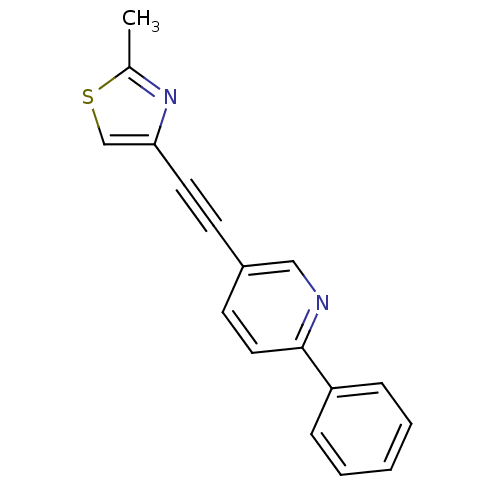 (2-Phenyl-5-(2-methylthiazol-4ylethynyl)pyridine | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement by compound of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from rat cortical membranes | Bioorg Med Chem Lett 14: 3993-6 (2004) Article DOI: 10.1016/j.bmcl.2004.05.037 BindingDB Entry DOI: 10.7270/Q2T43SKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50149805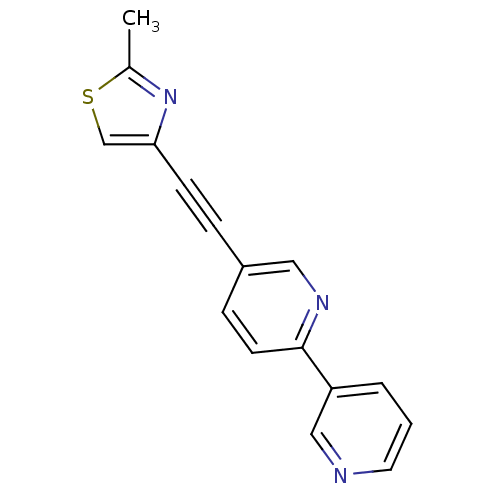 (2-(Pyridin-3-yl)-5-(2-methylthiazol-4ylethynyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement by compound of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from rat cortical membranes | Bioorg Med Chem Lett 14: 3993-6 (2004) Article DOI: 10.1016/j.bmcl.2004.05.037 BindingDB Entry DOI: 10.7270/Q2T43SKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (MOUSE) | BDBM50244416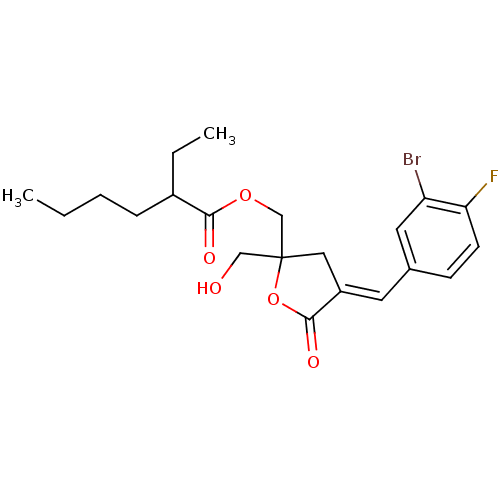 (CHEMBL527875 | rac-(E)-{4-[(3-Bromo-4-fluorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form mouse PKCalpha by scintillation counting | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (MOUSE) | BDBM50244416 (CHEMBL527875 | rac-(E)-{4-[(3-Bromo-4-fluorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form mouse PKCalpha by scintillation counting | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras guanyl-releasing protein 3 (Homo sapiens (Human)) | BDBM50245091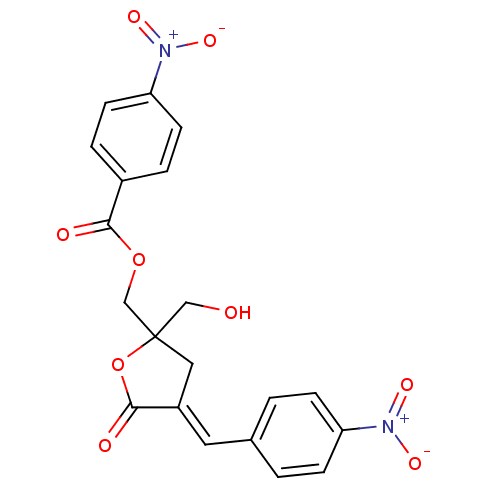 (CHEMBL519398 | rac-(E)-{2-(Hydroxymethyl)-4-[(4-ni...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Binding affinity to RasGRP3 (unknown origin) | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras guanyl-releasing protein 3 (Homo sapiens (Human)) | BDBM50244865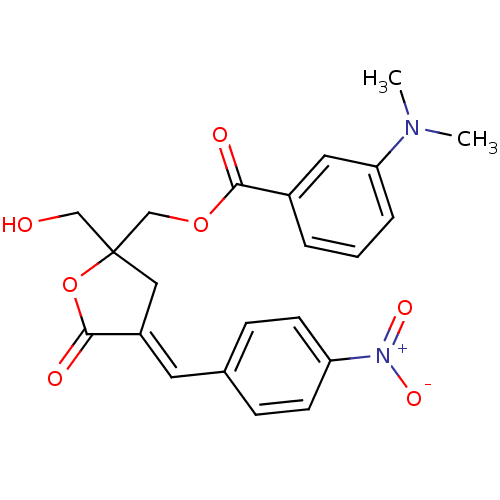 (CHEMBL471153 | rac-(E)-{2-(Hydroxymethyl)-4-[(4-ni...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Binding affinity to RasGRP3 (unknown origin) | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50247745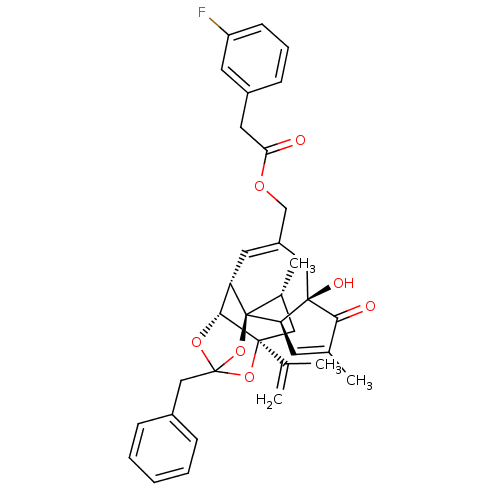 (CHEMBL455555 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (MOUSE) | BDBM50244693 (CHEMBL516638 | rac-(E)-{4-[(3-Chloro-4-fluoropheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form mouse PKCalpha by scintillation counting | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (MOUSE) | BDBM50244693 (CHEMBL516638 | rac-(E)-{4-[(3-Chloro-4-fluoropheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form mouse PKCalpha by scintillation counting | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (MOUSE) | BDBM50244961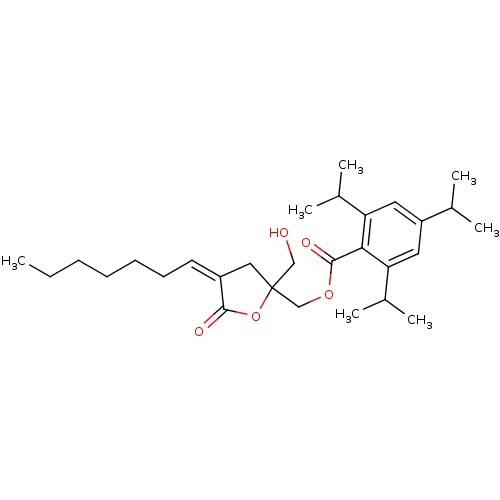 (CHEMBL472520 | rac-(E/Z)-(4-heptylidene-2-(hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form mouse PKCalpha by scintillation counting | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (MOUSE) | BDBM50244961 (CHEMBL472520 | rac-(E/Z)-(4-heptylidene-2-(hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form mouse PKCalpha by scintillation counting | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (MOUSE) | BDBM50244413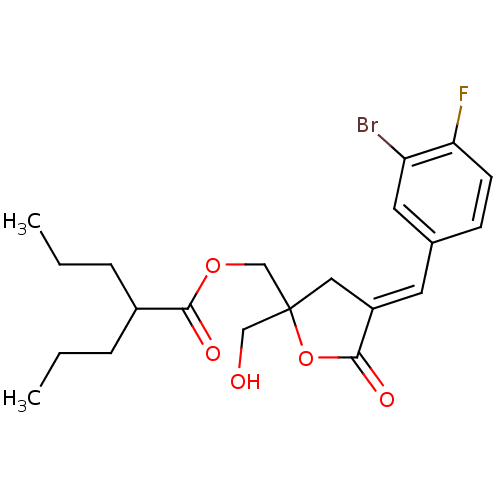 (CHEMBL488498 | rac-(E)-{4-[(3-Bromo-4-fluorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form mouse PKCalpha by scintillation counting | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (MOUSE) | BDBM50244413 (CHEMBL488498 | rac-(E)-{4-[(3-Bromo-4-fluorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form mouse PKCalpha by scintillation counting | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50247750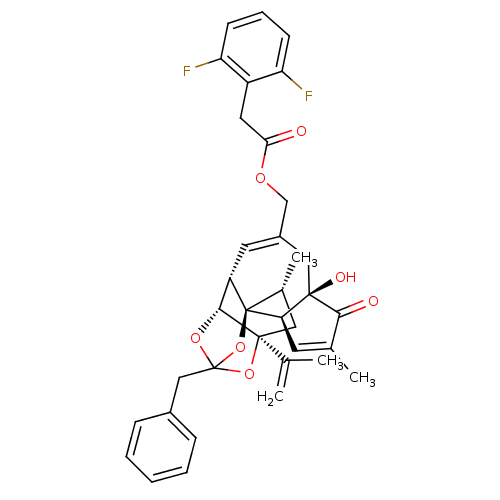 (CHEMBL455155 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50058163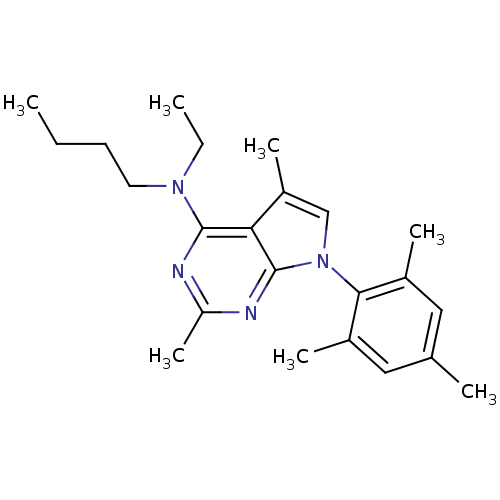 (Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against [125I]-Try0-o-Corticotropin-releasing Factor to Corticotropin releasing hormone receptor 1 from ovine | J Med Chem 40: 1749-54 (1997) Article DOI: 10.1021/jm960861b BindingDB Entry DOI: 10.7270/Q2SJ1JQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (MOUSE) | BDBM50244414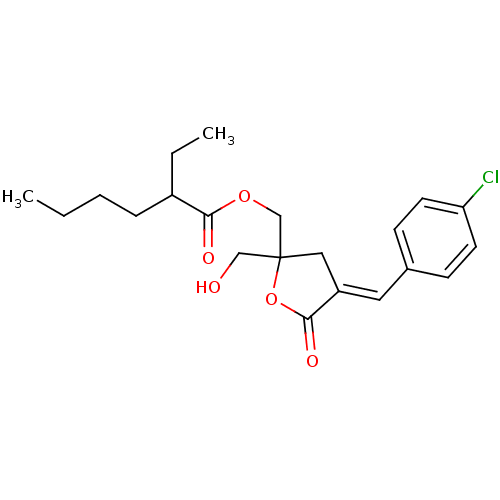 (CHEMBL488500 | rac-(E)-{4-[(4-Chlorophenyl)methyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form mouse PKCalpha by scintillation counting | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (MOUSE) | BDBM50244414 (CHEMBL488500 | rac-(E)-{4-[(4-Chlorophenyl)methyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form mouse PKCalpha by scintillation counting | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (MOUSE) | BDBM50244648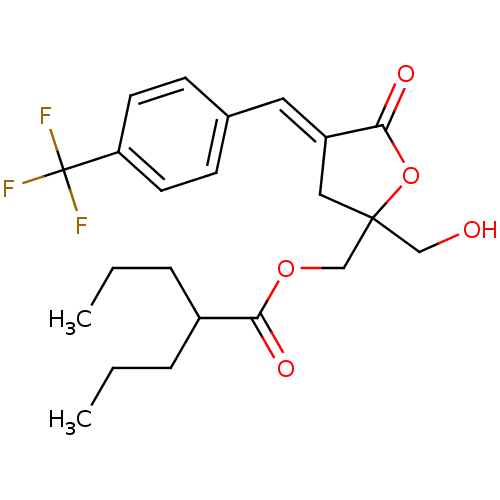 (CHEMBL453598 | rac-(E)-(2-(Hydroxymethyl)-5-oxo-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form mouse PKCalpha by scintillation counting | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (MOUSE) | BDBM50244648 (CHEMBL453598 | rac-(E)-(2-(Hydroxymethyl)-5-oxo-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form mouse PKCalpha by scintillation counting | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50247746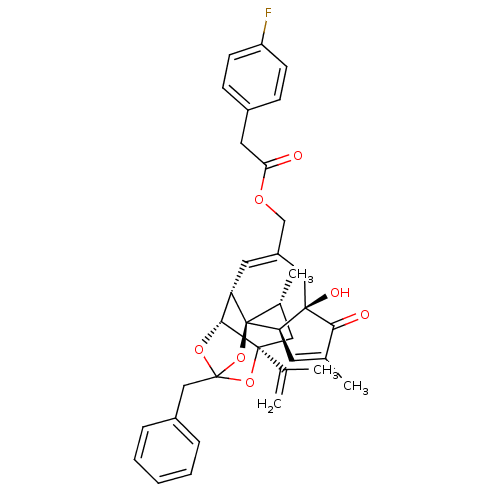 (CHEMBL502769 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50172119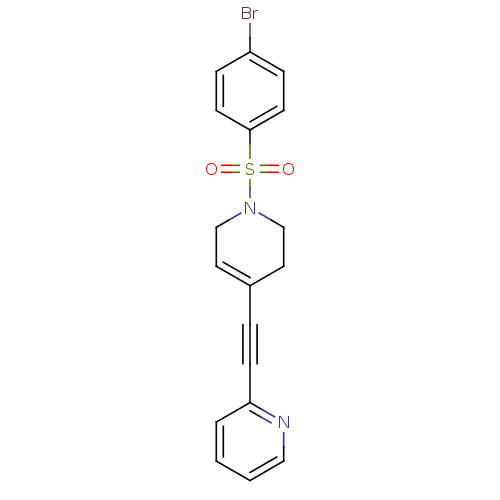 (2-[1-(4-Bromo-benzenesulfonyl)-1,2,3,6-tetrahydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from mGlu5 receptor of rat cortical membrane | Bioorg Med Chem Lett 15: 4589-93 (2005) Article DOI: 10.1016/j.bmcl.2005.06.099 BindingDB Entry DOI: 10.7270/Q2DB81C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (MOUSE) | BDBM50245049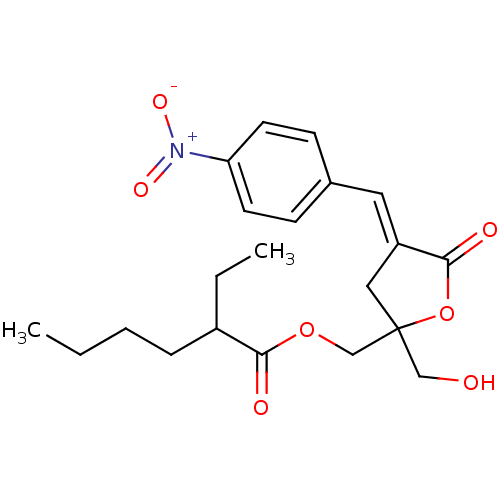 (CHEMBL488706 | rac-(E)-{2-(Hydroxymethyl)-4-[(4-ni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form mouse PKCalpha by scintillation counting | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50155000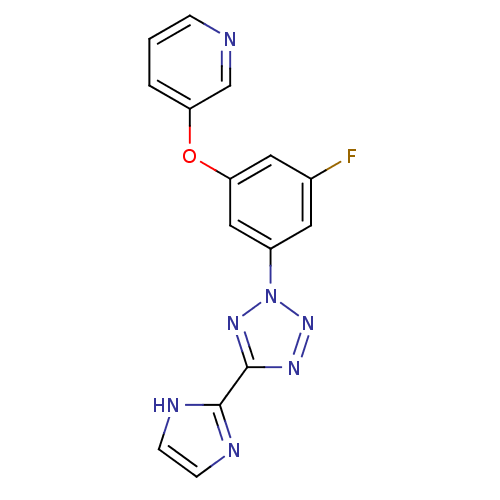 (3-{3-Fluoro-5-[5-(1H-imidazol-2-yl)-tetrazol-2-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Metabotropic glutamate receptor was determined by displacing [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from rat cortica... | Bioorg Med Chem Lett 14: 5481-4 (2004) Article DOI: 10.1016/j.bmcl.2004.09.018 BindingDB Entry DOI: 10.7270/Q25M657V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (MOUSE) | BDBM50244740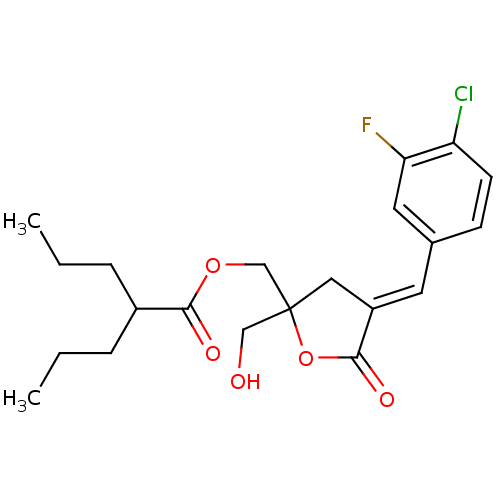 (CHEMBL461372 | rac-(E)-{4-[(3-Chloro-4-fluoropheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form mouse PKCalpha by scintillation counting | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (MOUSE) | BDBM50244740 (CHEMBL461372 | rac-(E)-{4-[(3-Chloro-4-fluoropheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form mouse PKCalpha by scintillation counting | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50155006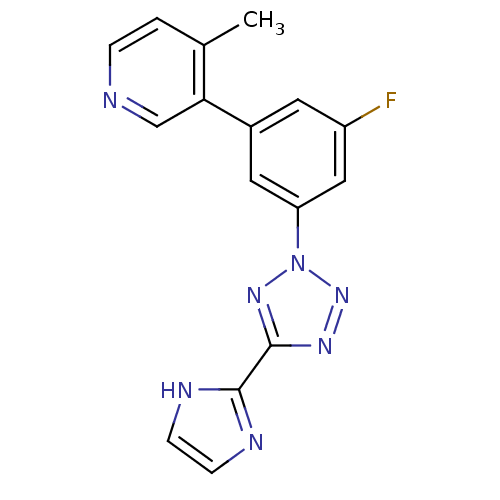 (3-{3-Fluoro-5-[5-(1H-imidazol-2-yl)-tetrazol-2-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Metabotropic glutamate receptor was determined by displacing [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from rat cortica... | Bioorg Med Chem Lett 14: 5481-4 (2004) Article DOI: 10.1016/j.bmcl.2004.09.018 BindingDB Entry DOI: 10.7270/Q25M657V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50247747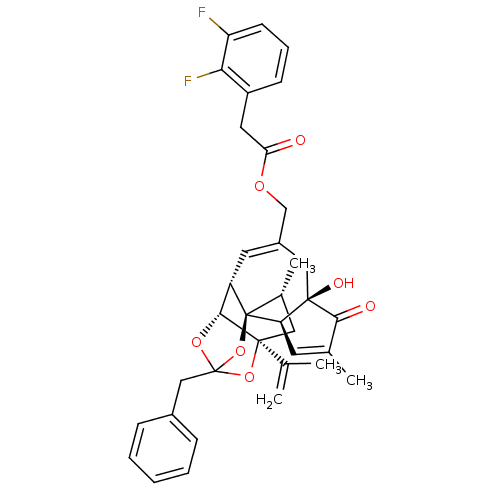 (CHEMBL509331 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (MOUSE) | BDBM50244908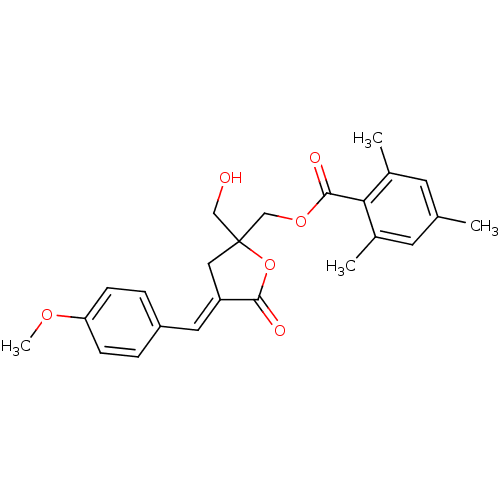 (CHEMBL511301 | rac-(E)-{2-(Hydroxymethyl)-4-[(4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form mouse PKCalpha by scintillation counting | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (MOUSE) | BDBM50244908 (CHEMBL511301 | rac-(E)-{2-(Hydroxymethyl)-4-[(4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form mouse PKCalpha by scintillation counting | J Med Chem 51: 5198-220 (2008) Article DOI: 10.1021/jm8001907 BindingDB Entry DOI: 10.7270/Q2G44Q4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2210 total ) | Next | Last >> |